Abstract
Avoidance of noxious ambient heat is crucial for survival. A well-known but enigmatic phenomenon is that animals are sensitive to the rate of temperature change. However, the cellular and molecular underpinnings through which animals sense and respond much more vigorously to fast temperature changes are unknown. Using Drosophila larvae, we found that nociceptive rolling behavior was triggered at lower temperatures and at higher frequencies when the temperature increased rapidly. We identified neurons in the brain that were sensitive to the speed of the temperature increase, rather than just the absolute temperature. These cellular and behavioral responses depended on the TRPA1 channel, whose activity was responsive to the rate of temperature increase. We propose that larvae use low threshold sensors in the brain to monitor rapid temperature increases as a protective alert signal to trigger rolling behaviors, allowing fast escape before the temperature of the brain rises to dangerous levels.
INTRODUCTION
An important class of molecules that contributes to thermosensation is Transient Receptor Potential channels (thermoTRPs)1-3. ThermoTRPs2 are activated directly or indirectly by changes in temperature, and enable animals to respond behaviorally to temperature fluctuations in the environment1, 4. The founding thermoTRP, mouse TRPV1, is activated by temperatures higher than 42°C, and is required for avoidance of noxious heat5, 6. Other mammalian thermoTRPs are activated with different thresholds, such as mouse TRPM8 and TRPA1, which are activated directly by low temperatures below ~23°C and ~17°C, respectively7-10.
The contribution of TRPs to thermosensation is evolutionarily conserved, and is well-documented in the invertebrate model organisms, C. elegans and Drosophila melanogaster4, 11. In Drosophila larvae, noxious heat is detected through direct activation of three TRPA channels, Painless, Pyrexia and TRPA112-15. The TRPA1 channel also enables larvae to sense exquisitely small deviations above the preferred temperature16. In the comfortable range, this fine thermal detection occurs through indirect activation of TRPA1 via a rhodopsin-dependent thermosensory signaling cascade17. This signaling cascade may serve to lower the threshold for direct activation of TRPA1.
The extensive studies on thermoTRPs in model organisms have contributed greatly to the theme that warm or hot temperatures of different thresholds are sensed by direct activation of TRP channels. However, a long-known but poorly understood phenomenon is that the rate of temperature change, rather than just the temperature threshold can affect the nociceptive response. Classic experiments on frogs performed >130 years ago demonstrate their highly sensitive escape response to fast rises in heat, and indifference to slow increases in temperature18. Stronger nociceptive reactions to fast temperature rises have been documented throughout the animal kingdom, in organisms as diverse as worms and humans19, 20. However, the mechanism underlying temperature rate detection is not clear.
To explore the mechanism through which an animal responds differentially to slow and fast elevations in temperature, we developed Drosophila larvae as an animal model. We found that if we challenged larvae with a rapid temperature rise, a very high proportion of the animals exhibited nociceptive rolling behavior. However, if the temperature increase was gradual, the percentage of larvae that rolled was much lower, even after we exceeded temperatures that induced robust nociceptive avoidance after a fast temperature increase. We found one of the TRPA1 isoforms was the key rate-sensor, and found that it was required in neurons in the brain that responded to the rate of temperature increase, rather than just the temperature threshold. Our results indicate that larvae use a TRPA1 dependent rate-sensing mechanism as to safeguard the brain from exposure to noxious heat.
RESULTS
Dependence of the nociceptive rolling response on the rate of temperature increase
In order to characterize the behavior of larvae in response to different rates of temperature change, we built an apparatus that allowed us to accurately control the heating speed, while monitoring larval movement. The temperature control system was comprised of a Peltier pad and a programmable integrated circuit responsible for voltage regulation. We used this apparatus to heat and cool an agarose surface for larval navigation. A video camera recorded the larvae behavior, and a computer program, MAGAT Analyzer21, recognized the larvae in each frame.
To automatically and objectively analyze the large volume of data, we wrote an algorithm that employed several parameters to discern rolling from non-rolling larvae. These included the speed of the larvae, their direction of movement perpendicular to the body, acceleration, and acceleration perpendicular to the body. We used a machine learning22, 23 approach to successively improve the ability of the computer to accurately identify rolling larvae, with minimal noise.
We exposed wild-type 2nd instar larvae to temperature ramps with different slopes, and determined how rolling was dependent on the rate of temperature change (dT/dt). In each experiment, we initially maintained the temperature at ~23.5°C for 30 seconds and then increased the temperature to 40°C. Larvae rolled when the temperature increased quickly (0.3°C/s; Supplementary Video 1). As the temperature approached 40°C, the larvae stopped moving and the rolling behavior ceased (Fig. 1a). However, the animals still responded to a mechanical stimulus (Supplementary Video 2).
Figure 1.
Rolling responses of wild-type larvae exposed to different rates of temperature increase. (a-i) The fraction of control (w1118) 2nd instar larvae that rolled (Frolling) as a function of the rate of temperature change (dT/dt). The dT/dt are indicated above each plot. The scale bars indicate the seconds required for the temperature to rise by 5°C. The rolling fraction was defined as Nrolling/Ntotal, where Nrolling was the number of larvae rolling and Ntotal was the total number of larvae. Fpeak was the maximum rolling fraction and Tmiddle was the temperature corresponding to the point when the rolling fraction rose to half the Fpeak. The curves (thicker lines) were fit using a sigmoid function. n=3, 5, 7, 3, 3, 6, 3, 7, 9 (≥30 larvae/experiment) corresponding to a-i, respectively. (j) Fpeak values as a function of dT/dt. The crosses indicate the average Fpeak values in response to the different temperature changing rates. (k) Tmiddle values as a function of dT/dt. The crosses indicate the average Tmiddle values. We performed one-way ANOVA on the Fpeak and Tmiddle obtained during the different temperature changing rates [Fpeak, F(8,37)=9.33, p=6.2 × 10−7; Tmiddle, F(8,37)=10.56, p=1.5 × 10−7]. We used the Tukey-Kramer test to ascertain statistically significant differences relative to the fastest temperature changing rate (0.5°C/s). The conditions with significant differences are indicated with asterisks (* p < 0.05, ** p < 0.01). The following are significant differences: 1) 0.5°C/s versus 0.02°C/s: Fpeak, q(37,9)=6.47, p=0.0016; Tmiddle, q(37,9)=8.75, p=1.2 × 10−5, 2) 0.5°C/s versus 0.05°C/s: Tmiddle, q(37,9)=7.30, p = 0.00027, 3) 0.5°C/s versus 0.06°C/s: Tmiddle, q(37,9)=6.07, p=0.0035 and 4) 0.5°C/s versus 0.07°C/s: Tmiddle, q(37,9)=4.90; p=0.033. The error bars indicate S.E.M.s.
