Abstract
Purpose
The inhibition of tumor growth by anti-CD20 antibody (Ab) treatment is mediated by antibody- and complement-dependent cytotoxicity in xenograft tumor models. Additionally, anti-CD20 therapy for B-cell lymphoma can result in intrinsic and extrinsic tumor resistance to further Ab treatment. However, adaptive immune response-related resistance has not been well studied in anti-CD20-mediated tumor control, and adaptive immunity has long been underestimated. The purpose of this study was to explore whether T cells are involved in mediating the effects of anti-CD20 therapy and what factors contribute to adaptive immune response-related resistance.
Experimental Design
Using a syngeneic mouse B-cell lymphoma model, we investigated the role of CD8+ T cells in anti-CD20-mediated tumor regression. Furthermore, we revealed how the tumor-specific T-cell response was initiated by anti-CD20. Finally, we studied adaptive immune response-related resistance in advanced B-cell lymphoma.
Results
CD8+ T cells played an essential role in anti-CD20-mediated tumor regression. Mechanistically, anti-CD20 therapy promoted DC-mediated cross-presentation. Importantly, macrophages were also necessary for the increase in the tumor-specific CTL response after anti-CD20 treatment, via the production of type I IFN to activate DC function. Furthermore, adaptive resistance is gradually developed through the CTLA-4 pathway in Treg cells in larger lymphomas. Further blockade of CTLA-4 can synergize with anti-CD20 treatment in anti-tumor activities.
Conclusions
The therapeutic function of anti-CD20 depends on tumor-specific CD8+ T-cell responses initiated by anti-CD20 through macrophages and DCs. CTLA-4 blockade can synergize with anti-CD20 to overcome adaptive immune response-related resistance in advanced B-cell lymphoma.
Keywords: anti-CD20, B-cell lymphoma, checkpoint blockade, cancer immunotherapy, adaptive immune response-related resistance
Introduction
Rituximab, the first monoclonal antibody (Ab) used for cancer, was approved by the FDA for treating non-Hodgkin’s B-cell lymphoma almost 20 years ago. Rituximab is a murine-human chimeric immunoglobulin G1 (IgG1) Ab that recognizes the human CD20 antigen on B cells, with a primary response rate from 40% to 70% (1-4). Rituximab can induce the death of tumor cells through antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and direct induction of apoptosis in vitro and in animal models (5). However, the exact contribution of each mechanism to the observed clinical activity of anti-CD20 Ab remains unclear. Using the receptor for the Fc region of immunoglobulin G (FcγRs)-deficient mice, the essential role of ADCC has been confirmed in the therapeutic function of anti-CD20 (6). Clinically, it has been observed that the FCGR3A polymorphism limits NK cell-mediated cytotoxicity in rituximab treatment (7). An enhanced therapeutic function was observed by enhancing the binding affinity of anti-CD20 for CD16 (8). Further study showed that anti-CD20-induced lymphoma depletion is mediated by macrophage FcγRI, FcγRIII, and FcγRIV (9, 10), and FcγRIIB inhibits its therapeutic function (11). These studies collectively showed that ADCC plays an important role in anti-CD20 therapy. Effective control of B-cell lymphoma by anti-CD20 in xenograft models further suggests direct killing or innate-mediated killing may be sufficient for the control of this type of tumor, while the role of the adaptive immune system has not been defined.
The role of adaptive immunity in anti-CD20 therapy had been long ignored until recently. Using the huCD20-EL4 tumor model, a murine T-cell lymphoma transfected with the human CD20 molecule, one group reported that the induction of cellular immune responses might contribute to long-lasting protection by anti-human CD20 treatment. Intriguingly, only CD4+ T cells, not CD8+ T cells, are required for the control of the tumor (12). Using the same model, another group showed that anti-CD20 treatment could generate protective memory T cell responses through different FcγRs, but the role of T cells in the primary treatment was not clear (13). Recent studies have shown that CD8+ T cells are essential for the anti-neu therapy of TUBO, a solid tumor model for breast cancer (14, 15). T-cell lymphoma might have abnormally high levels of cytokine expression, and the expression of human CD20 in mouse T-cell lymphoma has created many different antigenic epitopes and cytokine milieu after treatment in immune-competent mouse models. The above two conditions raise the possibility that human CD20-transfected EL4 could induce stronger immunity for tumor control, while the natural B-cell lymphoma might not induce CD4+ T-cell-dependent tumor control.
Anti-CD20 therapy for B-cell lymphoma can result in intrinsic and extrinsic tumor resistance to further Ab treatment (16, 17). Extrinsic resistance was observed to be related to a defective natural immune response (17). Recent studies in blocking the co-inhibitory signaling (CTLA-4 and PD-1/PD-L1) of T cells demonstrated that reversing T-cell suppression is important for effective cancer immunotherapy against solid tumors (18-24). However, adaptive immune response-related resistance has not been well studied in anti-CD20-mediated tumor control, and adaptive immunity has long been underestimated. We explored whether and what type of host response is essential for the therapeutic function of anti-CD20 against actual B-cell lymphoma. Intriguingly, we observed that CD8+ T cells, but not CD4+ T cells, were essential for the anti-mouse CD20 Ab therapy against A20, a B-cell lymphoma in a syngeneic mouse model. We have further observed an essential role of CTLA-4 in Tregs within advanced B-cell lymphoma in limiting anti-CD20-mediated tumor regression. Thus, anti-CTLA-4 and anti-CD20 combined treatment is a possible new strategy in overcoming adaptive resistance and preventing relapse in the clinical treatment of B-cell lymphoma.
Materials and Methods
Mice
WT BALB/c and BALB/c-nude mice were purchased from the Vital River Laboratories (Beijing, China). CL-4 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). CD11c-DTR mice were bred and housed at the Institute of Biophysics. Eight- to ten-week-old female mice were used in all experiments unless otherwise specified. All mice were maintained under specific pathogen–free conditions in the animal facility of the Institute of Biophysics. Animal care and use were in accordance with the Institutional Animal Care guidelines of the Institute of Biophysics, and all studies were approved by the Animal Care and Use Committee of the Institute of Biophysics.
