Summary
Background
Structured exergaming with prescribed moderate intensity physical activity has reduced adiposity among adolescents. The extent to which adolescents reduce adiposity when allowed to self-select intensity level is not known.
Objective
The objective of the study was to examine the influence of exergaming on adolescent girls’ body composition and cardiovascular risk factors.
Methods
This randomized controlled trial assigned 41 overweight and obese girls aged 14 to 18 years to group-based dance exergaming (36 h over 3 months) or to a self-directed care control condition. Body size and composition were measured by anthropometry, dual-energy X-ray absorptiometry [%fat and bone mineral density {BMD}] and magnetic resonance imaging. Cardiovascular risk factors included blood pressure, cholesterol, triglycerides, glucose and insulin.
Results
Attrition was 5%. Using analysis of covariance controlling for baseline value, age and race, there were no significant condition differences. Per protocol (attended >75%), the intervention group significantly decreased abdominal subcutaneous adiposity and increased trunk and spine BMD (ps < 0.05). Per protocol (>2600 steps/session), the intervention group significantly decreased leg %fat and decreased abdominal subcutaneous and total adiposity (ps < 0.05).
Conclusion
Exergaming reduced body fat and increased BMD among those adolescent girls who adhered. Further research is required before exergaming is recommended in clinical settings.
Keywords: video games, exercise, body fat, bone density
Only 27% of adolescents achieve 60 min of daily moderate-to-vigorous physical activity (MVPA) (1) as recommended by the Physical Activity Guidelines for Americans (2). Insufficient physical activity contributes to obesity, which affects 18% of adolescent girls in the USA (3). Two-thirds of obese adolescents have at least one risk factor for cardiovascular disease (4), and obesity places children at increased life-time risk of skeletal fractures because of disrupted bone development (5). The majority of children lose interest in traditional exercise during adolescence, with up to 80% of children dropping out of organized-sports activities between the ages of 12 and 17 years and even higher drop-out rates among adolescent girls (6). There is a need for non-traditional physical activity interventions to engage overweight and obese adolescent girls for sufficient duration to result in significant, lasting health improvements.
Exergames, which are active video games that require gross motor movement, provide a unique opportunity to increase physical activity in adolescents’ daily lives. Over 60% of adolescents play video games for an average of 73 min/d (7). Systematic reviews indicate that exergames elicit moderate intensity levels of physical activity (8) while being perceived as fun and enjoyable (9). Engaging the lower limbs during exergaming achieves light-to-moderate intensity physical activity (10), and dance exergaming has produced MVPA levels among adolescent girls (11).
To accrue weight loss and cardiovascular health benefits, adolescents would need to exergame at levels of MVPA for a sufficient duration and on a continual basis. A 20-week school-based exergaming trial used pre-assigned exergaming routines and observed 1.6 kg weight loss among overweight and obese adolescents who played exergames in a team (12). However, providing adolescents with control over game play, i.e. allowing them to select games to play and intensity level of play, is a key intrinsic motivator. Most importantly, allowing children to play at their self-selected level represents a naturalistic depiction of how the exergames are actually played. The use of pre-assigned routines produces boredom during exergaming (13) and is not characteristic of how adolescents typically play exergames, whereas allowing adolescents to select intensity level to tailor to their capabilities is important to encourage adherence (14). It is not known if allowing adolescents to self-select intensity level will achieve the same health improvements as observed with pre-assigned routines.
The current study aimed to (i) determine the feasibility of completing a 12-week supervized, group-based exergaming intervention with overweight and obese adolescent girls self-selecting intensity level and (ii) examine the intervention’s effects on body composition and cardiovascular risk factors. Dance exergames were selected for the appeal of dance to adolescent girls (15). The hypotheses were as follows:
Hypothesis 1: It will be feasible to recruit, enroll and sustain adolescent girls’ participation in a 12-week group-based exergaming programme.
Hypothesis 2: Participants randomized to the exergaming intervention will exhibit improvements in body composition and cardiovascular risk factors compared with a self-directed care control group.
