Introduction
A class of novel therapies leverages regenerative cell types in disease microenvironments.[1] This complex interplay challenges established good manufacturing practices, as standards and analytical tools to measure regenerative potency are missing.[2] That is, we can build the product right, but we do not know if we are building the right product. Here, we suggest that organ-chips, biomimetic in-vitro phenotyping platforms,[3,4] can serve as key quality assurance systems in regenerative medicine.[1,2,5]
Regenerative cells for tissue and organ repair
Basic, translational and clinical research have contributed to partial/palliative treatments for many acute and severe diseases. At the same time, this medical success created survived patients that suffer from degenerative and chronic disorders that are difficult and expensive to treat with conventional approaches. An interesting case study is myocardial infarction: an acute occlusion of the coronary circulation that causes widespread cell death in the heart. The mortality associated with the primary event has dropped to 5 % in hospitalized patients. Unfortunately, patients develop adverse post-ischemic remodeling that, together with the typical co-morbidities of the aging population, may degenerate into heart failure. Dramatically, survival at five years is ~30%.[1]
Regenerative medicine aims at restoring health in these chronic patients by providing the necessary stimuli to (re)activate organ-specific repair and regeneration mechanisms. In addition, and often in combination, with tissue-engineered scaffolds, regenerative cell types were tested for a variety of indications. For example, there are available cell-based regenerative products to treat cartilage defects, corneal damage, and for immunotherapy.[1] Stem cells with the ability to become heart cells directly, or to promote endogenous healing mechanisms are of particular interest for cardiac protection, repair, and regeneration.[1,5]
While a consensus is emerging that cell therapy is safe and technically feasible in humans, a comprehensive characterization of the regenerative phenotype of these cells and their mechanism of action remains elusive.[2] Interestingly, as translational scientists face the design and implementation of first-in-man and larger clinical studies, manufacturing considerations such as scalability, supply chain, and quality control have become important.[1,2] Here, we address how the incomplete characterization of cell phenotypes constitutes a major manufacturing difference between traditional and regenerative medicinal products. Further, we discuss how this difference may limit the effectiveness of current Good Manufacturing Practice (cGMP). Finally, we suggest that recently introduced organ-chips, engineered biomimetic in-vitro assays, may provide key potency tests for regenerative cells.
Good Manufacturing Practices in Regenerative Medicine
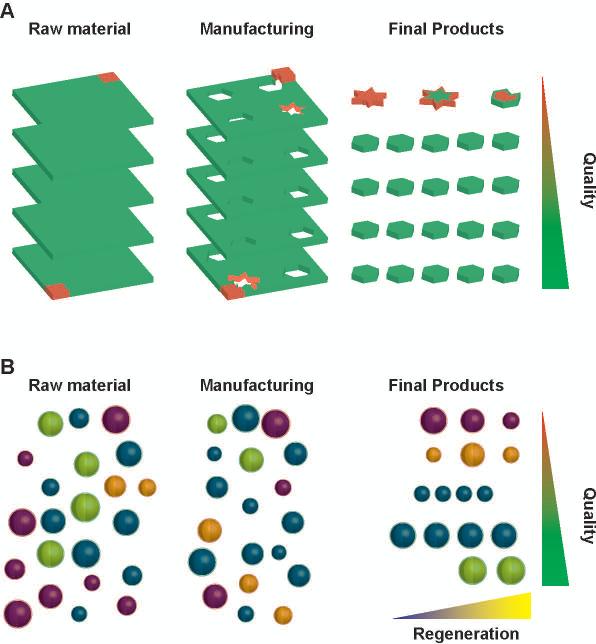
Shifting from making to manufacturing a product requires rethinking process control. The best practice evolved from controlling all aspects of the final product to ensure the identification of defective items, to controlling the fabrication process to minimize the total number of faulty items produced. Central to the definition of cGMP is the concept that the product is the process: if one were to start from identical raw materials and proceed through the same steps under the same conditions, then identical products would be obtained. Practically (Fig. 1A), we start from qualified lots of materials whose properties are defined within specific tolerances; we then use Standard Operating Procedures, user-unbiased protocols optimized to reduce variability, to obtain products whose properties lie within statistically-predictable tolerances. This approach offers a practical way to measure, optimize, and price quality. In Life Sciences, cGMP-compliant manufacturing mandates optimal quality by adopting the best raw materials, with the tightest tolerances, tested with the best available methods.[1,2]
Figure 1. Quality control in sourcing and manufacturing.