Cell lines and reagents
A20, Raji and 4T1 cells were purchased from ATCC and were maintained according to the method of characterization used by ATCC (Manassas, VA). The cell lines were authenticated by flow cytometry and morphology. In addition, the growth ability of A20, A20-HA, and 4T1 in immunocompetent syngeneic mice (BALB/c) was monitored in each experiment. A20, A20-HA and Raji cells remained CD20+ cells without mycoplasma contamination throughout the experiments. A20-HA was selected as a single clone with 2 μg/ml of puromycin (InvivoGen, San Diego, CA) after infection of A20 with lentivirus expressing huCD20-HA. A20, A20-HA, 4T1 and Raji cells were cultured in 5% CO2 and were maintained in vitro in RPMI 1640 medium (Corning, Manassas, VA), supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 2 mmol/l L-glutamine, 0.1 mmol/l MEM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin. Anti-CD8-depleting Ab (clone TIB210) and anti-CD4-depleting Ab (clone GK1.5) were produced in house. Anti-ifnar1 Ab (clone MAR1-5A3), anti-CTLA-4 Ab (clone 4F10) and hamster IgG were purchased from BioXcell (West Lebanon, NH). Isotype antibody (hIgG) was purchased from Sigma (St Louis, MO).
Production of anti-mouse CD20 antibody
The V region of the heavy chain and light chain of anti-mouse CD20 Ab was linked with a GGGGSGGGGSGGGGSGGGGS linker and was cloned into pEE12.4. The plasmid containing both the heavy chain and light chain was transfected into CHO cells, and stable clones were established according to the manual. The supernatant containing the anti-mouse CD20 Ab was purified using a Protein A column according to the manufacturer’s protocol.
Tumor growth and treatments
A20 cells (1-5*106) were injected subcutaneously (s.c.) into the backs of 8- to 10-week-old mice. The tumor volumes were measured along three orthogonal axes (a, b, and c) and were calculated as follows: tumor volume = abc/2. The mice were treated with intraperitoneal (i.p.) or intratumoral (i.t.) injections of 100 μg of anti-mouse CD20 Ab. For CD8+ or CD4+ T-cell-depletion experiments, 200 μg of anti-CD8 Ab (TIB210) or 200 μg of anti-CD4 Ab (GK1.5) was injected i.p. one day before anti-CD20 treatment. Finally, 100 μg of anti-ifnar1 Ab was used for type I IFN pathway blockade one day before anti-CD20 Ab treatment.
Measurement of IFN-γ-secreting T cells by ELISPOT assay
Spleen or lymph node (LN) cells were resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/l L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. A total of 3*105 spleen or 2*105 LN cells were used for the assay. A20 cells were irradiated with a single dose of 60Gys (10Gys/min for 6 minutes). The ratio of A20 and spleen or LN cells was 1:4. After 48 hours of incubation, the IFN-γ production was determined with an IFN-γ ELISPOT assay kit according to the manufacturer’s protocol (BD Biosciences). The visualized cytokine spots were enumerated with the ImmunoSpot Analyzer (CTL).
Ex vivo DC cross-presentation assay
A20-HA tumor-bearing BALB/c-nude mice were treated with 100 μg of anti-CD20 Ab or isotype control Ab (hIgG) on day 14. Three days later, the mice were sacrificed and DLN cells were digested with 1 mg/ml collagenase VIII (Sigma-Aldrich) and 200 μg /ml DNaseI (Sigma-Aldrich) at 37°C for 30 minutes. DCs (CD11b+CD11c+B220−) were sorted out by FACS. Approximately 1*103 DCs were mixed together with 1*104 purified CL4 T cells. Two days later, the supernatants were collected and IFN-γ, IL-2, and TNFa were measured by cytometric bead array assay (BD Biosciences).
Generation of bone marrow chimeras
WT BALB/c mice were lethally irradiated with a single dose of 1000 rad. The next day, the irradiated mice were adoptively transferred i.v. with 5*106 CD11c-DTR Tg donor bone marrow cells. The mice were maintained on sulfamethoxazole and trimethoprim (Bactrim) antibiotics diluted in drinking water for 4 weeks after reconstitution. The mice were injected with tumor cells approximately 12 weeks after reconstitution.
In vitro culture and function assay of BMDCs and BMDMs
Single-cell suspensions of bone marrow cells were obtained from BALB/c mice. The cells were plated in 10-cm Petri dishes and were cultured in RPMI-1640 medium containing 10% fetal bovine serum, supplemented with 200 U/ml GM-CSF or 1000 U/ml M-CSF. Fresh media with GM-CSF or M-CSF was added to the culture on day 3. BMDCs and BMDMs were harvested for stimulation assay on day 7. BMDCs or BMDMs were co-cultured with A20-HA cells at the ratio of 1:1 in the presence of either 10 μg/ml anti-CD20 Ab or hIgG for 3 days. To determine the role of Fc and FcR interaction, FcR blocking Ab (2.4G2) was used at the same time. The expression of type I IFN was measured by ELISA. Next, the tumor cells were removed, and DCs or macrophages were incubated with isolated CD8+ T cells from naïve CL-4 or BALB/c mice for 3 days. In one experiment, BMDMs were co-cultured with Raji cells in the presence of 10 μg/ml rituximab or hIgG for 3 days. Next, the Raji cells were removed, and macrophages were incubated with isolated CD8+ T cells from BALB/c mice that were immunized 3 times with Raji cells.
In vivo expression of type I IFN
A20-bearing WT BALB/c mice were administered 100 μg of anti-mCD20 or hIgG on day 12. Three days later, DCs and macrophages from the tumor were sorted by FACS. RNA from sorted cells was extracted by RNeasy Plus Mini Kit (Qiagen). RNA was digested with DNaseI and reverse transcribed into cDNA for real-time PCR. The levels of gene expression were normalized to β-actin. The following primers were used: β-actin, forward primer: 5’- ACACCCGCCACCAGTTCGC, reverse primer: 5’- ATGGGGTACTTCAGGGTCAGGGTCAGGATA; IFN-α, forward primer: 5’- TCCCCTGACCCAGGAAGATGCC, reverse primer: 5’- ATTGGCAGAGGAAGACAGGGCT.
Flow cytometry
Single-cell suspensions of cells were incubated with anti-CD16/32 (anti-FcgIII/ II receptor, clone 2.4G2) for 30 min, then were stained with conjugated Abs. All fluorescently labeled mAbs were purchased from BioLegend or eBioscience. Samples were analyzed on a FACSFotassa flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (TreeStar).