We calculated the fraction of larvae displaying rolling behavior using two parameters: Fpeak and Tmiddle. Fpeak was the maximum fraction of larvae that rolled during the temperature ramp. Tmiddle was the temperature in which the fraction of rolling was halfway between the baseline rolling behavior (at the start of the experiment) and Fpeak. We found that the relation between rolling and temperature (T) was dependent on the rate of temperature change. When the temperature rose at the fastest rate tested (0.5°C/s), the maximum fraction of larvae that rolled (Fpeak) was 0.89 ±0.09, and the Tmiddle temperature was 29.1 ±0.3°C (Fig. 1a,j,k). As the rate of temperature change declined, the Fpeak decreased, and the Tmiddle rose (Fig. 1b-k). At the slowest rate examined (0.02°C/s), the Fpeak fell to 0.47 ±0.08, and the Tmiddle increased to 34.5 ±0.6°C (Fig. 1j,k; Supplementary Video 3). Thus, when the rate of temperature increase was very gradual, only about half as many larvae rolled at a temperature that was >5°C hotter. These results demonstrate that under conditions in which the temperature rose slowly, the tendency for the larvae to initiate an escape response was diminished greatly.
Requirement for TRPA1-A for heat-induced rolling
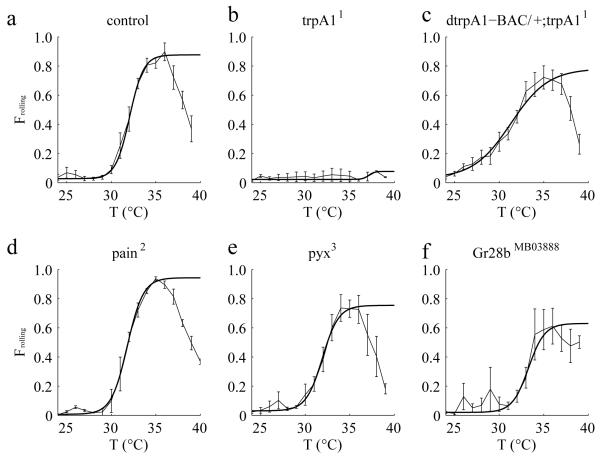
To identity a channel that might enable larvae to sense the fast temperature increases that stimulate rolling behavior, we screened for mutations in genes encoding channels known to detect temperatures in the noxious heat range (trpA1, painless and pyx)12-15, 24-26. We found that trpA11 mutant flies exhibited severe defects in heat-induced rolling behavior (Fig. 2a,b, Supplementary Fig. 1), and this phenotype was suppressed by a duplication that included the wild-type trpA1 gene (Fig. 2c). In contrast, the pain2 and pyx3 mutations had no or minimal effects in this temperature change assay (Fig. 2d,e), although loss of painless has been reported to elevate the threshold for a thermal escape response from 29° to 33°C)27. We also found that Gr28bMB03888—a mutation affecting a receptor protein required for sensing innocuously warm temperatures28, did not impact significantly on rolling behavior (Fig. 2f).
Figure 2.
Effects of eliminating different thermoTRPs on Frolling values in response to the same dT/dt. Frolling values exhibited by 2nd instar larvae of the indicated genotypes. dT/dt=0.1°C/s. The control flies in (a) were w1118. n=3, 4, 5, 3, 3, 4 (≥30 larvae/experiment) corresponding to a-f, respectively. The error bars indicate S.E.M.s.
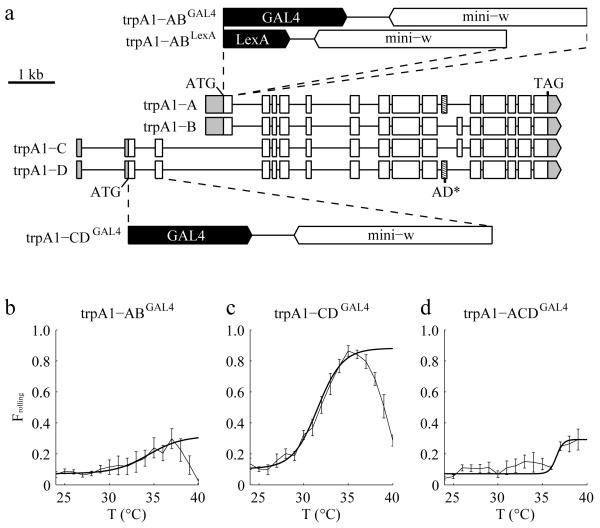
The trpA1 gene encodes four mRNA isoforms: trpA1-A, B, C and D (Fig. 3a)14, 16. The trpA1-A and trpA1-B isoforms (trpA1-AB) share one promoter and the trpA1-C and trpA1-D isoforms (trpA1-CD) share another promoter14. trpA1-A and trpA1-D have a common exon (Fig. 3a; boxes with diagonal lines), which enables them to be activated by elevated temperatures. The different N-termini in TRPA1-A and TRPA1-D influence their temperature thresholds (25°C for TRPA1-A and 36°C for TRPA1-D)14, 29.
Figure 3.