Methods
Participants
Overweight and obese adolescent girls were recruited for study participation through school health clinics, doctors’ offices, community events, news media, social media sites and listserv emails. Out of 200 adolescents who expressed interest in the study, 41 met eligibility criteria and were randomly assigned to either the exergaming intervention (n = 22) or control group (n = 19; See Supporting information Figure S1). Eligible participants were between 14 and 18 years of age, female, post-menarcheal and had a body mass index (BMI) percentile ≥85th. Exclusion criteria included being pregnant, suffering from a serious medical condition that contraindicated physical activity or video game play (i.e. physical disability, cardiovascular disease, severe asthma and epilepsy), recent hospitalization for mental illness or inability to comply with study procedures. All study procedures were approved by the Pennington Biomedical Research Center Institutional Review Board.
Procedures
Parents of interested participants completed a screening form online or over the phone. If participants met basic eligibility criteria, parents were contacted by a study coordinator to schedule a clinic visit. At this baseline visit, participants consented to study participation (or a parent/legal guardian provided consent and the participant provided written assent if the participant was a minor). Participants who met all eligibility criteria were randomly assigned by an interventionist into either the exergaming or control group. A biostatistician generated the allocation sequence using block randomization to balance race distribution within condition. Each participant in the exergaming condition started the 12-week intervention within 4 weeks of baseline assessment. Investigators and assessment staff remained blinded to the participant’s condition for the duration of the study.
Participants randomized to the exergaming intervention joined ‘Klub Kinect’and were scheduled to attend three 1-h exergaming sessions per week for 12weeks. Sessions occurred during the afternoon and early evening at Pennington Biomedical. Exergaming participants danced for 60 min at each session concurrently with other participants, using dance video games compatible with the Kinect® for the Xbox 360® (Microsoft, Redmond, WA) gaming console. Three to four exergaming stations were set up in the classroom, and participants could choose among the games Just Dance (versions 3, 4, 2014, and Greatest Hits) and Dance Central (versions 2 and 3). Participants were allowed to self-select the games, songs, dance mode, intensity level and dance partner(s); the only requirement was that they play dance video games for 60 min at each session. Three ‘Gaming Coaches’ supervised the sessions to provide ongoing motivation to participants. Incentive items that totaled less than $15 per participant were provided throughout the 12 weeks to encourage attendance and motivate participants to exercise at a high level.
Participants randomized to the control condition did not take part in the exergaming intervention and were asked to maintain their normal level of physical activity for 12 weeks. Following the 12-week intervention period, participants from both conditions completed a follow-up clinic visit. Follow-up visits occurred within 2.5 weeks of intervention end-date. All participants received up to $300 in compensation throughout the study to partially offset travel costs and were offered a 1-h nutrition counselling session after the final clinic visit.
Measures
Screening
Race/ethnicity was parent-reported. A brief physical examination was completed by a physician, physician’s assistant or nurse practitioner during the baseline visit to determine if the participant was physically capable of participating in the intervention without safety concerns. A resting electrocardiogram was completed at baseline and was reviewed by a study physician. No participants had abnormal electrocardiograms.
Baseline visit
Anthropometry was measured to the nearest 0.1 cm (0.1 kg for weight) at baseline and follow-up. All measurements were taken twice with the reported value being the average of both measurements. If the measures differed by greater than 0.5 units, a third measurement was taken to replace the discrepant value. Standing height was measured using a Harpenden stadiometer (Holtain Limited, Crymych, UK), and weight was measured using a Michelli GSE 460 scale (G.T. Michelli Co., Baton Rouge, LA). Waist circumference was measured at the natural waist (halfway between inferior border of rib cage and superior aspect of iliac crest). BMI percentile was calculated based on the participant’s age, height and weight using the CDC 2000 growth chart.
Blood pressure was measured at baseline and follow-up after the participant rested for 5 min in a quiet room. The average of two systolic and diastolic measurements was used for analysis, and blood pressure percentile was calculated based on age, sex and height (16). Blood samples were collected at baseline and follow-up after a 10-h fast. Samples were assessed for total cholesterol, triglycerides, glucose, insulin and high-density lipoprotein (HDL)-cholesterol. Low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald equation: LDL = total cholesterol − [{triglycerides/5} + HDL].