Schematics of the quality control practices in traditional (A) and stem cell (B) manufacturing. The amounts of good (green) and defective (red) products in each phase is pre-determined through complete qualification of materials (fill color) and manufacturing (edge color) steps. Unfortunately, patient-specific pools of regenerative cells are sorted based on incomplete characterization, such as the expression of surface markers (outer shell), as key parameters underlying regenerative (yellow core) and non-regenerative (blue core) phenotypes are difficult to characterize. Further how manufacturing and the interaction with the host affect the regenerative cell phenotype is also unclear. As a result, manufacturing of cGMP-grade non-regenerative cell products for regenerative medicine application may occur.
Here, we argue that this quality by design framework relies on a hidden assumption that has held true for industrial, chemical and pharmaceutical manufacturing, but that may prove challenging in regenerative medicine applications. That is, our ability to assess the quality of the final product based on the quality of its building blocks and the manufacturing steps depends on a mathematically-formalized, measurable understanding of the system. For example, we can measure the resistivity of a material (ρ) and calculate the resistance (R) of a cable manufactured from it, by virtue of the simple equation R = ρL/A (where L is the length and A the cross-section of the cable). Furthemore, measuring the resistance of a given cable is sufficient to predict the flow of direct electric current (I) given the applied voltage (V = RI) in any electrical apparatus, and so on to more complex systems. This chain of mathematical relationships, together with the completeness of the experimental characterization, enables quantitative estimates of defect propagation in the system. In the absence of such an elegant, actionable, conceptual model, the use of best practices accomplishes only half of its mandate in regenerative medicine: we can make the product right, but we do not know if we are making the right product.
For many regenerative therapies, cells are the raw material and the final product (Fig. 1B).[1,2,5] Regenerative cells are sourced based on the expression of certain surface markers (e.g. CD41, CD133 or CD117), and this antigen-based characterization becomes the reference point for further quality control. Unfortunately, this initial selection may result in pools of cells with heterogeneous regenerative potentials. Since this patient-specific heterogeneity is overlooked at sourcing, cGMP-grade manufacturing of cell-based regenerative products often start with poorly qualified materials. Furthermore, we do not possess a mathematical framework to relate measured parameters with system performance: in fact, the mechanistic relationships between cell antigen expression and tissue repair and regeneration are incompletely understood.[2] Finally, how manufacturing variables, such as culture and genetic engineering, or the interaction with the host microenvironment affect the regenerative cell phenotype is not known. Notably, cell priming and other second-generation approaches are effective at reducing the initial source of heterogeneity, but continue to suffer of the other mentioned issues. [1] Ultimately, in the absence of comprehensive assessments and mathematically rigorous frameworks to model repair and regeneration, perfectly cGMP-compliant manufacturing systems may still release cells that are safe, optimally manufactured to express the chosen surface markers, and yet ultimately ineffective regenerative products.
Organ-chips are biomimetic in-vitro platform for analytical phenotyping
In this final section, we advocate the use of organ-chips as quality assurance systems in regenerative medicine. Organ-chips are in-vitro platforms engineered to recapitulate key aspects of tissue- and organ-level physiology. Using these platforms, we can comprehensively assess cell phenotype by measuring parameters such as gene expression, structure, metabolism, and function of cells seeded on microenvironments that recapitulate healthy or diseased conditions. Furthermore, the use of patient-specific or genome-edited human cells (e.g. stem cells and their differentiated progenies) permits modeling of inherited pathologies at pre-clinical costs and on human genetic backgrounds. Finally, these simplified systems are easier to describe mathematically and offer a unique platform to validate computational modeling frameworks for regenerative medicine.[3]
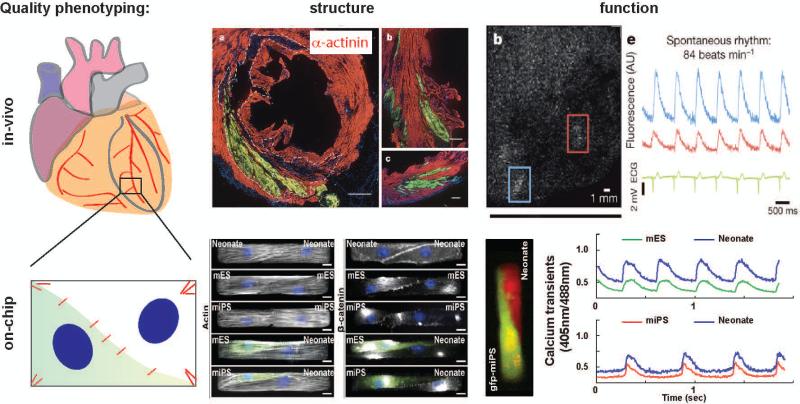