Effects of eliminating different TRPA1 isoforms on Frolling values in response to dT/dt. (a) Cartoon depicting the intron-exon organization of the four isoforms encoded by trpA1. The structures of the trpA1 alleles are also indicated. trpA1-ABGAL4 and trpA1-ABLexA both contain a deletion of 178 nucleotides spanning the trpA1-AB translation initiation codon and trpA1-CDGAL4 contains a 732 nucleotide deletion spanning the trpA1-CD translation initiation codon. To create flies that expressed only the trpA1-B isoform (trpA1-ACDGAL4), we introduced a two-nucleotide deletion in the exon common to trpA1-A and trpA1-D (boxes with diagonal lines) in the trpA1-CDGAL4 background. The 5′ and 3′ untranslated regions are indicated in gray. (b-d) Frolling values exhibited by 2nd instar larvae of the indicated genotypes. dT/dt=0.1°C/s. n=4, 5, 3 (≥30 larvae/experiment) corresponding to b-d, respectively. The error bars indicate S.E.M.s.
To determine which TRPA1 isoform was required for heat-induced rolling, we first assessed the effects of knocking out each isoform pair (trpA1-AB and trpA1-CD). To address the requirements for the TRPA1-AB, we took advantage of the trpA1-ABGAL4 allele, which eliminates TRPA1-A and TRPA1-B30. To disrupt TRPA1-CD, we used homologous recombination to create an allele containing a GAL4 reporter in place of 732 nucleotides spanning the trpA1-CD translation initiation codon (Fig. 3a). We then exposed the mutant larvae to a temperature heat ramp (0.1°C/s). We found that the trpA1-ABGAL4 larvae displayed significantly reduced rolling behavior, indicating that either the A or B isoform was required (Fig. 3b). In contrast, the trpA1-CDGAL4 larvae showed rolling behavior that was more reminiscent of the control larvae (Fig. 3c).
To address whether TRPA1-A or TRPA1-B was required for heat-induced nociception, we deleted two nucleotides in the exon that was present in trpA1-A, but not in trpA1-B (Fig. 3a; exons labeled with diagonal lines). We introduced this mutation (AD*) in the trpA1-CDGAL4 background using the CRISPR/Cas9 system31-34. In this mutant, only the B isoform of trpA1 remained (trpA1-ACDGAL4). We found that trpA1-ACDGAL4 displayed a deficit in responding to the heat ramp, similar to trpA1-ABGAL4 flies (Fig. 3b,d). These findings indicated in this assay the key temperature sensor required for inducing rolling behavior was trpA1-A.
We attempted to rescue the trpA1-ABGAL4 mutant phenotype by expressing trpA1-A in trpA1-AB neurons. However, driving trpA1-A expression in trpA1-AB neurons decreased the Tmiddle to 27.0 ±0.2°C (0.1°C/s), which was ~5°C lower than control animals (31.9 ±0.5°C; Supplementary Fig. 2a,b). This increased rolling behavior at lower temperatures suggested that the threshold for this avoidance behavior might be sensitive to expression levels, since the GAL4/UAS system potentially drove higher expression levels than the endogenous trpA1 promoter. Moreover, the rolling declined and then ceased at a lower temperature than control flies due to an increasing proportion of heat-induced locomotor arrest. (Fpeak: control, 0.90 ±0.06; UAS-trpA1-A/+;trpA1-ABGAL4/trpA11, 0.35 ±0.05). We observed a similar effect of expressing trpA1-A in a heterozygous background (UAS-trpA1-A/+; trpA1-ABGAL4/+; Supplementary Fig. 2d; Fpeak = 0.44 ±0.03; Tmiddle = 25.9 ±0.2°C). In contrast to the effects of driving expression of trpA1-A, we found that introducing trpA1-B in trpA1-AB neurons had no effect (Supplementary Fig. 2c).
To determine whether ectopic expression of trpA1-A could endow sensitivity to the rate of temperature change, we expressed trpA1-A in class IV multidendritic neurons (mdIV neurons) in a trpA11 mutant background (UAS-trpA1-A/ppk-GAL4;trpA11; Supplementary Fig. 3). The trpA11 null mutant larvae or trpA11 larvae harboring only the ppk-GAL4 or the UAS-trpA1-A transgene were virtually unresponsive to slow or fast temperature ramps (Supplementary Fig. 3a,j). However, upon applying a fast heat ramp (0.5°C/s) to larvae ectopically expressing UAS-trpA1-A under control of the ppk-GAL4 and, the fraction of animals that rolled (Fpeak) was 0.75 ±0.01 (Supplementary Fig. 3a,j). As the rate of temperature change declined, the Fpeak decreased (Supplementary Fig. 3b-j). At the slowest rate examined (0.02°C/s), the Fpeak fell to 0.35 ±0.04 (Supplementary Fig. 3i,j). However, the Tmiddle was not significant different (Supplementary Fig. 3k).
trpA1-AB neurons critical for sensing rapid temperature change
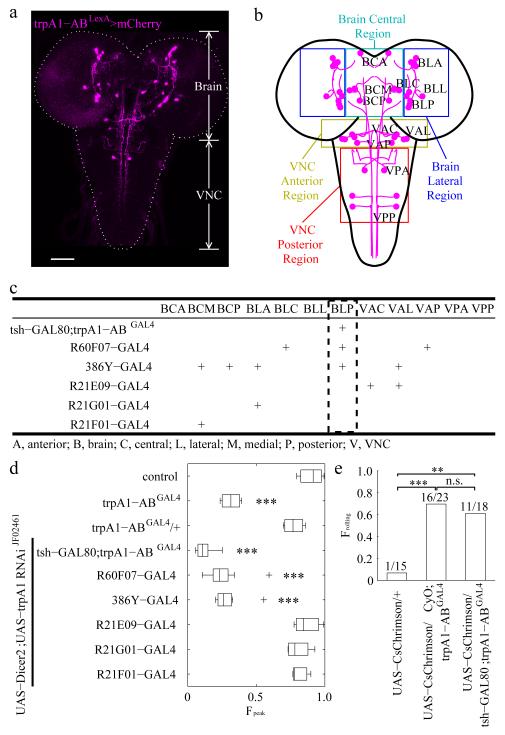
We performed homologous recombination using the CRISPR/Cas9 system31-34 to introduce the LexA gene into the trpA1-AB translation initiation codon, so that we could subsequently use this trpA1-ABLexA reporter in combination with GAL4 reporters (Fig. 3a). The LexA gene and the w+ marker replaced the same genomic region as in trpA1-ABGAL430. The trpA1-AB LexA reporter (trpA1-ABLexA/+) was expressed in a variety of neurons in the brain and ventral nerve cord (VNC) (Fig. 4a,b and Supplementary Fig. 4). We observed an indistinguishable expression pattern in the trpA1-ABLexA homozygous larvae (Supplementary Fig. 4a,b and Supplementary Fig. 5) indicating that the trpA1-AB phenotype was not due to loss of the trpA1-AB-expressing neurons.