Body composition was assessed by whole-body dual-energy X-ray absorptiometry (DXA) using a GE Lunar iDXA scanner (GE Medical Systems, Milwau-kee, WI) to quantify total and regional body fat. Regions were automatically delineated using the software enCore 2011 version 13.60.033. Percent fat (% fat) of the whole body and specific regions [i.e. trunk {shoulder to pelvis region}, gynoid {hip to thigh region}, android {waist to pelvis region} and leg] were calculated as fat mass divided by the sum of fat and lean mass. Bone mineral density (BMD in g cm−2) and bone mineral content (in g) were calculated for total (whole body) and specific regions [i.e. trunk {shoulder to pelvis region} and spine].
Body composition was also measured with magnetic resonance imaging using a General Electric Signa Excite 3.0 Tesla (GE Medical Systems, Milwaukee, WI) to quantify abdominal total (TAT), subcutaneous (SAT), and visceral adiposity (VAT). An eight-channel torso array coil passed over the abdomen, capturing a series of five to eight cross-sectional scans (4.78 cm apart) from the top of the liver to the bottom of the right kidney. Visceral adipose tissue was quantified using the CNS Software Analyze package, with each slice’s area multiplied by the slice gap (28 slices) by 0.000001 by voxel depth. The volumes were then summed to estimate total visceral adipose tissue volume (litres) and multiplied by 0.9193 to calculate total mass (kilogrammes). A negative urine pregnancy test was required prior to undergoing dual-energy X-ray absorptiometry or magnetic resonance imaging scans.
A medical history survey was completed by participants with parental assistance at baseline to collect information about the participant’s individual and family medical history. This survey was administered on a computer using research electronic data capture electronic data capture tools hosted at Pennington Biomedical Research Center (17).
Intervention fidelity
Participant weight and attendance were recorded at each session by an interventionist. At the end of each session, participants reported the amount of time spent exergaming and number of steps taken. Participants used stopwatches and wore Omron GoSmart pedometers (Omron Healthcare Inc., Bannockburn, Illinois) during each session to aid in reporting this information.
Statistical analyses
To test Hypothesis 1, descriptive statistics were calculated to describe the recruitment, enrollment and retention of participants. For the intervention participants, mean steps/session were calculated to determine amount of physical activity attained.
To test Hypothesis 2, analysis of covariance controlling for baseline value, age and race was computed for each variable of interest (i.e. body composition: BMI z-score, waist circumference, total body fat, regional body fat, visceral and subcutaneous abdominal adiposity, and total and regional bone mineral content and BMD; and cardiovascular risk factors: blood pressure percentile, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides and glucose). Analyses were performed as intent-to-treat and per protocol based on intervention attendance and steps/session. Adherence was considered to be attendance of ≥75% of sessions (≥27 of 36 sessions) or achievement of an average of >2600 steps/session (to align with the median step count).
Results
Forty-one participants were successfully recruited and enrolled into the study. The enrolled sample was 64.3% Black/African American and 35.7% White, with 2.4% of participants reporting Hispanic ethnicity. Average age at enrollment was 16 ± 1.4 years (see Table 1 for baseline descriptive characteristics of the sample).