Statistical analysis
Data are shown as the mean ± SEM. Statistical analyses were performed using a 2-tailed unpaired Student’s t test and Prism software (version 6.0, GraphPad Software, San Diego, California, USA). P<0.05 was considered statistically significant.
Results
The therapeutic effect of anti-CD20 treatment is CD8+ T cell-dependent
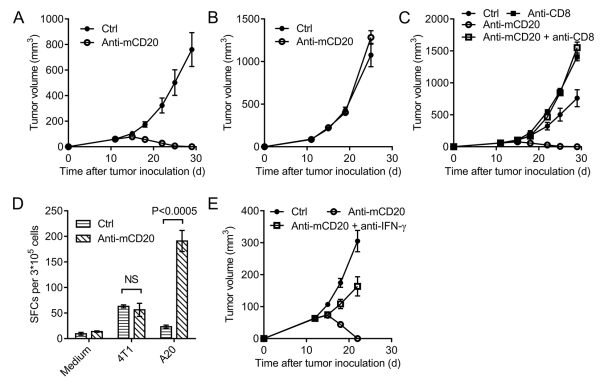
A20 is a mouse B-cell lymphoma cell line derived from a spontaneous neoplasm found in an advanced-age BALB/c mouse (25). To investigate the role of anti-CD20 in mouse B-cell lymphoma, WT BALB/c mice were inoculated with A20 and were treated with anti-mouse CD20 Ab or isotype control Ab (hIgG). Similar to the clinical outcome of B-cell lymphoma treated with rituximab, anti-mouse CD20 Ab could control the tumor effectively (Fig. 1A). However, surprisingly, anti-CD20 Ab failed to control A20 in BALB/c-nude mice (Fig. 1B), suggesting that T cells play an essential role in anti-CD20 therapy. To further investigate which subset of T cells is needed for the anti-CD20-initiated elimination of lymphoma, we depleted either CD4+ or CD8+ T cells during anti-CD20 treatment. The depletion of CD4+ or CD8+ T cells was verified by FACS. CD4+ T cells were not necessary for the function of anti-CD20 (Supplementary Fig. S1). However, the therapeutic function of anti-CD20 was almost abolished after CD8+ T-cell depletion (Fig. 1C). Together, the results suggest that CD8+ T cells are essential for the function of anti-CD20 in controlling B-cell lymphoma.
Figure 1. The therapeutic effect of anti-CD20 treatment is CD8 T cell dependent.
(A) WT BALB/c mice (n = 6/group) or (B) BALB/c-nude mice (n = 6/group) were injected subcutaneously (s.c.) with 1.8*106 A20 cells and were treated with 100 μg of anti-mCD20 or 100 μg of hIgG (Ctrl) by i.p. on days 12 and 15 post A20 injection. This is the basic procedure for C to E. (C) A20-bearing BALB/c mice (n = 6/group) were treated as in A. CD8+ T-cell-depleting Ab (TIB210, 200 μg/mouse) was administered twice a week starting on day 11. (D) Eleven days after the final Ab treatment, draining lymph node (DLN) cells were collected, and the IFN-γ ELISPOT assay was performed with medium or irradiated A20 cell restimulation. Irradiated 4T1 cells were used as the control. (E) A20-bearing BALB/c mice (n = 6/group) were treated as in A. Mouse IFN-γ Ab (R4-6A2, 200 μg/mouse) was administered twice a week starting on day 11. The mean ± SEM values are shown. Two-tailed Student’s t test was used for statistical analysis.
To study the mechanism for the generation of the tumor-specific T-cell response, T cells from the draining lymph node (DLN) were isolated 11 days after Ab treatment to evaluate their responses against tumor antigens using the IFN-γ ELISPOT assay. Irradiated A20 cells were used to stimulate the tumor-specific T cell response, and irradiated 4T1 cells were used as a control. We observed that there was a ten-time increase in immunospots after anti-CD20 treatment (Fig. 1D). These data further support the induction of the anti-CD20-mediated tumor-specific T-cell response in the B-cell lymphoma. CD8+ T cells are the major cytotoxic T cells capable of lysing tumor cells. To investigate whether IFN-γ is not only a biomarker for tumor-reactive T cells but also a key cytokine that can control tumors, we administered a specific neutralizing Ab soon after anti-CD20 therapy. The anti-tumor activity of anti-CD20 decreased dramatically after IFN-γ neutralization, and the tumor became resistant to anti-CD20 therapy (Fig. 1E). Collectively, these data demonstrate that the increased tumor-specific T-cell response after anti-CD20 treatment is necessary for its function in the control of B-cell lymphoma.
The increased cross-presentation function of DC is required for anti-CD20 treatment
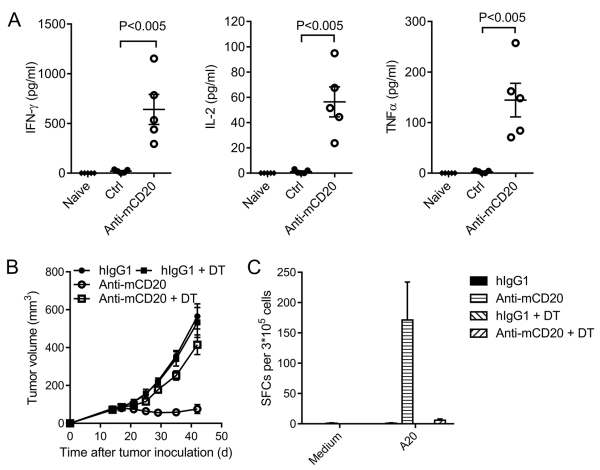
Considering that CD8+ T cells are crucial for effective anti-CD20 therapy, we further investigated the mechanism by which anti-CD20 enhanced tumor-specific T-cell responses. Cross-priming is an essential pathway for the formation of the CD8+ T-cell response. To determine whether the cross-presentation function of APC is enhanced after anti-CD20 treatment, we transfected an antigen epitope hemagglutinin (HA) into A20 cells. We sorted the DCs from the DLN after anti-CD20 treatment and incubated them with TCR-matched naïve T cells from CL4 Tg mice capable of recognizing HA. Two days after incubation, the supernatants were collected and measured using the cytometric bead array (CBA) assay. There was more IFN-γ, IL-2 and TNFα secretion after anti-CD20 treatment (Fig. 2A), suggesting that the cross-presentation function of DC was enhanced after anti-CD20 treatment. To further study whether DCs are essential for effective anti-CD20 therapy, DCs were depleted by DT in CD11c-DTR mice. Complete depletion was achieved, without affecting macrophages that much both in the tumor (Supplementary Fig. S2A, B) and DLN (Supplementary Fig. S2C, D). Depletion of DCs impaired the anti-tumor effect (Fig. 2B) and the T-cell response in anti-CD20 treatment (Fig. 2C). These data indicate that the increased cross-presentation function of APC is indeed essential for anti-CD20-mediated tumor regression.