Figure 4.
Identifying trpA1-AB neurons in the larval brain required for heat-induced rolling. (a) Expression of the trpA1-AB reporter in the CNS of 3rd instar larvae. The fly line used for the immunostaining with anti-DsRed was trpA1-ABLexA,LexAop-frt-mCherry-STOP-frt-ReaChR::Citrine/+. The trpA1-AB isoforms were expressed in the brain and ventral nerve cord (VNC). The dashed line outlines the brain and VNC. The scale bar represents 50 μm. (n=7). (b) Map of trpA1-AB neuronal clusters in the brain and VNC. The colored boxes show the regions that define the first two letters of the three letter nomenclature. The first letter indicates whether the neurons were in the brain (B) or the VNC (V). The second letter indicates the general region within the brain or VNC that contained the neuronal cell bodies: A, anterior; C, central; L, lateral; P, posterior. The third letter indicates the relative positions of the neuronal clusters within the general regions of the brain and VNC: A, anterior; C, central; L, lateral; M, medial, P, posterior. For example, “BLP” indicates the posterior neuronal cluster in the lateral region of the brain. (c) Summary of the expression patterns of the indicated GAL4 reporters in trpA1-AB positive neurons of 3rd instar larvae. “+” denotes expression in the indicated neurons. The expression patterns are shown in Supplementary Fig. 6 and Supplementary Fig. 7. (d) Effect on rolling behavior (FPeak) of 2rd instar larvae resulting from knockdown of trpA1, using the UAS-Dicer2;UAS-trpA1 RNAi line and the indicated GAL4 drivers. The temperature increased from 23.5°C to 40°C with a dT/dt=0.1°C/s. The center lines of the boxes represents the median value. The left and right edges of the boxes represent the 25th percentiles (q25%) and 75th percentiles (q75%) of the sample data, respectively. The “+” indicate points as outliers if they are greater than q75% + 1.5 (q75% – q25%) or less than q25% − 1.5 (q75% – q25%). The whiskers indicate the minimum and maximum value of data points except for outliers. We performed one-way ANOVA on the Fpeak values to test for significant different distributions between genotypes [n = 3, 4, 4, 8, 9, 8, 3, 4, 3. The n represents the number of independent experiments. F(8,37)=45.38; p=8.1 × 10−17]. We used the Tukey-Kramer test to ascertain statistically significant differences between the control (w1118) and other samples. Significant differences relative to the control are indicated (*** p < 0.001): 1) trpA1-ABGAL4, q(37,9)=10.45, p=3.9 × 10−7, 2) UAS-Dicer2;UAS-trpA1-RNAiJF02461 × tsh-GAL80;trpA1-ABGAL4: q(37,9)=15.68, p=9.0 × 10−8, 3) UAS-Dicer2;UAS-trpA1-RNAiJF02461 × R60F07-GAL4: q(37,9)=13.12, p=9.1 × 10−8, and 4) UAS-Dicer2;UAS-trpA1-RNAiJF02461 × 386Y-GAL4: q(37,9)=12.19, p=9.7 × 10−8. (e) Triggering rolling behavior of 2rd instar larvae by optogenetically activating trpA1-AB neurons. We manually recognized the rolling behaviors of each larvae. We stimulated the larvae with light and scored the larvae with either 1.0 or 0 depending on whether or the not the larvae rolled. The bar heights provide the fraction of larvae showed rolling behavior. The numbers above the bars are Nrolling/Ntotal, where Nrolling represents the number of larvae rolling and Ntotal provides the total number of larvae. We performed Fisher’s exact test between each genotype. The comparisons with significant differences are indicated with asterisks (** p < 0.01, *** p < 0.001). n.s., not significant. We obtained the following values for the following comparisons: 1) UAS-CsChrimson/+ versus UAS-CsChrimson/CyO;trpA1-ABGAL4 (odds ratio=32, p=0.00016), 2) UAS-CsChrimson/+ versus UAS-CsChrimson/tsh-GAL80;trpA1-ABGAL4 (odds ratio=22, p=0.0028), 3) UAS-CsChrimson/CyO;trpA1-ABGAL4 versus UAS-CsChrimson/tsh-GAL80;trpA1-ABGAL4 (odds ratio=0.69, p=0.74).
To determine the specific trpA1-AB expressing neurons required for rolling, we used RNAi-mediated gene silencing to assess the effects of knocking down trpA1 expression in different subsets of trpA1-AB neurons. To identify GAL4 lines to conduct the RNAi screen, we took advantage of the trpA1-ABLexA reporter to test for overlap, by performing co-labeling and intersectional FLP-out strategies. The GAL4 lines we screened were from the Bloomington Drosophila Stock Center (BDSC) that label neurotransmitter- or neuropeptide-releasing neurons. We also reviewed the staining patterns of the Janelia collection of ~7000 GAL4 lines by employing an image processing program we wrote to narrow down the candidates, and then manually checked their expression patterns. Furthermore, we combined GAL80 lines from the BDSC with the trpA1-ABGAL4 to label a subset of trpA1-AB neurons.
We identified ~70 candidate GAL4 lines, which showed overlap with the trpA1-ABGAL4. To clearly detect the neurons that expressed the GAL4 reporter and the trpA1-ABLexA, we used a “flipped out” approach. We crossed the GAL4 lines into a genetic background such that the trpA1-ABLexA positive neurons that did not express a given GAL4, were marked by mCherry. If the trpA1-ABLexA positive neurons also expressed GAL4, then the mCherry cassette was removed genetically flipped out, thereby leading to expression of the Citrine marker. We found six GAL4 lines that overlapped with less than 10 pairs of trpA1-ABLexA positive neurons (Fig. 4c, Supplementary Fig. 6 and Supplementary Fig. 7), and were therefore useful tools to manipulate small subsets of trpA1-ABLexA positive neurons.