Table 1.
Baseline descriptive characteristics of sample
| Intervention (n = 22) | Control (n = 19) | |
|---|---|---|
| Age (years) | 15.3 (1.2) | 16.1 (1.4) |
| Race | ||
| White (%) | 36 | 39 |
| African American (%) | 64 | 61 |
| Anthropometry | ||
| Weight (kg) | 97.2 (27.3) | 101.1 (26.1) |
| Height (cm) | 160.4 (5.9) | 164.9 (5.8) |
| BMI (z-score) | 2.1 (0.5) | 2.1 (0.5) |
| BMI (percentile) | 97.4 (2.9) | 97.1 (3.3) |
| Waist circumference (cm) | 107.3 (22.0) | 107.7 (21.1) |
| DXA | ||
| Total body fat (kg) | 47.0 (19.7) | 48.0 (17.0) |
| Total body fat (%) | 48.3 (7.7) | 48.2 (6.3) |
| Leg fat (kg) | 16.2 (5.6) | 16.2 (4.7) |
| Leg %fat | 48.3 (5.6) | 48.0 (5.2) |
| Gynoid fat (kg) | 7.6 (2.5) | 8.1 (2.2) |
| Gynoid %fat | 49.8 (6.5) | 50.1 (4.9) |
| Android fat (kg) | 4.2 (2.2) | 4.4 (2.3) |
| Android %fat | 53.8 (10.5) | 54.3 (9.2) |
| Trunk fat (kg) | 25.1 (13.6) | 26.1 (12.1) |
| Trunk %fat | 50.6 (9.5) | 50.5 (8.1) |
| BMC (kg) | 2.6 (0.3) | 2.7 (0.3) |
| BMD (g cm−2) | 1.2 (0.1) | 1.2 (0.1) |
| BMD trunk (g cm−2) | 1.1 (0.1) | 1.1 (0.1) |
| BMD spine (g cm−2) | 1.1 (0.1) | 1.2 (0.1) |
| BMD leg (g cm−2) | 1.3 (0.1) | 1.3 (0.1) |
| MRI † | ||
| Visceral adipose tissue (kg) | 1.0 (0.7) | 1.0 (0.7) |
| Subcutaneous adipose tissue (kg) | 12.1 (5.6) | 12.3 (5.7) |
| Total adipose tissue (kg) | 13.1 (5.9) | 13.3 (6.3) |
| Cardiovascular risk factors | ||
| SBP (percentile) | 63.5 (25.2) | 56.6 (30.1) |
| DBP (percentile) | 60.9 (22.3) | 66.4 (20.5) |
| Cholesterol (mg dL−1) | 149.6 (21.6) | 163.1 (27.3) |
| HDL-C (mg dL−1) | 47.4 (7.3) | 52.7 (10.5) |
| LDL-C (mg dL−1) | 86.4 (19.2) | 94.4 (22.9) |
| Triglycerides (mg dL−1) | 78.9 (49.4) | 80.0 (42.4) |
| Glucose (mg dL−1) | 87.6 (6.4) | 91.8 (21.8) |
| Insulin (μU mL−1) | 24.2 (15.2) | 19.4 (10.4) |
Note. Mean values (standard deviation).
MRI sample size was n = 21 in intervention, n = 17 in control.
BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SBP, systolic blood pressure.
Attrition was 5%, with two participants dropping out of the intervention condition: one dropped out at the first session because of lack of interest, and one dropped out due to mental health hospitalization. One control participant was removed from the analysis because of returning more than 2.5 weeks past the final visit end-date. No study-related adverse events or side effects were reported.
Participants exergamed for an average of 61.2 ± 6.5 min per session. On average, intervention participants performed 2756 steps per 60 min (see Supporting information Figure S2) and attended 79% of the 36 sessions. Thirteen of the 20 intervention participants (65%) adhered to ≥75% of sessions, and 11 intervention participants (55%) achieved >2600 steps/session. Seven participants met both adherence metrics.
In the intent-to-treat analyses, there were no significant condition differences in any body composition or cardiovascular risk factor variables. Effect estimates from analysis of covariance models are reported in Table 2. In the per protocol analysis based on attendance, compared with the control group, the intervention group significantly decreased SAT (p = 0.048) (Fig. 1). The intervention group significantly increased trunk BMD (p = 0.03) and spine BMD (p = 0.008). No other body composition or cardiovascular risk factor variable was significantly different by condition in the per protocol analysis based on attendance.
Table 2.