Figure 2. The increased cross-presentation function of APC is necessary for antibody treatment.
(A) A20-HA-bearing BALB/c-nude mice (n = 5/group) were treated with 100 μg of anti-mCD20 or hIgG on day 14. Three days later, DCs from the DLN were sorted by FACS and then were co-cultured with CL4 T cells. IFN-γ, IL-2, and TNFα production was measured two days later. (B) A20-bearing CD11c-DTR reconstituted BALB/c mice (n = 4/group) were treated with 100 μg of anti-mCD20 or hIgG on day 15. DT was administered every two days starting on day 14. (C) Twenty-five days after the final Ab treatment in (B), splenocytes were collected, and the IFN-γ ELISPOT assay was performed with medium or irradiated A20 cell restimulation. The mean ± SEM values are shown. Two-tailed Student’s t test was used for statistical analysis.
Macrophages increase the cross-presentation function of DCs through type I IFN
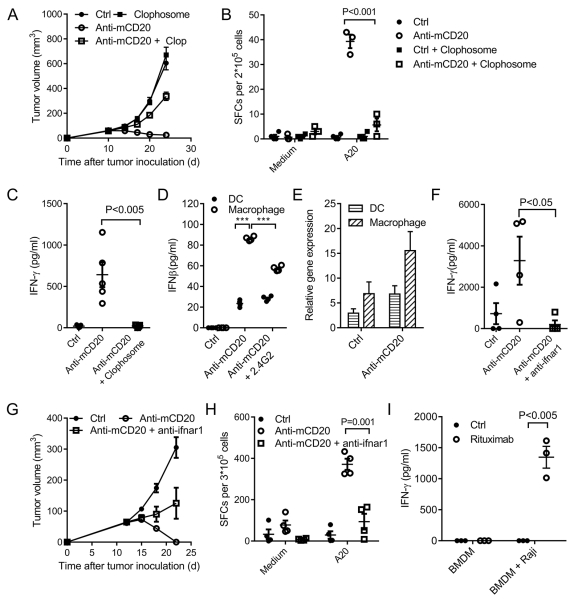
To directly rule out the role of macrophages, we depleted macrophages using Clophosome. After macrophage depletion, we unexpectedly observed that the therapeutic function of anti-CD20 was also largely abolished (Fig. 3A). The Clophosome depletion experiment showed it could eliminate CD11c and F4/80 double-positive cells in the tumor (Supplementary Fig. S3A, B). Recently, the cellular and molecular origin of this subset of cells was reported (26). They proved it is a type of macrophage that differentiates from CCR2+ inflammatory monocytes (26). Because macrophages, DC, and tumor-specific T cells are all essential in anti-CD20 therapy, we hypothesized that macrophages may be the upstream stimulating cells of DCs or T effector cells. To check whether macrophages are necessary for the T-cell response, tumor-specific T cells were monitored in macrophage-depleted mice treated with anti-CD20. The tumor-specific T-cell response was significantly decreased after macrophage depletion (Fig. 3B). We also observed that the therapeutic function of anti-CD20 is NK cell independent when using anti-asialo GM1 Ab for NK cell depletion (Supplementary Fig. S4A). Furthermore, the depletion of NK cells did not affect the tumor-specific T-cell response (Supplementary Fig. S4B). Together, the data suggest that macrophages are essential for the tumor-specific T-cell response.
Figure 3. Macrophages increase DC function through type I IFN.
(A) A20-bearing WT BALB/c mice (n = 5/group) were administered 100 μg of anti-mCD20 or 100 μg of hIgG on days 11 and 15. Macrophage-depleting reagent (Clophosome, 200 μg/mouse) was administered on day 10 and day 15. (B) Eleven days after Ab treatment, DLN cells were collected, and the IFN-γ ELISPOT assay was performed with medium or irradiated A20 cell restimulation. (C) A20-HA-bearing BALB/c-nude mice (n = 5/group) were treated with anti-mCD20 and/or Clophosome on day 14. Three days later, DCs from DLN were sorted by FACS and then were co-cultured with CL4 T cells. IFN-γ production was measured two days later. (D) Bone marrow-derived dendritic cells or macrophages were co-cultured with A20-HA cells in the presence of anti-mCD20 and/or 2.4G2 for 3 days. IFNβ production was measured by ELISA. ***P<0.0001. (E) A20-bearing WT BALB/c mice (n = 5/group) were administered 100 μg of anti-mCD20 or hIgG on day 12. Three days later, DCs and macrophages from the tumor were sorted by FACS. Total RNA was extracted and used for the real-time PCR detection of IFN-α. (F) A20-HA-bearing BALB/c-nude mice (n = 5/group) were treated with anti-mCD20, or anti-mCD20 and anti-ifnar1, or hIgG on day 14. Three days later, DCs from the DLN were sorted by FACS and then were co-cultured with CL4 T cells. IFN-γ production was measured two days later. (G) A20-bearing WT BALB/c mice (n = 5/group) were administered 100 μg of anti-mCD20 or hIgG on days 12 and 15. Mouse ifnar1-blocking Ab (anti-ifnar1, 100 μg/mouse) was administered twice a week starting on day 11. (H) Eleven days after the Ab treatment, DLN cells were collected, and the IFN-γ ELISPOT assay was performed with medium or A20 cell restimulation. (I) Bone marrow-derived macrophages were co-cultured with Raji cells in the presence of either Rituximab or hIgG for 3 days. Next, the tumor cells were washed out, and CD8+ T cells from Raji-immunized mice were added to the culture. IFN-γ production was measured 3 days later. The mean ± SEM values are shown. Two-tailed Student’s t test was used for statistical analysis.