We examined the requirements for different trpA1-AB neurons for rolling behavior in response to noxious heat by knocking down trpA1 expression by RNAi. We then performed temperature ramps at 0.1°C/s and measured the FPeak. Knockdown of trpA1 using the R60F07-GAL4 elicited severe rolling deficits (Fig. 4d and Supplementary Fig. 8a). The R60F07-GAL4 was expressed in just three of the twelve classes of trpA1-positive neurons: BLC, BLP and VMA (Fig. 4b,c and Supplementary Fig. 7a-c). The 386Y-GAL4, which overlapped with R60F07 in only the BLP class (Fig. 4b,c and Supplementary Fig. 7d-f), also caused strong impairment in rolling, when we used it to knockdown trpA1 (Fig. 4d and Supplementary Fig. 8b). The tsh-GAL80;trpA1-ABGAL4 only labeled trpA1-AB BLP neurons (Fig. 4b,c and Supplementary Fig. 6g-i). Using this latter GAL4 in combination with the tsh-GAL80 to knock-down trpA1 expression reduced the Fpeak significantly comparing to control and trpA1-ABGAL4/+ (Fig. 4d and Supplementary Fig. 8c,d). We did not observe a significant reduction in rolling behavior after suppressing trpA1 using any of three GAL4 lines that were not expressed in BLP neurons (Fig. 4b-d; Supplementary Fig. 7 and Supplementary Fig. 8e-g). Thus, we conclude that expression of trpA1 in BLP neurons influences rolling behavior.
To test whether BLP were sufficient to trigger rolling behaviors, we performed optogenetic experiments. We found that expression of UAS-CsChrimson35 under control of the trpA1-ABGAL4 (UAS-CsChrimson/CyO;trpA1-ABGAL4) triggered rolling behavior but not paralysis (Supplementary Video 4; Fig. 4e). Using the tsh-GAL80, we suppressed trpA1-ABGAL4-dependent expression of UAS-CsChrimson, but retained expression in two of the three BLP neurons (Supplementary Fig. 6g-i). We found that light also triggered rolling in these larvae (Supplementary Video 5; Fig. 4e), but not in control larvae harboring UAS-CsChrimson/+ alone (Supplementary Video 6; Fig. 4e).
trpA1-AB and trpA1-CD neurons function in a common circuit
We showed above that trpA1-AB was required for the rolling behavior evoked by rapid heating of the entire larvae body. However, the trpA1-CD neurons expressing trpA1-C and painless are necessary for the rolling induced by touching a localized spot on the larvae with a hot (>39°C) probe12, 14. Even though the temperature thresholds were different, trpA1-AB neurons and trpA1-CD neurons both trigger rolling behavior in response to noxious heat. This raises the possibility that the trpA1-AB and trpA1-CD neurons function in a common neuronal circuit.
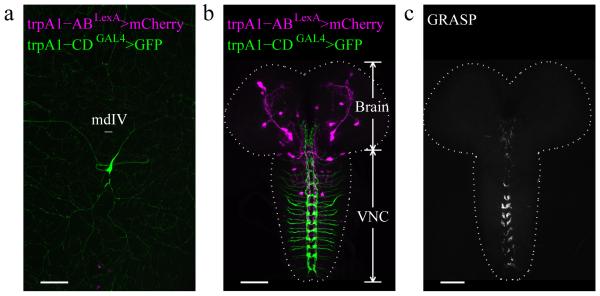
In order to determine the relative expression patterns of the trpA1-AB and trpA1-CD neurons, we performed double-labeling experiments. The trpA1-CDGAL4/+ reporter stained mdIV neurons in the body wall (Fig. 5a), which extended axonal projections to the ventral nerve cord (VNC) (Fig. 5b). In contrast, the trpA1-ABLexA/+ reporter stained neurons in the larval brain and VNC, but not in body wall neurons (Fig. 4a and Fig. 5b). Therefore, there was no apparent overlap between the expression patterns for the trpA1-AB and trpA1-CD reporters.
Figure 5.
Relative expression of trpA1-AB and trpA1-CD in the body wall and CNS of 3rd instar larvae. (a,b) The fly line used was UAS-GFP/+;trpA1-ABLexA,LexAop-frt-mCherry-STOP-frt-ReaChR::Citrine/trpA1-CDGAL4. The tissues were immunostained with anti-DsRed and anti-GFP. (a) Testing for expression of the trpA1-AB and trpA1-CD reporters in the larval body wall. The trpA1-CD but not the trpA1-AB reporter labeled peripheral neurons (type IV multidendritic neurons; mdIV) near the body wall. (n=4). (b) Testing for expression of the trpA1-AB and trpA1-CD reporters in the CNS. The trpA1-AB isoforms were expressed in the brain and ventral nerve cord (VNC). The trpA1-CD neurons near the body wall projected to the VNC. The dashed line outlines the brain and VNC. (n=16). (c) Application of the GFP reconstitution across synaptic partners (GRASP) technique to investigate whether the trpA1-CD neurons and trpA1-AB neurons form synapses, using LexAop-CD4::spGFP11/+;UAS-CD4::spGFP1-10,trpA1-ABLexA/trpA1-CDGAL4. (n=3). The scale bars represent 50 μm.
Since the localization of trpA1-AB neurons and trpA1-CD neurons were close to each other in the VNC, we wondered whether they formed synapses. GFP reconstitution across synaptic partners (GRASP) is a system to label membrane contacts between two cells in living animals36. We used trpA1-ABLexA and trpA1-CDGAL4 to drive LexAop-spGFP11 and UAS-spGFP1~10 and found that they form functional GFP in the VNC (Fig. 5c). These results indicate that trpA1-CD and trpA1-AB neurons are in close proximity, and possibly form synaptic connections.