Model effects of changes in body composition and cardiovascular risk factors by condition
| Change in control |
Intent-to-treat | Per protocol (>75% attendance) | Per protocol (>2600 steps/session) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Change in intervention |
Difference between conditions |
p value | Difference between conditions |
p value | Difference between conditions |
p value | ||
| Anthropometry | n = 18 | n = 20 | n = 13 | n = 11 | ||||
| BMI (z-score) | 0.004 (0.02) | −0.002 (0.02) | −0.01 (0.03) | −0.01 (0.03) | −0.04 (0.03) | |||
| BMI (%) | 0.1 (0.2) | −0.1 (0.2) | −0.2 (0.3) | −0.2 (0.4) | −0.4 (0.4) | |||
| WC (cm) | 0.5 (1.3) | 0.5 (1.2) | −0.04 (1.7) | 1.0 (1.9) | −0.1 (2.3) | |||
| DXA | ||||||||
| Total body fat (g) | 1593.8 (536.6) | 994.9 (525.4) | −599.0 (742.2) | −1004.1 (874.1) | −1483.9 (821.5) | 0.08 | ||
| Total body fat (%) | 0.8 (0.3) | 0.5 (0.3) | −0.3 (0.5) | −0.68 (0.53) | 0.8 (0.5) | 0.10 | ||
| Gynoid fat (g) | 337.6 (130.2) | 194.9 (127.5) | −142.7 (180.2) | −254.7 (205.6) | −316.8 (212.6) | 0.15 | ||
| Gynoid fat (%) | 1.1 (0.4) | 0.7 (0.3) | −0.4 (0.5) | −0.9 (0.5) | 0.06 | −0.9 (0.5) | 0.06 | |
| Leg fat (g) | 581.6 (505.1) | 352.2 (494.5) | −299.5 (698.6) | −586.8 (803.6) | −672.6 (831.0) | |||
| Leg fat (%) | 0.8 (0.4) | 0.3 (0.4) | −0.6 (0.5) | −1.1 (0.5) | 0.06 | −1.1 (0.5) | 0.049 | |
| Android fat (g) | 78.6 (66.9) | 113.9 (65.5) | 35.2 (92.5) | −23.2 (112.4) | −92.3 (112.7) | |||
| Android fat (%) | 0.7 (0.5) | 0.9 (0.5) | 0.2 (0.7) | −0.3 (0.8) | −0.4 (0.8) | |||
| Trunk fat (g) | 911.3 (404.8) | 476.2 (396.3) | −435.1 (559.9) | −435.5 (638.2) | −669.4 (637.1) | |||
| Trunk fat (%) | 0.8 (0.5) | 0.7 (0.5) | −0.04 (0.6) | −0.4 (0.8) | −0.6 (0.7) | |||
| BMC (g) | 13.0 (10.8) | 14.0 (10.6) | 1.0 (14.9) | 22.9 (14.3) | 0.12 | −12.4 (17.2) | ||
| BMD total (g cm−2) | 0.01 (0.004) | 0.01 (0.004) | 0.003 (0.01) | 0.01 (0.01) | 0.07 | 0.0 (0.01) | ||
| Trunk, (g cm−2) | 0.003 (0.01) | 0.01 (0.01) | −0.01 (0.01) | 0.02 (0.01) | 0.03 | 0.002 (0.01) | ||
| Spine, (g cm−2) | 0.002 (0.01) | 0.02 (0.01) | 0.01 (0.01) | 0.03 (0.01) | 0.008 | 0.001 (0.01) | ||
| Leg, (g cm−2) | 0.01 (0.01) | 0.002 (0.01) | −0.01 (0.01) | −0.01 (0.01) | −0.004 (0.01) | |||
| MRI | n=15 | n=18 | n=13 | n=10 | ||||
| VAT (kg) | 0.04 (0.03) | 0.02 (0.03) | −0.01 (0.1) | 0.02 (0.1) | −0.1 (0.1) | |||
| SAT (kg) | 0.6 (0.2) | 0.3 (0.2) | −0.3 (0.2) | 0.12 | −0.5 (0.2) | 0.048 | −0.6 (0.2) | 0.02 |
| TAT (kg) | 0.7 (0.2) | 0.3 (0.2) | −0.4 (0.2) | 0.13 | −0.5 (0.3) | 0.08 | −0.7 (0.2) | 0.01 |
| CV risk factors | n=18 | n =20 | n=13 | n=11 | ||||
| SBP, % | −1.75 (6.4) | −13.3 (6.3) | −11.5 (8.9) | −7.2 (10.8) | −7.7 (10.9) | |||
| DBP, % | −7.70 (4.9) | −3.8 (4.8) | 3.9 (6.8) | 4.1 (8.3) | −2.8 (8.4) | |||
| Cholesterol (mg dL−1) | −0.31 (4.1) | 8.0 (4.0) | 8.3 (5.6) | 0.15 | 3.0 (5.7) | −2.2 (5.4) | ||
| HDL-C (mg dL−1) | −1.08 (1.7) | 1.5 (1.6) | 2.6 (2.3) | 1.2 (2.3) | 1.1 (2.5) | |||
| LDL-C (mg dL−1) | 0.78 (3.0) | 3.9 (2.9) | 3.1 (4.2) | 0.4 (4.4) | −4.5 (4.0) | |||
| Triglycerides (mg dL−1) | −0.03 (9.2) | 13.0 (9.0) | 13.0 (12.7) | 6.9 (14.9) | 6.0 (13.2) | |||
| Glucose (mg dL−1) | 11.61 (6.7) | 1.5 (6.5) | −10.1 (9.2) | −8.6 (11.6) | −10.6 (12.6) | |||
| Insulin (μU mL−1) | 4.07 (2.2) | 1.9 (2.2) | −2.2 (3.0) | −4.4 (2.7) | 0.12 | −4.4 (2.3) | 0.07 | |
Note. Values are model effects (standard error). Difference between conditions represents intervention change score minus control change score. P values indicate significant differences between intervention vsersus control based on estimated means in analysis of covariance models controlling for age, race and baseline value. P values < 0.20 are reported.
BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; CV, cardiovascular; DBP, diastolic blood pressure; DXA, dual-energy X-ray absorptiometry; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Figure 1.
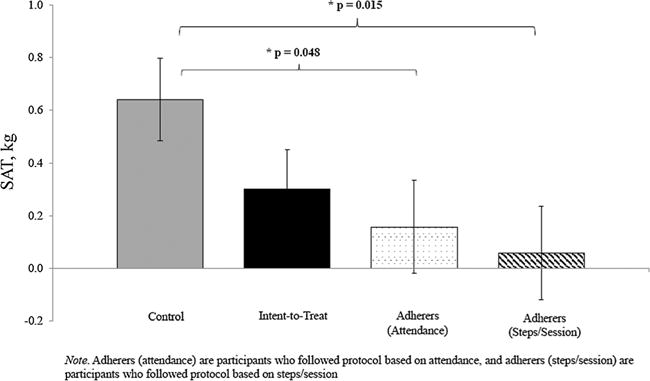
Change in subcutaneous adipose tissue after 12-week exergaming intervention.
In the per protocol analysis based on steps/session, compared with the control group, the intervention group significantly decreased SAT (p = 0.02), TAT (p = 0.01), and leg %fat (p = 0.049). No other body composition or cardiovascular risk factor variable was significantly different by condition in the per protocol analysis based on steps/session.
Discussion
The purpose of this study was to examine the feasibility of recruiting, enrolling and sustaining participation among overweight and obese adolescent girls in a 12-week exergaming intervention that involved 36 h of group-based dance exergaming and to examine the intervention’s effects on body composition and cardiovascular risk factors. Recruitment and enrollment were successful, and the average attendance of 79% in the exergaming condition to 36 sessions over 12 weeks was higher than prior exergaming trials (12). Home-based gaming has not been successful in achieving children’s adherence to the prescribed amount of exergaming, with sharp declines in exergaming within the first several weeks (18–20). The present study provided coach support in a group-based context, which was previously demonstrated to increase intrinsic motivation and energy expenditure during bouts of exergaming (13).
As predicted, participants assigned to the exergaming intervention reduced adiposity compared with those in the control condition, but these significant findings were only observed in the subset who adhered to the protocol (i.e. attended at least 75% of the sessions; achieved at least 2600 steps/session), and there were no other significant effects on cardiovascular risk factors. Whereas prior interventions that successfully decreased adolescents’ body weight prescribed the intensity of the exergaming (12), the present study allowed the participants to select intensity level. The present intervention was structured on a physical activity trial by Lee et al. that provided the same duration of aerobic activity (12 weeks of 180 min/week) and significantly reduced adolescent girls’ total body fat and visceral adiposity without weight change (21). However, the prior trial involved exercise on treadmills and ellipticals, and heart rate monitors were used to ensure adolescents met intensity levels of 60–75% of Vo2peak. Based on calculations that adolescent girls should take between 11 500 and 14 000 steps/day to achieve the recommended 60-min of MVPA (22), the average 2756 steps/session from the exergaming sessions represented between 20 and 24% of the steps needed to achieve an equivalent aerobic intensity dose as delivered in the Lee et al. intervention (21). The present study provides evidence that dance-based exergames can provide sufficient aerobic intensity to reduce adiposity among those who adhere, even when the intensity is self-selected. Yet the self-selected intensity did not produce a sufficient BMI z-score decrease (e.g. at least 0.25 to 0.50) (23,24) or adiposity reduction (21) to achieve the cardiometabolic health improvements observed in prior trials.