In addition to previously developed lung-, heart-, kidney-, liver-, and even multi-organ chips for drug discovery application,[3] we recently presented a cardiac cell therapy on-a-chip platform (Fig. 2).[4] We utilized a computational model of muscle mechanics and mechanotransduction to design a minimal in-vitro model of the infarct border zone. We then created microenvironments mimicking the elasticity and extra-cellular matrix composition of healthy and fibrotic myocardium. Finally, on these substrates we engineered cell pairs composed of primary and stem cell-derived cardiomyocytes, as proxies for speared and newly formed myocytes, respectively. Using this platform, we were able to recapitulate in-vitro important observations from pre-clinical and clinical studies.[4,5] For example, all pairs expressed β-catenin and connexin-43 at the cell-cell junction and the contractile architectures of cell pairs containing newly-formed myocytes were indistinguishable from that of primary pairs. These findings were consistent with the ability of most pre-/clinically injected cells to engraft in the myocardium and contribute to a reduction in infarct size.[1] For example, stem cell-derived cardiomyocytes were found to consistently engraft in a non-human primate study, where they restored ~40% of the infarct volume after ~80 days. [5] At the same time, newly-formed myocytes exhibited a large heterogeneity in calcium cycling properties, consistent with the pro-arrhythmic risk associated with cardiac cell therapy. In the same non-human primate study, stem cell-derived cardiomyocytes were found to undergo synchronous electromechanical coupling with host myocardium. [5] Finally, we could show that the intrinsic difference in contractile strength between primary and stem cell-derived cardiomyocytes was associated with the presence of substrate adhesions at the junction that preserved structural integrity but limited force transmission. We speculated that this mechanotransductive mechanism might contribute to the limited amelioration of ejection fraction observed in many in-vivo studies.[4,5] Together, these findings support the notion that organ-chips can be utilized to much more comprehensively phenotype regenerative cell products in complex microenvironments.
Figure 2. Organ-chips as quality assurance systems for regenerative medicine.
Organ-chips are engineered in-vitro platforms that enable multiparametric assessment of the phenotype of cells exposed to healthy and diseased microenvironments. We recently developed a cardiac cell therapy on-a-chip platform using primary and stem cell-derived cardiomyocytes, as proxies for speared and newly formed myocytes. Our characterization of the cell structural and functional phenotype was consistent with previously published in-vivo observations suggesting organ-chips may enable comprehensive characterization of cell products for regenerative medicine. Figure panels were reproduced with permission [4, 5].
In conclusion, we suggest that organ-chips may be valuable quality assurance systems to complement cGMP by providing in-vitro, biomimetic, comprehensive potency tests for regenerative medicine products. Additionally, organ-chips simplified biology facilitates the development of flexible mathematical and computational frameworks to link comprehensive phenotyping with mechanisms of repair and regeneration. To completely fulfill this role, we envision upgraded organ-chips featuring i) healthy and diseased micro-environments, to recapitulate genetic and acquired diseases; ii) sensors and actuators, to control the pathophysiological responses in real-time; and iii) multi-tissue and multi-organ architectures, to capture complex systemic interactions.
Acknowledgments
We acknowledge support from the following grants: DARPA BAA-11-73 “Human Microphysiological Systems Program”; NIH/NCATS UH3-TR000522 “Human Cardio-Pulmonary System on a Chip”; and NIH/NHLBI U01 HL100408 “Human Pluripotent Stem Cells and Progenitor Models of Cardiac and Blood Diseases”.
References
- 1.Terzic A, Behfar A. Stem cell therapy for heart failure: Ensuring regenerative proficiency. Trends Cardiovasc Med. S1050-1738(16):00021–9. doi: 10.1016/j.tcm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimmelman J, et al. Policy: Global standards for stem-cell research. Nature. 533(7603):311–3. doi: 10.1038/533311a. [DOI] [PubMed] [Google Scholar]
- 3.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 14(4):248–60. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aratyn-Schaus Y, Pasqualini FS, et al. Coupling primary and stem cell-derived cardiomyocytes in an in vitro model of cardiac cell therapy. J Cell Biol. 212(4):389–97. doi: 10.1083/jcb.201508026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]