Abstract
Combating antimicrobial resistance is one of the most serious public health challenges facing society today. The development of new antibiotics or alternative techniques that can help combat antimicrobial resistance is being prioritised by many governments and stakeholders across the globe. Antimicrobial photodynamic therapy is one such technique that has received considerable attention but is limited by the inability of light to penetrate through human tissue, reducing its effectiveness when used to treat deep-seated infections. The related technique sonodynamic therapy (SDT) has the potential to overcome this limitation given the ability of low-intensity ultrasound to penetrate human tissue. In this study, a Rose Bengal–antimicrobial peptide conjugate was prepared for use in antimicrobial SDT (ASDT). When Staphylococcus aureus and Pseudomonas aeruginosa planktonic cultures were treated with the conjugate and subsequently exposed to ultrasound, 5 log and 7 log reductions, respectively, in bacterial numbers were observed. The conjugate also displayed improved uptake by bacterial cells compared with a mammalian cell line (P ≤ 0.01), whilst pre-treatment of a P. aeruginosa biofilm with ultrasound resulted in a 2.6-fold improvement in sensitiser diffusion (P ≤ 0.01). A preliminary in vivo experiment involving ASDT treatment of P. aeruginosa-infected wounds in mice demonstrated that ultrasound irradiation of conjugate-treated wounds affects a substantial reduction in bacterial burden. Combined, the results obtained from this study highlight ASDT as a targeted broad-spectrum novel modality with potential for the treatment of deep-seated bacterial infections.
Keywords: Sonodynamic therapy, Antimicrobial, Sensitiser, Peptide
1. Introduction
Although the threat of antibiotic resistance has been prophesised for years, the issue has recently been described as an ‘apocalyptic scenario’ that presents ‘one of the most significant public health challenges facing society today’ [1].With 80% of gonorrhoeal infections now resistant to antibiotics and a reported 440,000 new cases of drug-resistant tuberculosis per year, it has been suggested that we are fast approaching a post-antibiotic era [2,3]. This threat is not confined to systemic infections, with the problem equally apparent in localised wound infections. Surgical wound infections account for 25% of nosocomial infections and result in a 2.5 times longer hospital stay with additional costs of ca. £5000 per patient [4]. The overall impact of this on both the patient and the health service provider is significant and highlights an urgent need for alternative therapies.
Photodynamic therapy (PDT) is a clinical treatment that uses a combination of light, molecular oxygen and a photosensitising drug to generate cytotoxic reactive oxygen species (ROS) [5]. Whilst predominantly used in the treatment of cancer, antimicrobial PDT (APDT) has also received considerable interest for the treatment of microbial infections [6–8]. The major attraction of APDT over conventional antibiotics is that multidrug-resistant strains are as easily killed as sensitive strains; moreover, because treatment results in the production of multiple forms of ROS, resistance to APDT is less likely to occur [9]. However, PDT is severely limited by the inability of light to penetrate to depth through mammalian tissue. This is due to endogenous pigments such as haem or melanin competing for light absorption with the sensitiser and is a particular problem in localised infection where the wound area may be severely discoloured due to bruising or inflammation, or in ethnic groups where the skin is naturally heavily pigmented [10]. Currently approved sensitisers absorb in the visible region of the electromagnetic spectrum, limiting light penetration to only a few millimetres and reducing the ability of APDT to eradicate bacteria localised deeper within infected wounds [11].
In recent years it has been demonstrated that many of the existing clinically-used photosensitisers can be activated by ultrasound, although the precise mechanism(s) by which this occurs remain(s) unknown [12–15]. This approach has become known as sonodynamic therapy (SDT). Ultrasound can be tightly focused, with penetration in soft tissue exceeding 10 cm depending on the frequency used [16]. The efficacy of SDT as an anticancer treatment has been demonstrated in numerous preclinical and clinical studies [17–20]. Antimicrobial SDT (ASDT) has also emerged as an active area of research, but reports to date have used clinically-unsuitable ultrasound equipment/conditions and have not explored the potential damage of the treatment to host tissue [21–23]. As the cytotoxic agent(s) involved in APDT/ASDT are indiscriminate in their action on bacterial cells and host cells, it is imperative that the sensitiser is preferentially directed to bacterial cells rather than host cells before activation with light or ultrasound. One method to achieve sensitiser selectivity is to exploit the differential binding exhibited by cationic species to the surface of bacterial and mammalian cells. For example, it has been demonstrated that light irradiation of wounds in mice treated with a poly-l-lysine–chlorin(e6) conjugate exhibited a greater bacterial kill and less host tissue damage than the free sensitiser alone [24]. Similarly, when the antimicrobial peptide (KLAKLAK)2 was conjugated to the sensitiser eosin, its antimicrobial photodynamic activity was enhanced with negligible photodamage observed to host cells [25].
Inspired by these results, we have developed a Rose Bengal–(KLAKLAK)2 conjugate for use in targeted ASDT. The potential of the conjugate to generate ROS during exposure to ultrasound was determined in cell-free solution, and the antimicrobial efficacy was established using both Staphylococcus aureus and Pseudomonas aeruginosa as target micro-organisms. The ability of the conjugate to preferentially target bacteria over healthy mammalian cells was also determined. Finally, the effectiveness of ultrasound to enhance the diffusion of sensitisers through bacterial biofilms and to impart antibacterial action was investigated.
2. Materials and methods
Detailed Materials and methods and characterisation of the Rose Bengal–C(KLAKLAK)2 conjugate are provided in the Supplementary material.