The exercise and reduced adiposity involved in this exergaming intervention was sufficient to protect against BMD loss in obese adolescent girls who adhered to the protocol. Retaining bone mass is particularly important among adolescents with obesity, as obesity is inversely associated with whole-body and spine BMD in adolescent girls, especially girls with one or more cardiometabolic risk factors (25). Obesity thereby places adolescents at higher risk for bone fractures and osteoporosis (26). Sustained exercise and weight loss attenuate BMD loss in adults (27), but the optimal amount of weight loss or exercise to attenuate BMD loss in adolescents is unknown (25). A physical training programme (3 h/week over 6 months) was not sufficient to alter BMD among obese pre-pubertal children aged 9 to 12 years (28), whereas the present intervention of 36 h of exergaming did attenuate BMD loss in post-pubertal girls. Further research should investigate the intensity and frequency of exergaming needed to achieve clinically significant increases in BMD in this population and the underlying mechanisms that drive these associations.
Strengths of this study included the randomized controlled trial design and the gold standard measurements of body fat, bone mineral density and cardiovascular risk factors. By allowing adolescents to self-select intensity level, the study was designed for generalizability such that this intervention could be implemented in the school, gym or home setting with other students, friends and family members, without the need for prescribed routines or heart rate monitoring. The sample was two-thirds African American, and all participants were overweight or obese, including several who were severely obese. Participants self-selected their intensity level which was both a strength for generalizability and a limitation for internal validity. A potential limitation for the generalizability of this intervention in other settings is the reliance on coaches to support gaming, although similar support could be provided by teachers or family members. To enhance the potential for adiposity reduction and improvement in cardiovascular health, future studies should examine ways to increase the intensity level of exergaming to match the aerobic dose delivered in exercise training studies (21). Additionally, future studies should investigate the role of exergaming as a physical activity tool imbedded in a multidisciplinary weight loss intervention that includes both dietary and behavioural counselling (29).
Conclusion
In conclusion, 36h of group-based dance exergaming over 12weeks attenuated adiposity gain and increased BMD among participants who adhered to the intervention, even when adolescents, were allowed to self-select intensity levels. Exergames may address a key challenge to adolescent girls’ participation in physical activity by providing a fun and enjoyable activity that will be sustained over time. Ensuring that adolescents play these exergames with sufficient frequency, intensity and duration is essential to reap cardiovascular health benefits.
Supplementary Material
Acknowledgments
We gratefully acknowledge the efforts of the Preventive Medicine team and the clinic staff at Pennington Biomedical Research Center. AES and RAB had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding source
All phases of this study were supported by Pennington Biomedical Research Center. This work was partially supported by a NORC Center Grant # P30DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ and 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. PTK is supported, in part, by the Marie Edana Corcoran Endowed Chair in Pediatric Obesity and Diabetes. The funders did not play a role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the publication.
Footnotes
Conflicts of interest
The authors have indicated they have no potential conflicts of interest to disclose.
Author contributions
AES conceptualized and designed the study and drafted the initial manuscript. AMM coordinated data collection and the intervention and critically reviewed and revised the manuscript. RAB carried out the randomization and all statistical analyses and critically reviewed and revised the manuscript. DSH provided medical oversight of the clinic visits and intervention and critically reviewed and revised the manuscript. PTK and RLN provided scientific contributions to the conception, design and execution of the intervention and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Clinical trial registration
ClinicalTrials.gov, NCT02003963, https://clinicaltrials.gov/ct2/show/NCT02003963?term=klub+kinect&rank=1
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
CONSORT diagram.
Average steps per exergaming session over 36 sessions.