BLP trpA1-AB neuronal activity increased by steep temperature ramps
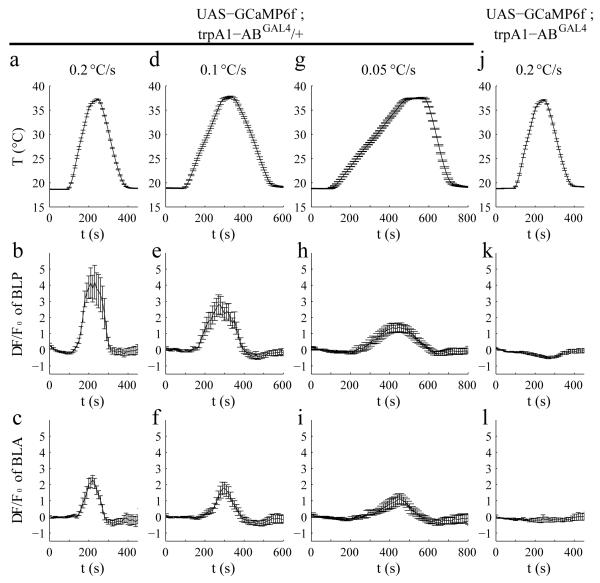
The trpA1-AB neurons in the central nervous system (CNS) might directly sense the rate of temperature change. Alternatively, these brain neurons may not be thermally activated, but receive signals from temperature-activated peripheral neurons. Drosophila TRPA1 is a Ca2+-permeable channel24. To monitor Ca2+ increases in response to different rates of temperature change, we expressed UAS-GCaMP6f37 under the control of the trpA1-ABGAL4 (trpA1-ABGAL4/+), and dissected out the CNS from the transgenic larvae. We varied the temperature ramps from 0.05 to 0.2°C/s and monitored the changes in fluorescence (ΔF/Fo). We found that the rise of Ca2+ in BLP neurons correlated with the steepness of the temperature ramps (0.2°C/s, ΔF/Fo =4.9 ±1.0; 0.1°C/s, ΔF/Fo =3.2 ±0.6; 0.05°C/s, ΔF/Fo =1.7 ±0.2; Fig. 6a,b,d,e,g,h. p =4.7 × 10−7 by one-way ANOVA). The Ca2+ increase was due to TRPA1, since it was eliminated in trpA1-ABGAL4 homozygous mutant flies (Fig. 6j,k). The BLA neurons also showed Ca2+ changes in response to the faster ramps (Fig. 6c,f,i,l), and were eliminated in the trpA1-ABGAL4 mutant (Fig. 6l). However, the peak responses of the BLA neurons were much smaller than those exhibited by the BLP neurons (Fig. 6c,f,i). These findings indicate that TRPA1-AB expressing neurons are activated by rapid temperature changes, and that the BLP neurons respond most robustly, especially when the rate of temperature change was the most rapid. Consistent with the conclusion that BLP and BLA are intrinsically responsive to temperature, these neurons also responded to temperature in the presence of tetrodotoxin (TTX; Supplementary Fig. 9), which depresses synaptic transmission due to blocking voltage-gated Na+ channels and nerve conduction.
Figure 6.
Effects of the rate of temperature change (dT/dt) on the activities of BLP and BLA neurons of 3rd instar larvae. To assay the activities of BLP and BLA neurons in response to different dT/dt, we expressed the Ca2+ sensor, UAS-GCaMP6f, under the control of the trpA1-ABGAL4 and measured the ΔF/F0. The dT/dt are indicated in the top row (a, d, g, j). The response profiles of BLP neurons (second row) and BLA neurons (third row) to different dT/dt are indicated. (b,c,e,f,h,i) ΔF/F0 exhibited by neurons from trpA1-ABGAL4/+ heterozygous control brains. (k,l) ΔF/F0 exhibited by neurons from trpA1-ABGAL4 homozygous mutant brains. The error bars indicate S.E.M.s. n≥6. We performed one-way ANOVA on the maximum ΔF/F0 values of BLP neurons obtained during the different temperature changing rates (b,e,h,k) n=15, 13, 18, 21 corresponds to b,e,h,k, respectively. [F(3,63)=13.86; p=4.7 × 10−7]. We used the Tukey-Kramer test to ascertain statistically significant differences between two samples. [b versus h: q(63,4)=6.07, p=3.6 × 10−4, b versus k: q(63,4)=8.72, p=3.3 × 10−7 and e versus k: q(63,4)=5.10, p=0.034]. We also performed one-way ANOVA on the maximum ΔF/F0 values of BLA neurons obtained during the different temperature changing rates (c,f,i,l) n=10, 11, 6, 6 corresponds to c,f,i,l, respectively. [F(3,29)=8.01; p=4.9 × 10−4]. We used the Tukey-Kramer test to ascertain statistically significant differences between two samples. [c versus i: q(29,4)=3.80, p=0.054, c versus l: q(29,4)=6.65, p=3.2 × 10−4 and f versus l: q(29,4)=4.85, p=0.0093].
TRPA1-A activity enhanced by rapid changes in temperature
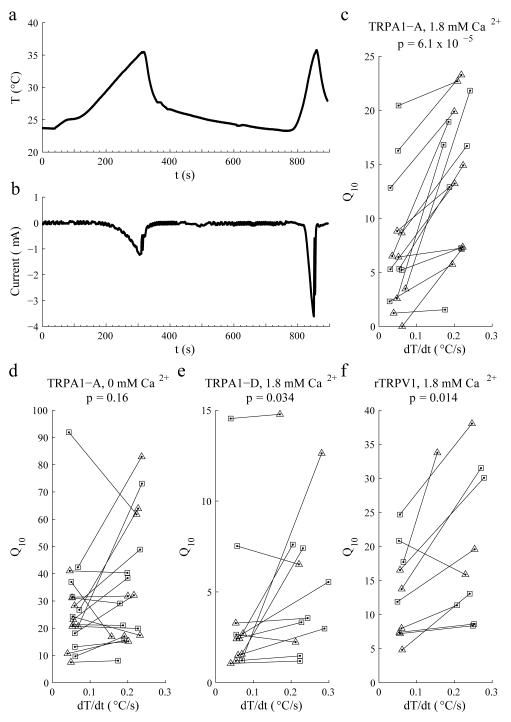
To address whether TRPA1 was sensitive to the rate of temperature change, we expressed TRPA1-A in Xenopus oocytes and performed two-electrode voltage clamp experiments. We increased the temperature at either slow (0.05 ±0.02°C/s) or fast (0.2 ±0.02°C/s) rates and measured the currents (24°C to 35°C for TRPA1-A, 24°C to 40°C for TRPA1-D). When the temperature increased slowly, the peak current was −1.2 μA (Fig. 7a,b). However, when we employed the steeper temperature ramp, the peak current increased ~3-fold to −3.7 μA (Fig. 7a,b). Q10 is defined as the fold increase in activity caused by a 10°C rise in temperature. In each pair of experiments, the Q10 increased significantly with the faster temperature changing rate (p=6.1 × 10−5; Fig. 7c). This suggested that the activity of the TRPA1-A channel was controlled both by the rate of temperature increase, and by the absolute temperature. While the peak current was much larger during the fast heat ramp, there was more total current during the slow ramp (Supplementary Fig. 10). Nevertheless, we suggest that the peak current is the more relevant parameter since the lower peak during the slow ramp may be insufficient to cross the threshold to trigger action potentials.