We have shown that both macrophages and the increased cross-presentation function of DCs are responsible for stimulating the tumor-specific T-cell response. To investigate if and how macrophages and DCs are cooperative in anti-CD20 treatment, we checked the cross-presentation function of DCs after macrophage depletion with anti-CD20 treatment. There was much less IFN-γ expression using DCs from the DLN of mice with macrophage depletion (Fig. 3C), suggesting the cross-presentation function of DCs decreased dramatically in the absence of macrophages. Additionally, NK cells are not responsible for increased DC function (Supplementary Fig. S4C). However, how macrophages activate the cross-presentation function of DC is unknown.
Type I IFN is known to be critical for the cross-priming of the tumor-specific T-cell response (27-29). Therefore, we tried to determine which cells are the major contributors for type I IFN, macrophages versus DCs. We observed that the macrophages expressed much higher levels of type I IFN than DCs after anti-CD20 treatment (Fig. 3D). Additionally, type I IFN expression of macrophages induced by anti-CD20 was dependent on Fc and FcR interaction (Fig. 3D). The in vivo expression of type I IFN after anti-CD20 treatment was also checked by RT-PCR (Fig. 3E). The essential roles of type I IFNs in initiating anti-tumor T-cell responses by various anti-tumor therapies have been demonstrated in many studies (27-29). Their data showed that tumor-specific T-cell responses were impaired after blocking the type I IFN pathway, and type I IFNs were necessary for the cross-presentation function of APC. We proposed that macrophages might improve the cross-priming of DCs by secreting type I IFNs after treatment. Furthermore, type I IFN might be essential for anti-CD20-mediated T-cell responses and tumor regression. To check the essential role of type I IFNs, the type I IFN pathway was blocked by anti-ifnar1 during anti-CD20 treatment. Indeed, blocking of the type I IFN pathway by antibodies resulted in an impaired cross-presentation function of DCs (Fig. 3F). Furthermore, the therapeutic function of anti-CD20 was largely abolished when the type I IFN pathway was blocked (Fig. 3G). Correspondingly, blockade of the type I IFN pathway diminished the tumor-specific T-cell response initiated by anti-CD20 (Fig. 3H). Collectively, these data indicate that anti-CD20 therapy can induce type I IFN, which could increase the cross-presentation function of DCs for potent T-cell responses against tumors. The increase in the APC cross-presentation function induced by anti-CD20 was also confirmed with human lymphoma cells in vitro. BMDMs presented an increased cross-priming function when co-cultured with human B-cell lymphoma in the presence of anti-human CD20 Ab (rituximab) (Fig. 3I), suggesting that rituximab might be capable of priming tumor-specific T cells in the patients.
Tumor-specific T cells are mainly primed in the draining lymph node after anti-CD20 therapy
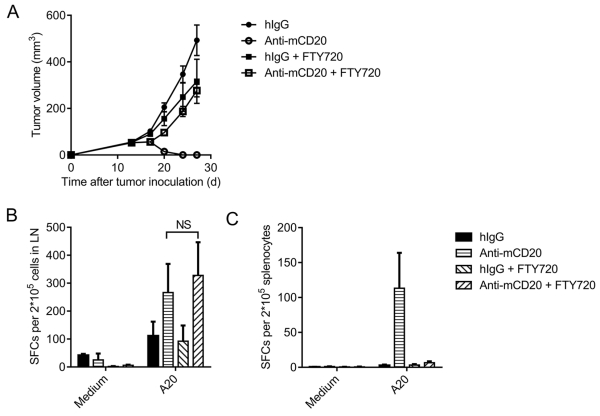
Several studies have indicated that anti-PD-1- or anti-CD47-mediated T-cell re-activation inside tumor tissues is essential for tumor regression (30, 31). Anti-CD20 can induce tumor cell apoptosis that triggers the cross-priming of tumor DCs to reactivate T cells. To investigate whether priming tumor-specific T cells in the tumor or DLN was essential for tumor regression, we utilized FTY720 treatment to block sphingosine 1-phosphate receptor-1, thereby prohibiting T cells from exiting the lymphoid organ (Supplementary Fig. S5). The therapeutic function of anti-CD20 was almost abolished after FTY720 administration (Fig. 4A), suggesting that the reactivation of T cells inside the tumor is not sufficient, and T cells in DLNs are essential for the therapeutic function. To monitor the tumor-specific T-cell changes after FTY720 administration, DLN cells and splenocytes were collected, and the IFN-γ ELISPOT assay was performed. FTY720 had no influence on the activation of tumor-specific T cells in the DLN when treated with anti-CD20 (Fig. 4B). There were no detectable tumor-specific T cells in the spleen when treated with FTY720 (Fig. 4C), suggesting that tumor-specific T cells were not stimulated in the spleen. Taken together, tumor-specific T cells are largely primed in the DLN after treatment. Recirculation of those primed T cells to the tumor tissue is essential to control tumors.
Figure 4. Tumor-specific T cells are primed in the draining lymph node.
(A) A20-bearing WT BALB/c mice (n = 5/group) were administered 100 μg of anti-mCD20 or hIgG on day 11. FTY720 was administered every two days starting on day 10 through the end of the experiment. (B-C) Eleven days after the Ab treatment, DLN cells (B) or splenocytes (C) were collected, and the IFN-γ ELISPOT assay was performed with medium or A20 cell restimulation. The mean ± SEM values are shown. Two-tailed Student’s t test was used for statistical analysis.