References
- 1.Kann L, Kinchen S, Shanklin SL, et al. Youth risk behavior surveillance – United States, 2013. MMWR Surveill Summ. 2014;63(Suppl 4):1–168. [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. US Government Printing Office; Washington, DC: 2008. 2008. [Google Scholar]
- 3.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–1376. doi: 10.1161/CIRCULATIONAHA.113.001559. [DOI] [PubMed] [Google Scholar]
- 4.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999 2008. Pediatrics. 2012;129:1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 5.Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J Bone Miner Res. 2010;25:527–536. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- 6.Slater A, Tiggemann M. ‘Uncool to do sport’: a focus group study of adolescent girls’ reasons for withdrawing from physical activity. Psychol Sport Exerc. 2010;11:619–626. [Google Scholar]
- 7.Rideout VJ, Foehr UG, Roberts DF. Generation M2: Media in the Lives of 8- to 18-year-olds. Menlo Park: California; 2010. [Google Scholar]
- 8.LeBlanc AG, Chaput JP, McFarlane A, et al. Active video games and health indicators in children and youth: a systematic review. PLoS One. 2013;8:e65351. doi: 10.1371/journal.pone.0065351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Z, Chen S, Pasco D, Pope Z. A meta-analysis of active video games on health outcomes among children and adolescents. Obes Rev. 2015;16:783–794. doi: 10.1111/obr.12287. [DOI] [PubMed] [Google Scholar]
- 10.O’Donovan C, Roche EF, Hussey J. The energy cost of playing active video games in children with obesity and children of a healthy weight. Int J Pediatr Obes. 2014;9:310–317. doi: 10.1111/j.2047-6310.2013.00172.x. [DOI] [PubMed] [Google Scholar]
- 11.Fawkner SG, Niven A, Thin AG, Macdonald MJ, Oakes JR. Adolescent girls’ energy expenditure during dance simulation active computer gaming. J Sports Sci. 2010;28:61–65. doi: 10.1080/02640410903369935. [DOI] [PubMed] [Google Scholar]
- 12.Staiano AE, Abraham AA, Calvert SL. Adolescent exergame play for weight loss and psychosocial improvement: a controlled physical activity intervention. Obesity. 2013;21:598–601. doi: 10.1038/oby.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staiano AE, Abraham AA, Calvert SL. Motivating effects of cooperative exergame play for overweight and obese adolescents. J Diabetes Sci Technol. 2012;6:812–819. doi: 10.1177/193229681200600412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deforche B, Lefevre J, De Bourdeaudhuij I, Hills AP, Duquet W, Bouckaert J. Physical fitness and physical activity in obese and nonobese Flemish youth. Obes Res. 2003;11:434–441. doi: 10.1038/oby.2003.59. [DOI] [PubMed] [Google Scholar]
- 15.Robinson TN, Killen JD, Kraemer HC, et al. Dance and reducing television viewing to prevent weight gain in African–American girls: the Stanford GEMS pilot study. Ethn Dis. 2003;13(1 Suppl 1):S65–S77. [PubMed] [Google Scholar]
- 16.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(Suppl 2):555–576. [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen KA, Yen S, Wlasiuk L, Newman TB, Lustig R. Feasibility of a dance videogame to promote weight loss among overweight children and adolescents. Arch Pediatr Adolesc Med. 2007;161:105–107. doi: 10.1001/archpedi.161.1.105-c. [DOI] [PubMed] [Google Scholar]
- 19.Baranowski T, Abdelsamad D, Baranowski J, et al. Impact of an active video game on healthy children’s physical activity. Pediatrics. 2012;129:e636–e642. doi: 10.1542/peds.2011-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman GJ, Adams MA, Ramirez ER, et al. Effects of behavioral contingencies on adolescent active video game play and overall activity: a randomized trial. Games Health J. 2013;2:158–165. doi: 10.1089/g4h.2013.0016. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305:E1222–E1229. doi: 10.1152/ajpendo.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams MA, Johnson WD, Tudor-Locke C. Steps/day translation of the moderate-to-vigorous physical activity guideline for children and adolescents. Int J Behav Nutr Phys Act. 2013;10:49. doi: 10.1186/1479-5868-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford AL, Hunt LP, Cooper A, Shield JPH. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95:256–261. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- 24.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419–422. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr. 2011;158:727–734. doi: 10.1016/j.jpeds.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Investig. 1988;81:1804. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 28.Rochefort GY, Rocher E, Aveline PC, et al. Osteocalcin–insulin relationship in obese children: a role for the skeleton in energy metabolism. Clin Endocrinol (Oxf) 2011;75:265–270. doi: 10.1111/j.1365-2265.2011.04031.x. [DOI] [PubMed] [Google Scholar]
- 29.Trost SG, Sundal D, Foster GD, Lent MR, Vojta D. Effects of a pediatric weight management program with and without active video games a randomized trial. JAMA Pediatr. 2014;168:407–413. doi: 10.1001/jamapediatrics.2013.3436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