Figure 7.
Effects of the rate of temperature change on the activities of the TRPA1-A and TRPA1-D channels. The indicated channels were expressed in Xenopus oocytes and the currents were recorded during two temperature ramps (dT/dt: slow, ~0.05°C/s; fast, ~0.2°C/s) in ND96 buffer containing either 1.8 mM Ca2+ or 1 mM EGTA (0 mM Ca2+) as indicated. (a) The oocytes were exposed to two temperature ramps as indicated by the red trace. (b) Representative total currents in an oocyte expressing TRPA1-A in the presence of 1.8 mM Ca2+ upon exposure a slow and fast dT/dt. (c-f) The indicated TRP channels were expressed in oocytes. Shown are the Q10 values as a function of dT/dt. We used linear regression to calculate the temperature changing rates and non-linear curve fitting to calculate the Q10 corresponding to the temperature ramp. In each experiment, we treated one oocyte with a slow and a fast temperature ramp. The black lines link the data from the same oocyte. We changed the order of the slow and fast temperature changes so they were similar in number. The squares and triangles indicate the first and second temperature ramp in each experiment, respectively. We compared Q10 values corresponding to the slow and fast temperature ramps using the Wilcoxon signed rank test. The p values are indicated. (c) TRPA1-A currents using a 24°—35°C ramp. (n=15, W=120, p=6.1 × 10−5). (d) TRPA1-A currents using a 24°—35°C ramp. (n=18, W=65, p=0.16). (e) TRPA1-D currents using a 24°—40°C ramp (n=12, W=54, p=0.034). (f) Rat TRPV1 (rTRPV1) currents using a 24°—48°C ramp (n=10, W=47, p=0.014).
We tested whether Ca2+ affected the differences in Q10 during the slow and fast temperature ramps, as well as the absolute Q10s under both conditions. When we eliminated Ca2+ from the external bath, the differences in Q10 at the slow and fast rates were not significant (p =0.16; Fig. 7d). However, the Q10 values were much higher than in the presence of Ca2+ (Fig. 7c,d). These findings suggest that inactivation of TRPA1-A is sensitive to Ca2+, as is the case for mammalian TRPA138-41.
In addition to TRPA1-A, one additional TRPA1 isoform, TRPA1-D, is thermosensitive14, 29. We found that TRPA1-D also exhibited a higher Q10 in response to the faster temperature ramp (p=0.034; Fig. 7e). However, the differences between the slow and fast ramps were not as significant as with TRPA1-A (Fig. 7c). Thus, the N-terminal exons that are distinct between dTRPA1-A and -D (Fig. 3a) may contribute to the magnitude of the sensitivity to rate of temperature change. We also tested whether rat TRPV1 (rTRPV1) is sensitive to the rate of temperature change. Reminiscent of TRPA1, rTRPV1 also exhibited significant differences in Q10 using slow and fast temperature ramps (p=0.014, Fig. 7f; 24°—48°C ramp).
DISCUSSION
In this work, we established Drosophila larvae as an animal model for dissecting the physiological basis through which an animal displays nociceptive behavior in proportion to the rate of heating. We found that if the temperature rose quickly, a much greater percentage of larvae initiated an escape response, which involved rolling perpendicular to its body axis. Moreover, they did so at much higher temperatures if the environmental temperature increased slowly. Thus, we conclude that Drosophila larvae respond to the rate of heating, rather than just the absolute temperature.
We demonstrated that the molecular sensor essential for detecting the rate of temperature change was a TRPA1 isoform, TRPA1-A. The peak TRPA1-A-dependent currents were larger when the heating was rapid, demonstrating that the activity of this thermoTRP was not strictly a function of the actual temperature, but was also impacted by the heating rate. This finding was consistent with the larval behavioral response to different heating slopes. Rapid heating not only increased the percentage of larvae that roll, but decreased the temperature at which the nociceptive behavior took place.
The trpA1-A-expressing neurons that were critical for sensing the heating speeds were in the brain. In support of this conclusion, the trpA1-AB reporter (trpA1-ABGAL4 and trpA1-ABLexA) was expressed in the brain in BLP neurons. Using a genetically encoded Ca2+ sensor, we found that BLP neurons exhibited larger Ca2+ responses when they were heated rapidly. The heat-induced Ca2+ dynamics were eliminated in trpA1-AB mutant larvae. Nevertheless, we cannot exclude that voltage-gated Ca2+-channels activated subsequent to TRPA1 contribute to the rise in Ca2+. Because the tissue that we used for these experiments were devoid of the peripheral nervous system, our data indicate that that the BLP neurons are directly sensing the rate of temperature change. Moreover, these neurons responded to temperature changes in the presence of TTX, which suppresses voltage-gated Na+ channels and synaptic transmission.
Neural accommodation is a potential mechanism through which BLP neurons could respond differentially to variations in rate of temperature change. Neural accommodation is a consequence of inactivation of voltage-gated Na+ channels, due to slow depolarization. However, the slow depolarization that leads to neural accommodation typically occurs on a sub-second timescale42, and our fastest temperature ramps (0.5°C/s) occurred over the course of many seconds. Thus, we suggest that the lower rolling propensity in response to the slow temperature ramps are not likely due neural accommodation, although our data do not formally rule this out. Nevertheless, a more likely mechanism is Ca2+-dependent inactivation of the TRPA1 channels themselves. Consistent with this latter possibility, we found that the peak TRPA1-A currents were similar in response to slow and fast heat ramps in the absence of external Ca2+. However, during slow heat ramps in the presence of external Ca2+, there is more time for Ca2+ to inactivate the TRPA1 channels, thereby reducing the peak currents.