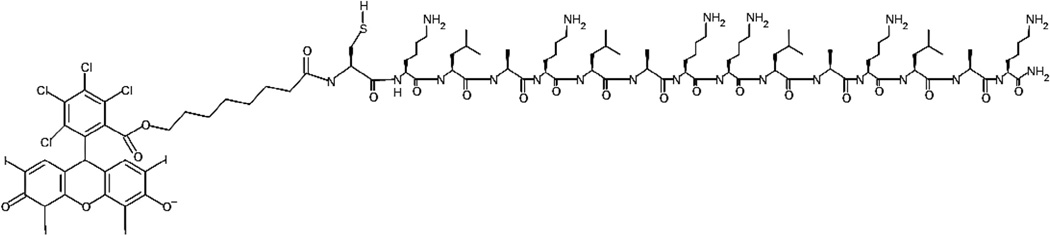
The Rose Bengal–C(KLAKLAK)2 conjugate (Fig. 1) was prepared by first synthesising the C(KLAKLAK)2 peptide using Fmoc solid-phase peptide synthesis on Rink amide resin. In parallel, a carboxylic acid derivative of Rose Bengal was also prepared by reacting Rose Bengal with 1-bromooctanoic acid. This carboxylic acid derivative was added to the N-terminus of C(KLAKLAK)2 whilst still on the resin using standard peptide coupling reagents (i.e. HOBt/HBTU). The Rose Bengal–C(KLAKLAK)2 conjugate was then cleaved from the resin and was purified using preparative reverse-phase high-performance liquid chromatography (RP-HPLC). Product formation was confirmed using matrix-assisted laser desorption/ionisation time-of-light (MALDI-TOF) and positive electrospray ionisation mass spectrometry (Supplementary Fig. S1).
Fig. 1.
Structure of Rose Bengal–C(KLAKLAK)2 conjugate.
3. Results and discussion
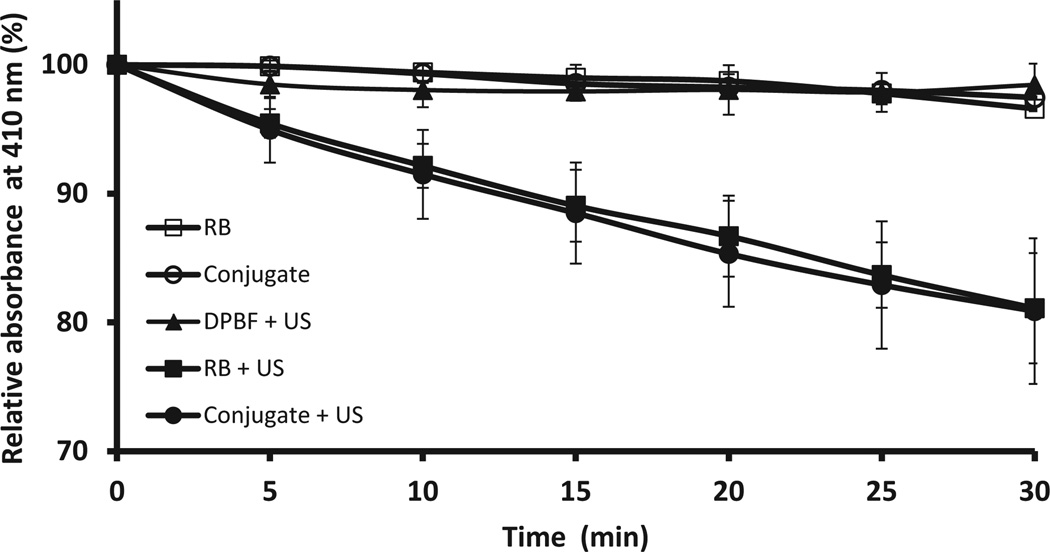
The ability of the Rose Bengal–C(KLAKLAK)2 conjugate to generate ROS upon exposure to low-intensity ultrasound was determined using the chromogenic ROS probe 1,3-diphenylisobenzofuran (DPBF) [26]. DPBF has an intense absorbance band centred at 410 nm in its native furan form but is readily bleached by ROS to the corresponding diketone. This conversion to the diketone is accompanied by a loss in absorbance at 410nmthat can be used to determine the amount of ROS produced. Solutions containing either Rose Bengal or Rose Bengal–C(KLAKLAK)2 conjugate and DPBF were treated with ultrasound for 30min and the DPBF absorbance at 410 nm was measured every 5 min. The results are shown in Fig. 2 and show a significant reduction in DPBF absorbance both for Rose Bengal and Rose Bengal–C(KLAKLAK)2 treated with ultrasound relative to the controls, indicating ROS generation upon application of ultrasound. In addition, the almost identical profile observed both for Rose Bengal and Rose Bengal–C(KLAKLAK)2 suggests that the presence of the peptide does not inhibit ultrasound-induced ROS production by the sensitiser.
Fig. 2.
Plot of 1,3-diphenylisobenzofuran (DPBF) absorbance at 410 nm against time for solutions containing Rose Bengal (RB), Rose Bengal–C(KLAKLAK)2 (conjugate), DPBF alone plus ultrasound treatment (DPBF + US), Rose Bengal plus ultrasound treatment (RB + US) and Rose Bengal–C(KLAKLAK)2 plus ultrasound treatment (conjugate + US). [RB] = [Rose Bengal–C(KLAKLAK)2] = 0.5 µM; [DPBF] = 20 µM. Ultrasound conditions: frequency 1 MHz, ultrasound power density 3.0 W/cm2, duty cycle 50%, 10 min.