CTLA-4 contributes to the adaptive resistance to anti-CD20 therapy
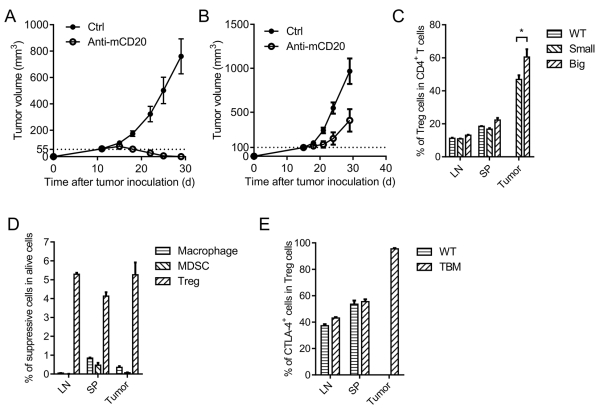
Anti-CD20 therapy was effective for controlling small tumors (Fig. 5A). However, when the tumors became more established, they became much more resistant to anti-CD20 treatment (Fig. 5B). Previous studies have shown that tumor intrinsic resistance and innate immune response resistance account for the tumor non-responsiveness to Ab treatment (16, 17). In addition, larger tumors might lead to an insufficient anti-CD20 molecule to contact each tumor cell. In light of the essential role of CD8+ T cells for initial tumor control in our current study, we wondered whether anti-CD20-initiated immunity was tempered by T cell-mediated resistance. We investigated possible changes among T-cell subsets and observed that there were far fewer tumor-specific T cells after Ab treatment in the advanced tumor (data not shown). We propose that increased Treg cells might contribute to the reduction of CTL. Indeed, the percentage of Treg cells in CD4+ T cells in the tumor was increased in the large established B-cell lymphoma (Fig. 5C). Additionally, Treg cells are the major immunosuppressive cells in the tumor compared with macrophages and MDSCs in large established tumors (Fig. 5D). Thus, Treg cells might contribute to the inhibition of CTL. CTLA-4 on Tregs is a major checkpoint for adaptive immunity (32-39). We observed that the CTLA-4-positive population of intratumoral Tregs was extremely high in advanced B-cell lymphoma, almost reaching 100%, which is much higher than that in the DLN and spleen (Fig. 5E). However, the percentage of CTLA-4-expressing CD8+ T cells in the tumor is much lower than that of Treg cells (Supplementary Fig. S6). This raises the possibility that CTLA-4 on Tregs might limit anti-CD20-mediated CD8+ T-cell expansion.
Figure 5. Adaptive immune response-related resistance accounts for antibody therapy resistance.
(A-B) A20-bearing WT BALB/c mice (n = 5/group) were administered 100 μg of anti-mCD20 or hIgG on day 11 (A) or day 25 (B). (C) The LN, spleen and tumor from naïve (WT) or tumor-bearing (small and large established) WT BALB/c mice were collected and stained for Treg cells. (D) Macrophages (CD11b+ F4/80+), MDSCs (CD11b+ Gr-1Hi) and Treg cells (CD4+ Foxp3+) in large advanced B-cell lymphoma were detected by FACS staining. (E) CTLA-4 expression of Tregs in the LN, spleen, and tumor of naïve and large established tumor-bearing WT BALB/c mice was detected by FACS staining. The mean ± SEM values are shown.
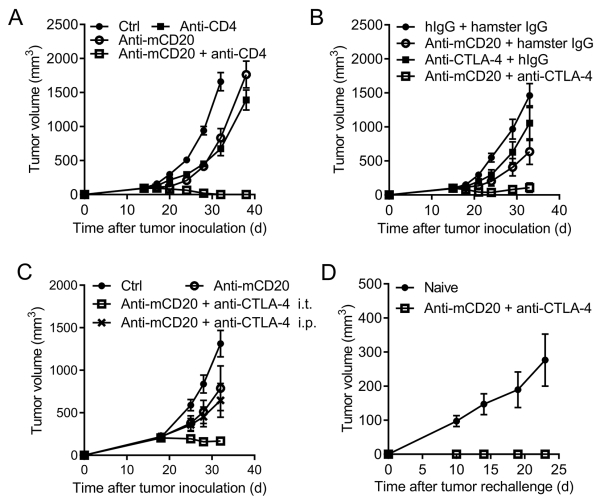
Because Treg is the major subset of CD4+ cells, we determined whether the dominant function of CD4+ T cells is suppressive. CD4+ T cells were depleted by GK1.5 systemically. CD4+ T cell depletion in large established lymphoma could slow down the growth of tumors, suggesting that, overall, CD4+ T cells play an immunosuppressive role in the large established tumor and Treg cells play a dominant role. Such depletion indeed enhanced the anti-CD20 therapeutic effect (Fig. 6A) and suggests that Treg might suppress anti-CD20-mediated immunity against tumors. CTLA-4 is highly expressed by intratumoral Treg. We further explored whether CTLA-4 is essential for adaptive resistance and whether anti-CTLA-4 could overcome anti-CD20 resistance in controlling advanced B-cell lymphoma. Anti-CTLA-4 was administered together with anti-CD20; as expected, the anti-CTLA-4 and anti-CD20 combined treatment had much more effective control of advanced B-cell lymphoma than single treatment (Fig. 6B). As T cells with high expression of CTLA-4 were mainly in the tumor, we compared the intratumoral treatment versus intraperitoneal treatment of anti-CTLA-4. We observed a much stronger synergy of the local injection of anti-CTLA-4 with anti-CD20 than the systemic treatment (Fig. 6C), suggesting that CTLA-4-induced suppression could occur mainly inside the tumor. Moreover, anti-CTLA-4 and anti-CD20 combined treatment could generate stronger protection from a re-challenge (Fig. 6D), indicating a protective memory response generated by the synergetic therapy. Thus, anti-CTLA-4 could be used to overcome adaptive immune response-related resistance to anti-CD20 therapy and protect against tumor relapse.
Figure 6. Anti-CTLA-4 synergizes with anti-CD20 for controlling advanced B-cell lymphoma.
(A) A20-bearing BALB/c mice (n = 4/group) were administered with 100 μg of anti-mCD20 or hIgG on day 15 and day 20. CD4+ T cell-depleting Ab (GK1.5, 200 μg/mouse) was administered twice a week, starting on day 14. (B) A20 bearing WT BALB/c mice (n = 5/group) were i.t. administered respectively with 100 μg of hIgG and 100 μg of hamster IgG, 100 μg of anti-mCD20 and 100 μg of hamster IgG, 100 μg of anti-CTLA-4 Ab and 100 μg of hIgG, or 100 μg of anti-mCD20 combined with 100 μg of anti-CTLA-4 Ab on day 15. (C) A20-bearing WT BALB/c mice (n = 5/group) were administrated i.t. as in A, but with an extra group of anti-CTLA-4 i.p. injection (Anti-CD20 + anti-CTLA-4 i.p.). (D) About 60 days after the tumor rejection in anti-mCD20 combing anti-CTLA-4 Ab treated mice, 5×106 A20 cells were injected for tumor rechallenging assay. Naïve WT BALB/c mice were used as control. The mean ± SEM values are shown.