In addition to a role for trpA1-AB-expressing BLP neurons in thermal nociception, trpA1-CD-positive neurons in the periphery also sense elevated temperatures14. However, the activation threshold for trpA1-AB neurons in the central brain is considerably below the temperature required for activation of the trpA1-CD neurons in the periphery, which is >40°C14. Nevertheless activation of either group of neurons elicits the same rolling behavior. Based on the GRASP analysis, it appears that the trpA1-AB and trpA1-CD neurons are in close proximity, suggesting that these neurons might function in a common neuronal circuit. Thus trpA1-AB neurons in the larval brain are not only primary thermosensors, but may also be transducers of peripheral signals.
We propose that having two groups of thermosensory neurons offers a dual defense. One class, the central brain trpA1-AB neurons, has a low threshold when heating is precipitous, thereby enabling the larvae to begin detecting rapid rates of temperature increase before the temperature rises to an acutely dangerous level. The faster the rate of temperature increase the lower the threshold, thereby providing the animals amble time to initiate rolling and escape a noxious environment. However, if the rate of temperature increase is very gradual, the larvae have more time to respond and do not need to initiate an abrupt escape. Once the absolute temperature reaches an acutely noxious temperature, such as 39°C, activation of the trpA1-CD neurons stimulates a rolling response, thereby providing a second line of defense, via activation of the same neuronal circuit. Nevertheless, another TRP channel, referred to as Painless participates in thermal nociceptive responses12, 27. Thus, this TRP might provide a distinct backup mechanism that allows the animals to respond to noxious temperature changes. Finally, our findings that the robust behavioral response of larvae to rapid heating is mediated by a low-threshold TRP channel raises questions as to whether similar mechanisms occur in vertebrate animals, ranging from amphibians to mammals.
METHODS
Methods and any associated references are available in the online version of the paper. Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Macdonald (UC Santa Barbara) and H. Luo (Shanghai Jiao Tong University) for assistance in generating the knock-in fly lines, and J. Liu and H. Chen (UC Santa Barbara) for assistance in performing blind optogenetic experiments. B. Afonso (Janelia Research Campus), M. Zlatic (Janelia Research Campus), M. Gershow (Harvard University) and A.D.T. Samuel (Harvard University) for help building the software and hardware for the larval tracking system, W.D. Tracey (Indiana University) for trpA1-BAC14, K. Scott (UC Berkeley) for GRASP flies36, P.A. Garrity (Brandeis University) for the pOX-trpA1-A construct43, and G.M. Rubin and J.W. Truman (Janelia Research Campus) for the expression data corresponding to the adult and larval Janelia GAL4 lines. W.L.S. was supported by National Nature Science Foundation of China (X-0402-14-002). This work was supported by grants to C.M. from the National Eye Institute (EY010852) and the National Institute on Deafness and Other Communication Disorders (DC007864).
Footnotes
DATA AND CODE AVAILABILITY
The scripts and data that support the findings of this study are available from the corresponding author upon request.
AUTHOR CONTRIBUTIONS
The study was designed by J.L., W.L.S. and C.M., and directed and coordinated by C.M. The behavioral experiments were performed by J.L. and W.L.S. J.L. generated the trpA1 alleles, performed the immunohistochemistry experiments and the Ca2+ imaging experiments. W.L.S. and J.L. performed two-electrode recordings in Xenopus oocytes. The manuscript was prepared by J.L., W.L.S. and C.M.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 2.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatachalam K, Luo J, Montell C. Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb. Exp. Pharmacol. 2014;223:937–962. doi: 10.1007/978-3-319-05161-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 7.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 8.McKemy DD, Nenhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 9.Peier A, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 10.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, Is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 11.Xiao R, et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L, et al. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neely GG, et al. TrpA1 Regulates Thermal Nociception in Drosophila. PLoS ONE. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 17.Shen WL, et al. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 18.Sedgwick WT. On variations of reflex-excitability in the frog, induced by changes of temperature. Stud. Biol. Lab., Johns Hopkins University. 1882;385 [Google Scholar]
- 19.Clark DA, Gabel CV, Lee TM, Samuel AD. Short-term adaptation and temporal processing in the cryophilic response of Caenorhabditis elegans. J. Neurophysiol. 2007;97:1903–1910. doi: 10.1152/jn.00892.2006. [DOI] [PubMed] [Google Scholar]
- 20.Green BG, Akirav C. Threshold and rate sensitivity of low-threshold thermal nociception. Eur. J. Neurosci. 2010;31:1637–1645. doi: 10.1111/j.1460-9568.2010.07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershow M, et al. Controlling airborne cues to study small animal navigation. Nat. Methods. 2012;9:290–296. doi: 10.1038/nmeth.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell TM. Machine Learning. McGraw-Hill; 1997. [Google Scholar]
- 23.Manning CD, Raghavan P, Schütze H. Introduction to information retrieval. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- 24.Viswanath V, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 25.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J. Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babcock DT, et al. Hedgehog signaling regulates nociceptive sensitization. Curr. Biol. 2011;21:1525–1533. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oswald M, Rymarczyk B, Chatters A, Sweeney S. A novel thermosensitive escape behavior in Drosophila larvae. Fly (Austin) 2011;5 doi: 10.4161/fly.5.4.17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni L, et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature. 2013;500:580–584. doi: 10.1038/nature12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang K, et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195:715–721. doi: 10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Z, et al. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics. 2013;195:289–291. doi: 10.1534/genetics.113.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gratz SJ, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klapoetke NC, et al. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 40.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat. Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 42.Stoney SD, Jr., Machne X. Mechanisms of accommodation in different types of frog neurons. J. Gen. Physiol. 1969;53:248–262. doi: 10.1085/jgp.53.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.