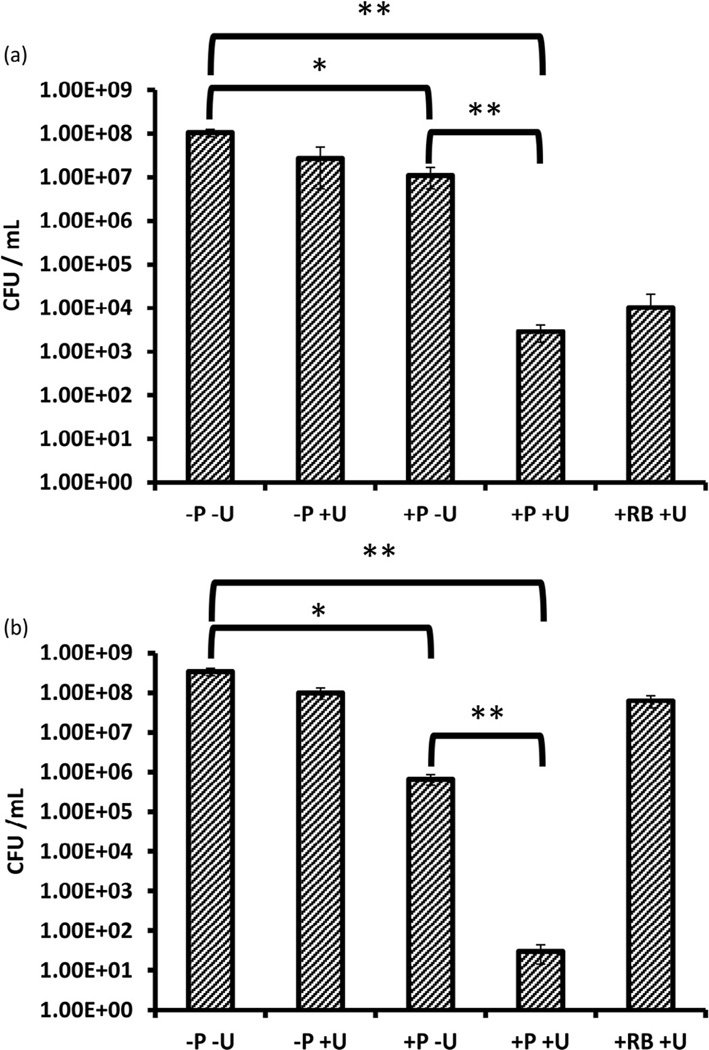
To determine the antimicrobial potential of this ROS generation, two candidate bacterial strains, the Gram-positive bacterium S. aureus and the Gram-negative bacterium P. aeruginosa, were subjected to ASDT treatment. In each case, suspensions containing 108 bacteria were added to the wells of a 96-well plate and were incubated with 10 µM Rose Bengal or Rose Bengal–C(KLAKLAK)2 for 30 min. The wells were then treated with ultrasound from the underside of the plate for either 10 min (S. aureus) or 6min (P. aeruginosa). These ultrasound exposure times represented the optimum in terms of the maximum ultrasound treatment duration that produced no toxic effects on its own (i.e. dark toxicity). Following treatment, the number of viable bacteria remaining was determined and was expressed as CFU/mL. The results reveal that ultrasound treatment of S. aureus produces only a minor reduction (ca. 0.5 log) in bacterial number that was not statistically significant (Fig. 3a). Treatment of S. aureus with Rose Bengal–C(KLAKLAK)2 in the absence of ultrasound produced an ca. 1 log reduction in bacterial number. This reduction was attributed to the antimicrobial effect from the antimicrobial peptide component of the Rose Bengal–C(KLAKLAK)2 conjugate as Rose Bengal alone in the absence of ultrasound produced no change in bacterial number (data not shown). The magnitude of this reduction is consistent with other literature where (KLAKLAK)2 alone has been shown to possess little activity against Gram-positive bacteria [25]. However, when Rose Bengal–C(KLAKLAK)2 was combined with ultrasound treatment, a statistically significant 5 log reduction in bacterial number was observed. This suggests that the ROS generated upon interaction of ultrasound with the Rose Bengal component of Rose Bengal–C(KLAKLAK)2 produces the desired antimicrobial effect. When this experiment was repeated using the same concentration of Rose Bengal (i.e. without antimicrobial peptide attached) and the same ultrasound conditions, the reduction in bacterial numbers was ca. 1 log less than for Rose Bengal–C(KLAKLAK)2 plus ultrasound. This difference, whilst not statistically significant, suggests that the slight antimicrobial effect observed for Rose Bengal–C(KLAKLAK)2 alone (i.e. no ultrasound) complements the ASDT effect of Rose Bengal.
Fig. 3.
Colony counts (CFU/mL) after treatment of (a) Staphylococcus aureus and (b) Pseudomonas aeruginosa with Rose Bengal–C(KLAKLAK)2 (P) or Rose Bengal (RB) with or without ultrasound (+/− U). [RB-C(KLAKLAK)2] = [RB] = 10 µM. Ultrasound conditions: frequency 1 MHz, ultrasound power density 3.0 W/cm2, duty cycle 50%, 10 min for S. aureus and 6 min for P. aeruginosa. *P ≤ 0.05; **P ≤ 0.01.