Discussion
Using xenograft models and in vitro assays, it has been well established that anti-CD20 therapy can increase CDC, ADCC or apoptosis of lymphoma (5). Some clinical studies have reported that tumor-specific T-cell responses could also be detected after the use of rituximab in clinical treatment (40, 41). However, whether T cells are essential for anti-CD20-mediated primary B-cell lymphoma regression has been largely ignored. Using EL4-huCD20, a mouse T-cell lymphoma transduced with human CD20, a xenoprotein, it has been shown that CD4+ T cells are essential for anti-CD20-mediated tumor regression (12). Another recent study demonstrated that long-lasting antitumor protection and ADCC against human CD20-transfected EL4 could be triggered by anti-CD20 therapy, likely through differential Fc-receptor engagement (13). However, strong immunogenicity of human CD20, a lack of depletion of B cells, and strong cytokine production in T-cell lymphoma by anti-human CD20 could complicate the comprehensive understanding of T-cell involvement for anti-CD20-mediated tumor control and relapse. In our study, anti-mouse CD20 Ab was used for the treatment of a mouse B-cell lymphoma (without transfection of human CD20) in the immunocompetent mouse model, to mimic the clinical use of rituximab in human B-cell lymphoma. We have observed that enhancing macrophage-dependent cross-priming for CTL induced by anti-CD20 is essential for anti-tumor therapy: 1) The anti-mouse CD20 Ab could control mouse B-cell lymphoma effectively in WT BALB/c mice but not in the BALB/c-nude mice, suggesting that T cells are required for anti-CD20 treatment; 2) The essential role of T cells was also confirmed by the loss of a treatment effect after CD8 T-cell depletion and an enhanced tumor-specific T-cell response after anti-CD20 treatment; 3) Type I IFN-associated cross-priming is essential; 4) Macrophages increase the cross-presentation function of DCs through type I IFN; and 5) Impressively, CTLA-4 from Tregs plays an essential role in adaptive resistance. These data reveal an essential role of the adaptive immune response in anti-CD20 therapy and resistance to this therapy.
Macrophages have been shown to induce ADCC in anti-CD20 therapy in a mouse model with T-cell lymphoma over-expressing human CD20 (9). Our data showed that macrophages also control mouse B-cell lymphoma treated with anti-mouse CD20. However, the function of macrophages depended on the induction of the tumor-specific T-cell response but not on ADCC alone. The function of macrophages is normal or increased in nude mice, but anti-CD20 failed to control B-cell lymphoma in the syngeneic model. In contrast to the xenograft model, innate cells against syngeneic tumors might not be as strong as xenograft tumors. In fact, T cells can suppress innate cell function (42). When macrophages were depleted, the therapeutic function of anti-CD20 was abolished in WT mice, and the tumor-specific T-cell response was also impaired. Our study suggests that tumor-specific T cells is the final effector cell in the control of tumors; in addition to killing some tumor cells by ADCC, macrophages play a critical role in inducing a tumor-specific T-cell response.
It is not easy to determine whether the tumor or lymphoid tissue is the major site essential for the generation of T-cell responses after Ab treatment because it might depend on the function of antibodies and/or type of tumor. Several studies have indicated that anti-PD-1 or anti-CD47-mediated T-cell re-activation inside tumor tissues is essential for tumor regression (30, 31). Blockade of T cells exiting from the LN during CD20 therapy could reduce the therapeutic effect, suggesting that the DLN might be the essential site for T-cell priming and expansion. Furthermore, increased activation of T cells against tumor cells can be detected in the DLN. The absence of A20 cells in the DLN suggests that APCs must carry antigens into the DLN to prime T cells, and cross-priming might be involved. Type I IFN is the best-known cytokine for the increased cross-priming of DCs to tumor-specific CD8+ T cells. Type I IFNs are critical in initiating anti-tumor T-cell responses induced by Ab against Her2/neu in mammary tumors that are sensitive to oncogenic receptors (14, 15). It is unclear whether anti-CD20 can induce sufficient stress to trigger type I IFN expression. We have demonstrated that type I IFN is also required for cross-priming, leading to the control of B-cell lymphoma with anti-CD20 therapy. DCs have been shown to be the major cell type for IFN in spontaneous tumor regression in various therapies (15, 27-29, 43). However, the expression of type I IFN in macrophages is much higher than that in DCs after anti-CD20 treatment, raising the possibility that macrophages could increase cross-priming via increased type I IFN production. Indeed, the depletion of macrophages impairs the cross-priming function of DCs.
It is unclear why CD8+ T cells are involved in this study while CD4+ T cells are involved in other studies. It was shown that the therapeutic function of anti-CD20 depended on CD4+ T cells instead of CD8+ T cells in huCD20-EL4, a T-cell lymphoma expressing human CD20, finding that are different from our data (12). The treatment includes anti-human CD20 that will not target the mouse B-cell population. In addition, human CD20 is a xenoprotein that triggers strong immune responses. Consistently, we observed that human CD20 is a high immunogenic antigen because human CD20-expressing A20 tumors were immunologically rejected in mice even when given 10-fold higher than the lethal dose (107 per mouse) at transplantation. Furthermore, the EL4-huCD20 model shows that the therapeutic function of anti-human CD20 depends on NK cells, which can strongly express IFN-γ for a rapid Th1 CD4 T-cell response. However, in our model, NK cells are not necessary, which is in accordance with another study also using mouse B-cell lymphoma treated with anti-mouse CD20 (9). Whether the xenoproteins or tumor antigens are the reasons for the different phenotypes of T-cell responses remains to be determined.
Ab-induced apoptosis and innate responses have been thought to be major mechanisms in antibody-initiated tumor regression (5). Therefore, studies on Ab resistance in tumor treatment have been mostly focused on the tumor intrinsic resistance and innate immune response-related resistance (such as absent CD20 expression and FcgRIIIA polymorphism) (16, 17). Our study has revealed that the adaptive immune response is essential for the initial control of tumors at the early phase, but tumor relapse could also be due to adaptive resistance in the adaptive immune system. CTLA-4 is one of the immune checkpoints of T cells after initial T-cell activation. CTLA-4 is important for the suppressive function of Tregs in tumor evasion (33-38). In our study, we observed a much higher percentage of Treg cells as the tumor progressed. Interestingly, these Tregs expressed a much higher level of CTLA-4 than other T cells. However, consistent with single anti-CTLA-4 treatment in the clinic (44), anti-CTLA-4 alone showed a minor effect for A20 regression in our mouse model. However, the function of anti-CTLA-4 was greatly enhanced after anti-CD20 treatment, suggesting that anti-CTLA-4 might release the brake of Tregs in anti-CD20 treatment. Anti-CTLA-4 could feasibly remove the suppression of Tregs. However, in anti-CTLA-4 single treatment, there are no functional T cells, so the tumor could not be controlled. Furthermore, in the anti-CD20 and anti-CTLA-4 combined treatment, it is possible that anti-CD20 initiates a tumor-specific T-cell response and anti-CTLA-4 removes Treg suppression to tumor-specific T cells, achieving a synergistic effect. Anti-CTLA-4 and anti-CD20 combined treatment could also generate a protective memory response against tumor re-challenge, which may also prevent tumor relapse. Our study raises the possibility that the combination of anti-CTLA-4 with anti-CD20 treatment may greatly reduce relapse in lymphoma patients because approximately half of the patients with a complete response to anti-CD20 alone will suffer relapse eventually, and pretreatment with anti-CD20 might overcome the resistance of advanced lymphoma against CTLA-4 blockade.