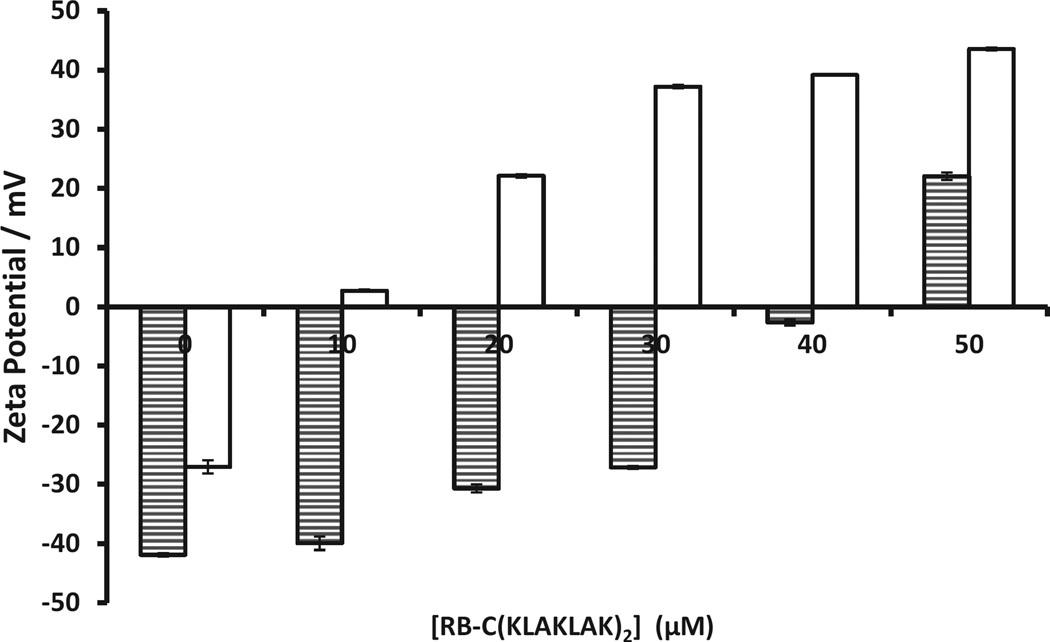
It is generally considered that PDT is more toxic to Gram-positive bacteria than Gram-negative bacteria and it has been suggested that this is due to structural differences in the cell wall composition [27]. Given that both the sensitisers used and the cytotoxic species generated (i.e. ROS) are the same in PDT and SDT, one would expect that Gram-negative bacteria would also be more difficult to kill using SDT. Indeed, when P. aeruginosa was treated with Rose Bengal and ultrasound, only a minor reduction in bacterial number was observed (ca. 0.5 log), which was considerably lower than for S. aureus. However, when P. aeruginosa was treated with the Rose Bengal–C(KLAKLAK)2 conjugate and ultrasound, the results were even more dramatic than for S. aureus, with a 7 log reduction in reduction in CFU viability observed (Fig. 3b). This large reduction in bacterial number cannot be explained by the antimicrobial nature of the peptide alone, as treatment of P. aeruginosa with Rose Bengal–C(KLAKLAK)2 in the absence of ultrasound produced a much lower 3.5 log reduction in bacterial number. To determine whether the net positive charge on the peptide could enhance uptake by the bacteria, suspensions of both P. aeruginosa and S. aureus were incubated with different amounts of the Rose Bengal–C(KLAKLAK)2 conjugate and the zeta potential was measured before and after conjugate addition. Both bacterial strains showed strongly negative zeta potentials (−42.0 mV and −27.0 mV, respectively), which are consistent with literature precedent [28,29]. Upon addition of increasing amounts of Rose Bengal–C(KLAKLAK)2, the net charge of both bacteria increased but with significantly different magnitudes (Fig. 4). For example, addition of 10 µM Rose Bengal–C(KLAKLAK)2 to P. aeruginosa resulted in a 2.0 mV increase in zeta potential, whilst for S. aureus an increase of 29.7 mV was observed. Indeed, only when 50 µM Rose Bengal–C(KLAKLAK)2 was added to P. aeruginosa did the charge become positive, whilst for S. aureus this occurred after only 10 µM. These results suggest an interaction between the positively charged peptide and the negatively charged bacterial cell wall, with P. aeruginosa requiring a significantly greater number of Rose Bengal–C(KLAKLAK)2 molecules to bind in order to titrate the more negative surface charge.
Fig. 4.
Plot of zeta potential for suspensions of Pseudomonas aeruginosa (shaded columns) and Staphylococcus aureus (clear columns) recorded after addition of increasing amounts of Rose Bengal–C(KLAKLAK)2.