In conclusion, we have characterized how anti-CD20 treatment initiated a potent tumor-specific T-cell response for tumor control. Ab could kill some tumor cells through ADCC by macrophages that produce type I IFN for cross-priming; DCs capture antigens; IFN binds IFNAR and activates DCs to better process tumor antigens for cross-priming T cells in the DLN; tumor-specific T cells travel to the tumor site for tumor control (Supplementary Fig. S7). Importantly, this study reveals the unappreciated contribution of adaptive immune responses in the early elimination and late resistance of anti-CD20 therapy, raising the potential of using checkpoint blockade to overcome adaptive resistance in the future.
Supplementary Material
Translational Relevance.
Rituximab (anti-CD20 antibody), the first monoclonal antibody used for cancer therapy, was approved by the FDA for treating non-Hodgkin’s B-cell lymphoma nearly 20 years ago. However, the role of the adaptive immune system in anti-CD20-mediated tumor regression has been ignored until recently. This study reveals the essential role of adaptive immunity for anti-CD20-mediated tumor regression and its underlying mechanism. Through studying the mechanism by which anti-CD20 mediates tumor regression, novel strategies for enhancing the anti-CD20 therapeutic effect can be designed and examined in the clinic. By exploiting the immunocompetent mouse model, we have further revealed that the adaptive immune system is essential for the adaptive resistance to anti-CD20 in advanced B-cell lymphoma, opening up a new avenue to combine anti-CD20 with immunotherapy to overcome the resistance of advanced B-cell lymphoma in the clinic.
Acknowledgments
We are grateful to Dr. Mingzhao Zhu (Institute of Biophysics, CAS) for helpful suggestions and comments on the project. We thank Daryl Harmon Harmon (University of Texas Southwestern Medical Center, Dallas, Texas) for helpful editing and discussions. We thank Junjing Yu, Hui Su and Qing Li (Institute of Biophysics, CAS) for technical assistance.
Grant Support: This work was supported by National Nature and Science Foundation of China grant (No. 81172814) to H. Peng, National Science and Technology Major Project of China grant (No. 2012ZX10001006) to H. Peng, National Nature Science Foundation of China grant (NO. 81202328) to Y. Luan and Ministry of Science and Technology of China grant (NO. 2011DFA31250) to Y.-X. Fu. This work was in part supported by the U.S. National Institutes of Health through National Cancer Institute grants CA141975 to Y.-X. Fu.
Footnotes
Authors’ Contributions
Conception and design: Z. Ren, H. Peng, Y.-X. Fu
Development of methodology: Z. Ren, Y.-X. Fu
Acquisition of data: Z. Ren, J. Guo, Z. Liu, Z. Sun, Y. Liang
Analysis and interpretation of data: Z. Ren, H. Peng, Y.-X. Fu
Writing, review and/or revision of the manuscript: Z. Ren, H. Peng, Y.-X. Fu
Administrative, technical, or material support: J. Liao, X. Liu, Y. Luan, H. Peng
Study supervision: H. Peng, Y.-X. Fu
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Maloney DG, Brown S, Czerwinski DK, Liles TM, Hart SM, Miller RA, et al. Monoclonal anti-idiotype antibody therapy of B-cell lymphoma: the addition of a short course of chemotherapy does not interfere with the antitumor effect nor prevent the emergence of idiotype-negative variant cells. Blood. 1992;80:1502–10. [PubMed] [Google Scholar]
- 2.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66. [PubMed] [Google Scholar]
- 3.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. [PubMed] [Google Scholar]
- 4.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:3266–74. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 5.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. The New England journal of medicine. 2012;366:2008–16. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 6.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature medicine. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 7.Dall’Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer research. 2004;64:4664–9. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 8.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–54. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, et al. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood. 2008;112:1205–13. doi: 10.1182/blood-2008-01-135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. The Journal of experimental medicine. 2004;199:1659–69. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roghanian A, Teige I, Martensson L, Cox KL, Kovacek M, Ljungars A, et al. Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer cell. 2015;27:473–88. doi: 10.1016/j.ccell.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–34. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- 13.DiLillo DJ, Ravetch JV. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell. 2015;161:1035–45. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–68. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 17.Cartron G, Trappe RU, Solal-Celigny P, Hallek M. Interindividual variability of response to rituximab: from biological origins to individualized therapies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:19–30. doi: 10.1158/1078-0432.CCR-10-1292. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. Journal of immunology (Baltimore, Md : 1950) 1979;122:549–54. [PubMed] [Google Scholar]
- 26.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science (New York, NY) 2014;344:921–5. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. The Journal of experimental medicine. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer research. 2011;71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. The Journal of experimental medicine. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. Journal for immunotherapy of cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nature medicine. 2015;21:1209–15. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science (New York, NY) 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 34.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer immunology research. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 36.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. The Journal of clinical investigation. 2013;123:2447–63. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. Journal of immunology (Baltimore, Md : 1950) 2006;177:4376–83. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. The Journal of experimental medicine. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. The Journal of clinical investigation. 2015;125:3377–83. doi: 10.1172/JCI80012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood. 2009;113:3809–12. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahlin BE, Sundstrom C, Holte H, Hagberg H, Erlanson M, Nilsson-Ehle H, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4136–44. doi: 10.1158/1078-0432.CCR-11-0264. [DOI] [PubMed] [Google Scholar]
- 42.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, et al. Adaptive immune cells temper initial innate responses. Nature medicine. 2007;13:1248–52. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6446–53. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.