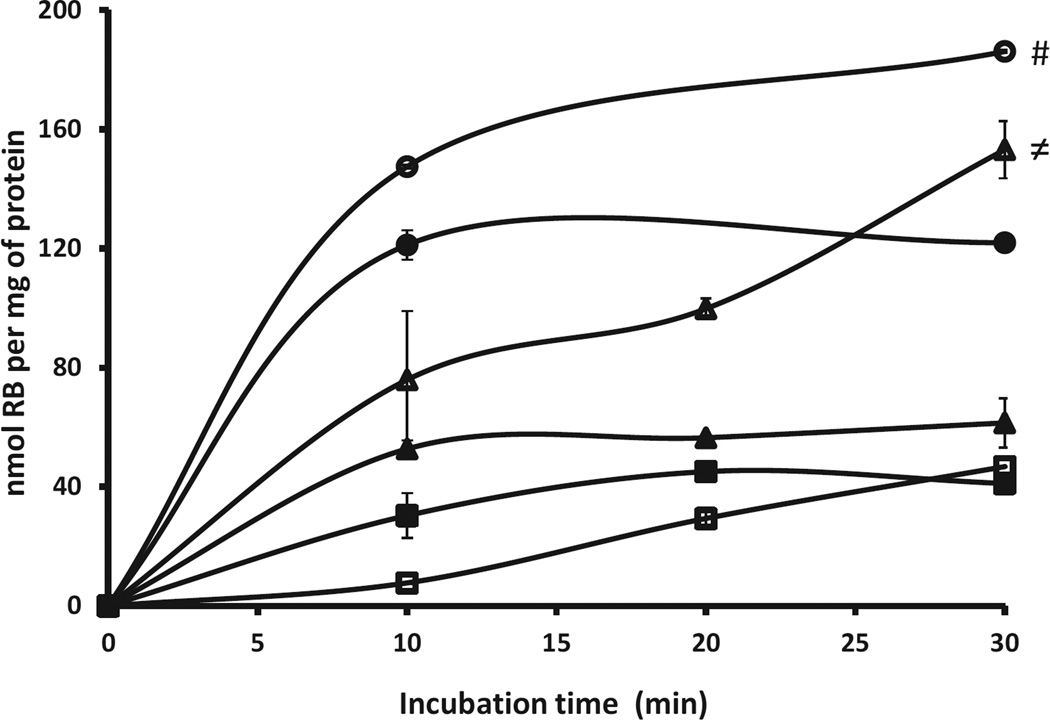
Systemic delivery of sensitisers is not normally considered in APDT as damage to capillaries and host cells directly supplied by them is undesirable [30]. Therefore, whilst local administration is preferred, this form of delivery still requires the sensitiser to be targeted to bacteria so that collateral damage to host tissue crucial to the healing process can be minimised. To determine the ability of Rose Bengal–C(KLAKLAK)2 to preferentially target bacteria over mammalian cells, solutions containing Rose Bengal or Rose Bengal–C(KLAKLAK)2 were incubated with suspensions containing S. aureus, P. aeruginosa or human fibroblast (Hs27) cells for either 10, 20 or 30 min. Following incubation, the suspensions were centrifuged, the cells were lysed and the Rose Bengal concentration was determined using ultraviolet–visible (UV–Vis) spectroscopy. The results are shown in Fig. 5 and reveal a significantly enhanced uptake of the Rose Bengal–C(KLAKLAK)2 in both bacteria compared with Hs27 cells at the time points tested. Indeed, uptake of the Rose Bengal–C(KLAKLAK)2 conjugate was also higher than Rose Bengal in both bacteria, whilst it was generally lower in the Hs27 cells, which is ideal for bacterial targeting.
Fig. 5.
Concentration of Rose Bengal (RB) per mg protein for suspensions of Staphylococcus aureus (circles), Pseudomonas aeruginosa (triangles) and human fibroblast (Hs27) cells (squares) incubated with Rose Bengal (filled symbols) or Rose Bengal–C(KLAKLAK)2 conjugate (open symbols) for 10, 20 or 30 min. #P ≤ 0.001 with respect to uptake of Rose Bengal alone or Rose Bengal–C(KLAKLAK)2 in HS27 cells; ≠P ≤ 0.01 with respect to uptake by Rose Bengal alone or Rose Bengal–C(KLAKLAK)2 in HS27 cells.
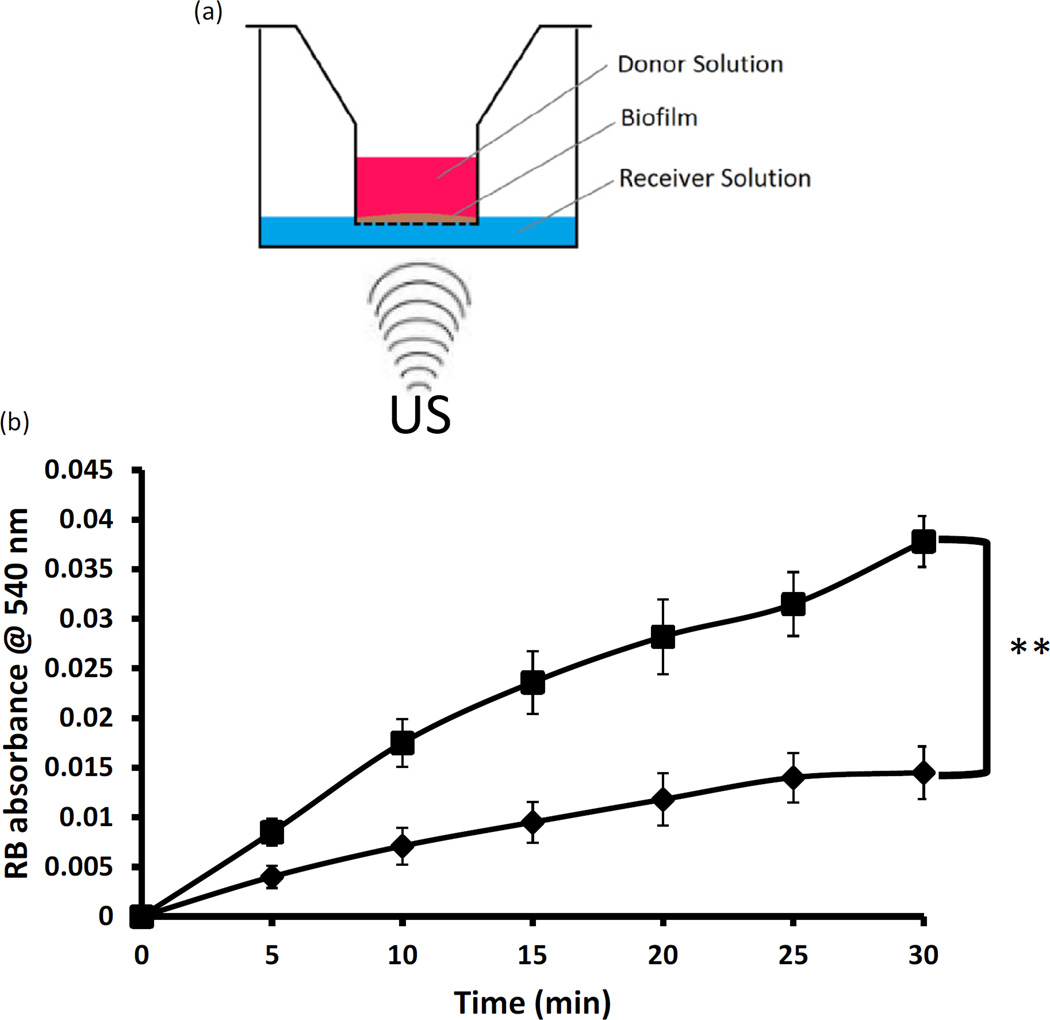
The presence of biofilms is a significant challenge associated with the local delivery of sensitiser drugs as it can act as a barrier between the applied sensitiser and bacteria. With as many as 80% of surgical site infections involving a microbial biofilm, strategies that can enhance the dispersion of drugs through biofilms offer a significant advantage. It has been demonstrated that in addition to increasing the permeability of membranes through sonoporation, shear forces induced by ultrasound cause membrane and biofilm disruption, enhancing the effectiveness of antibiotic treatment [31]. To test this hypothesis, P. aeruginosa biofilms were generated on the surface of Transwell® inserts and the diffusion of Rose Bengal through the biofilm in the presence and absence of ultrasound was tested (Fig. 6a). The data shown in Fig. 6b illustrate that pre-treatment of the biofilm with low-intensity ultrasound for 5 min before addition of Rose Bengal produced a 2.6-fold increase in sensitiser diffusion through the biofilm compared with the untreated biofilm control. These results suggest that ultrasound can facilitate the dispersion of sensitisers through biofilms and potentially improve the efficacy of ASDT.
Fig. 6.
(a) Schematic representation of biofilm diffusion experiment. Pseudomonas aeruginosa biofilms were generated on Transwell® inserts. The inserts were placed in wells containing phosphate-buffered saline (PBS) and the base of each well was irradiated or not with low-intensity ultrasound. Rose Bengal (RB) solution was added to the donor insert and the concentration of RB in the receiving PBS solution was determined at various time points using ultraviolet–visible (UV–Vis) spectroscopy. (b) Plot of RB absorbance against time for experiments performed in (a): ■, wells pre-treated with ultrasound; ♦, wells not pre-treated with ultrasound. **P ≤ 0.01. Ultrasound conditions: frequency 1 MHz, ultrasound power density 3.0 W/cm2, duty cycle 50%.
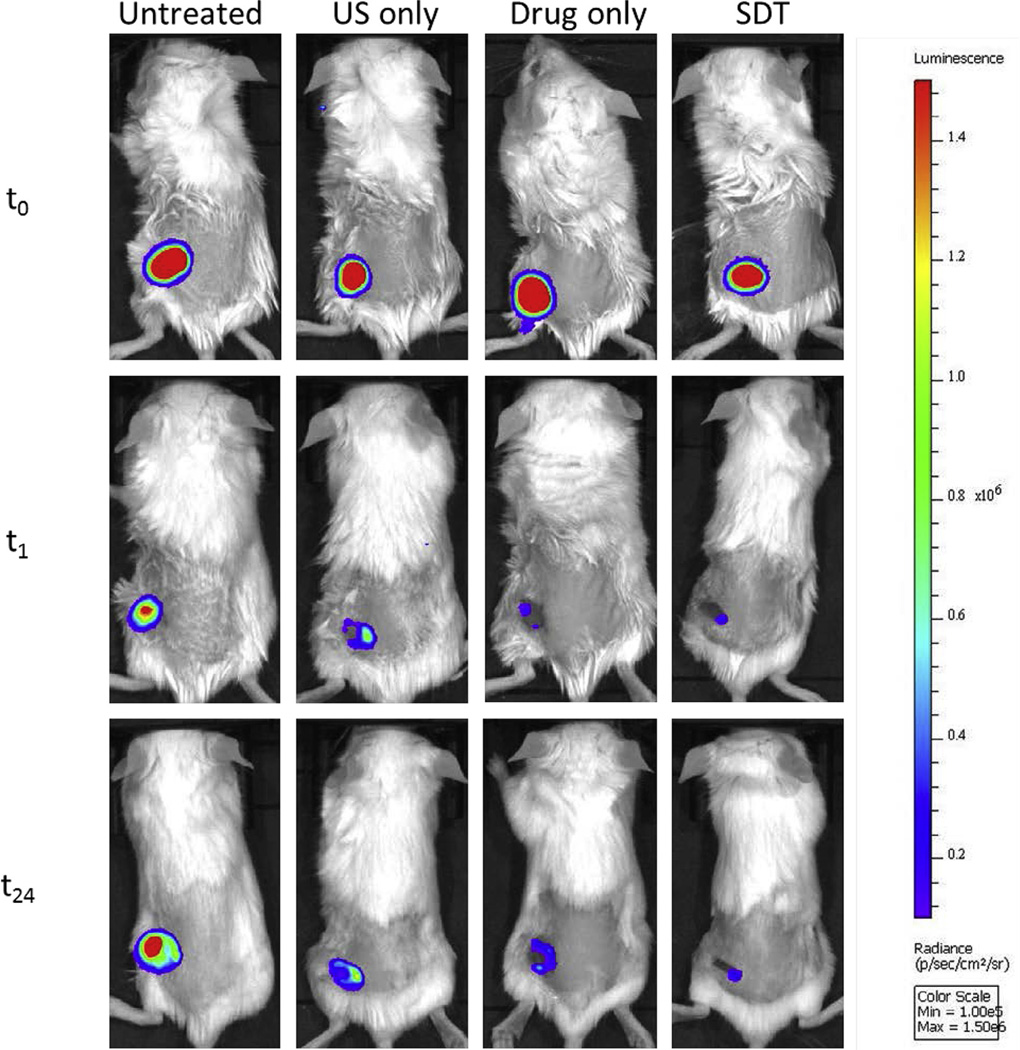
Having established the effectiveness of the SDT approach in vitro, we were also interested whether a similar effect would be observed in vivo. To determine this, wound abrasions (0.5 cm2) were established in the dorsum of BALB/c mice and were inoculated with a bioluminescent strain of P. aeruginosa. Once the infection had established, bioluminescent images were recorded using an IVIS Lumina Series III In Vivo Imaging System (Perkin Elmer Inc., Waltham, MA). The wound was then treated with a phosphate-buffered saline (PBS) solution containing the Rose Bengal–C(KLAKLAK)2 conjugate (4.5 mg/kg) and 10 min later was exposed to ultrasound. Bioluminescent images were recorded 1 h and 24 h after ultrasound treatment. Control groups involving no treatment or treatment with Rose Bengal–C(KLAKLAK)2 or ultrasound alone were also undertaken for comparative purposes. Representative images of the mice are shown in Fig. 7 and reveal substantial reductions in bioluminescent intensity for mice treated with the conjugate alone or SDT, with the SDT image being less intense, particularly after 24 h. In contrast, the bioluminescent intensity of the untreated and ultrasound only groups were substantially more intense than the Rose Bengal–C(KLAKLAK)2- or SDT-treated animals. This pattern follows a similar trend to the results obtained for the in vitro experiments undertaken using P. aeruginosa where Rose Bengal–C(KLAKLAK)2 alone produced a modest 3.5 log reduction whilst SDT treatment resulted in a much greater 7 log reduction. It is also apparent from the images presented in Fig. 7 that the size of the wound at 24 h following SDT treatment was much smaller compared with 1 h following SDT treatment, a feature that was not apparent in any of the other groups. Whilst there is an obvious limitation in the small sample size used in these experiments, the results do suggest that SDT using Rose Bengal–C(KLAKLAK)2 is capable of substantially reducing the bacterial burden in an in vivo model of localised infection. We are currently designing a larger animal study involving both methicillin-resistant S. aureus (MRSA) and P. aeruginosa infection models and will report on this in due course.
Fig. 7.
Whole-body bioluminescent images of mice bearing 0.5 cm2 wounds infected with a bioluminescent strain of Pseudomonas aeruginosa and receiving (i) no treatment, (ii) ultrasound (US) only, (iii) Rose Bengal–C(KLAKLAK)2 only or (iv) sonodynamic therapy (SDT), with images recorded immediately before and 1 h and 24 h after treatment. [RB−C(KLAKLAK)2] = 4.5 mg/kg. Ultrasound conditions: frequency 1 MHz, ultrasound power density 3 W/cm2, duty cycle 30% for 3.5 min, followed by a second dose using the same parameters 15 min later (i.e. total treatment time = 7 min).
4. Conclusions
In conclusion, a Rose Bengal–C(KLAKLAK)2 conjugate has been prepared for use in targeted ASDT. A broad-spectrum ASDT effect was observed when the conjugate was used to treat S. aureus and P. aeruginosa in the presence of low-intensity ultrasound. The conjugate also displayed improved uptake by these bacterial strains compared with a mammalian cell line, which promises to minimise damage to host tissue when considering in vivo ASDT applications. In addition, pre-treatment of a P. aeruginosa biofilm with low-intensity ultrasound before application of Rose Bengal enhanced diffusion of the sensitiser through the biofilm. A preliminary pilot in vivo experiment provided qualitative evidence of a substantial reduction in bacterial burden without collateral damage to host tissues when a P. aeruginosa-infected wound was treated with SDT using the Rose Bengal–C(KLAKLAK)2 conjugate. Combined, these results suggest that ASDT using Rose Bengal–C(KLAKLAK)2 is an effective broad-spectrum antimicrobial technique with the potential to activate sensitisers at a much greater depth in human tissue than APDT, enabling the treatment of more deep-seated infections.
Supplementary Material
Acknowledgments
JFC thanks Norbrook Laboratories Ltd. for an endowed chair.
Funding: None.
Footnotes
Competing interests: None declared.
Ethical approval: Not required.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2016.09.034.
References
- 1.Davies SC. Annual report of the Chief Medical Officer, volume two, 2011 Infections and the rise of antimicrobial resistance. London, UK: Department of Health; 2013. [Accessed 21 October 2015]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/138331/CMO_Annual_Report_Volume_2_2011.pdf. [Google Scholar]
- 2.Ison CA. Antimicrobial agents and gonorrhoea: therapeutic choice, resistance and susceptibility testing. Genitourin Med. 1996;72:253–257. doi: 10.1136/sti.72.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Tuberculosis global facts. Geneva, Switzerland: WHO; 2011. [Accessed 10 November 2015]. Updated 2012; Available from: http://www.who.int/tb/publications/2011/factsheet_tb_2011.pdf. [Google Scholar]
- 4.Jenks P, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect. 2014;86:24–33. doi: 10.1016/j.jhin.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AO, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod. 2010;36:322–328. doi: 10.1016/j.joen.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavares A, Carvalho C, Faustino MA, Neves MG, Tomé JP, Tomé AC, et al. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z, Xu H, Meyers AD, Musani AI, Wang L, Tagg R, et al. Photodynamic therapy for treatment of solid tumors—potential and technical challenges. Technol Cancer Res Treat. 2008;7:309–320. doi: 10.1177/153303460800700405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Riordan K, Akilov OE, Hasan T. The potential for photodynamic therapy in the treatment of localized infections. Photodiagnosis Photodyn Ther. 2005;2:247–262. doi: 10.1016/S1572-1000(05)00099-2. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi N, Mišík V, Fukuda M, Riesz P. Effect of gallium-porphyrin analogue ATX-70 on nitroxide formation from a cyclic secondary amine by ultrasound: on the mechanism of sonodynamic activation. Radiat Res. 1995;143:194–202. [PubMed] [Google Scholar]
- 13.Miyoshi N, Igarashi T, Riesz P. Evidence against singlet oxygen formation by sonolysis of aqueous oxygen-saturated solutions of hematoporphyrin and rose bengal. The mechanism of sonodynamic therapy. Ultrason Sonochem. 2000;7:121–124. doi: 10.1016/s1350-4177(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 14.Misik V, Riesz P. Free radical intermediates in sonodynamic therapy. Ann N Y Acad Sci. 2000;899:335–348. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomankova K, Kolarova H, Kolar P, Kejlova K, Jirova D. Study of cytotoxic effect of photodynamically and sonodynamically activated sensitizers in vitro. Toxicol In Vitro. 2009;23:1465–1471. doi: 10.1016/j.tiv.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 16.ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93:111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Nonaka M, Yamamoto M, Yoshino S, Umemura S, Sasaki K, Fukushima T. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res. 2009;29:943–950. [PubMed] [Google Scholar]
- 18.Kenyon JN, Fulle RJ, Lewis TJ. Activated cancer therapy using light and ultrasound—a case series of sonodynamic photodynamic therapy in 115 patients over a 4 year period. Curr Drug Ther. 2009;4:179–193. [Google Scholar]
- 19.Wang X, Zhang W, Xu Z, Luo Y, Mitchell D, Moss RW. Sonodynamic and photodynamic therapy in advanced breast carcinoma: a report of 3 cases. Integr Cancer Ther. 2009;8:283–287. doi: 10.1177/1534735409343693. [DOI] [PubMed] [Google Scholar]
- 20.McEwan C, Owen J, Stride E, Fowley C, Nesbitt H, Cochrane D, et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Control Release. 2015;203:51–56. doi: 10.1016/j.jconrel.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Dong J, Ip M, Wang X, Leung AW. Sonodynamic action of chlorin e6 on Staphylococcus aureus and Escherichia coli. Ultrasonics. 2016;64:54–57. doi: 10.1016/j.ultras.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Leung AW, Hua H, Xu C, Ip M. Sonodynamic action of hypocrellin B on biofilm-producing Staphylococcus epidermidis in planktonic condition. J Acoust Soc Am. 2015;138:2548–2553. doi: 10.1121/1.4932014. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang D, Hou C, Bi L, Han J, Hao Y, Cao W, et al. Sonodynamic effects of hematoporphyrin monomethyl ether on Staphylococcus aureus in vitro. FEMS Microbiol Lett. 2014;361:174–180. doi: 10.1111/1574-6968.12628. [DOI] [PubMed] [Google Scholar]
- 24.Soukos NS, Hamblin MR, Hasan T. The effect of charge on cellular uptake and phototoxicity of polylysine chlorin(e6) conjugates. Photochem Photobiol. 1997;65:723–729. doi: 10.1111/j.1751-1097.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson GA, Muthukrishnan N, Pellois J. Photoinactivation of Gram positive and Gram negative bacteria with the antimicrobial peptide (KLAKLAK)2 conjugated to the hydrophilic photosensitizer eosin Y. Bioconjug Chem. 2012;24:114–123. doi: 10.1021/bc3005254. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell SO, Hall MJ, Allen LT, Byrne A, Gallagher WM, O’Shea DF. Supramolecular photonic therapeutic agents. J Am Chem Soc. 2005;127:16360–16361. doi: 10.1021/ja0553497. [DOI] [PubMed] [Google Scholar]
- 27.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B. 1992;14:262–266. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 28.Wilson WW, Wade MM, Holman SC, Champlin FR. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods. 2001;43:153–164. doi: 10.1016/s0167-7012(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 29.Soni KA, Balasubramanian AK, Beskok A, Pillai SD. Zeta potential of selected bacteria in drinking water when dead, starved, or exposed to minimal and rich culture media. Curr Microbiol. 2008;56:93–97. doi: 10.1007/s00284-007-9046-z. [DOI] [PubMed] [Google Scholar]
- 30.Berg K, Golab J, Korbelik M, Russell D. Drug delivery technologies and immunological aspects of photodynamic therapy. Photochem Photobiol Sci. 2011;10:647–648. doi: 10.1039/c1pp90010b. [DOI] [PubMed] [Google Scholar]
- 31.He N, Hu J, Liu H, Zhu T, Huang B, Wang X, et al. Enhancement of vancomycin activity against biofilms by using ultrasound-targeted microbubble destruction. Antimicrob Agents Chemother. 2011;55:5331–5337. doi: 10.1128/AAC.00542-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.