Abstract
Background & Aims
In vitro studies suggest that vitamin D may reduce hepcidin expression and pro-inflammatory cytokine release from monocytes. However, data assessing the vitamin D-mediated effects on iron recycling in healthy individuals are lacking. We aimed to examine the effect of high-dose vitamin D3 on plasma hepcidin, inflammatory cytokine, and ferritin concentrations in healthy adults.
Methods
This was a pilot, double-blind, placebo-controlled trial in healthy adults (N=28) randomized to receive a one-time oral dose of 250,000 IU of vitamin D3 or placebo. Between-and within-group differences in plasma hepcidin, pro-inflammatory cytokine [interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1)], and ferritin concentrations at baseline and 1 week were determined using two-sample and paired t-tests, respectively.
Results
At baseline, plasma 25-hydroxyvitamin D [25(OH)D], hepcidin, pro-inflammatory cytokine, and ferritin concentrations did not differ between the two groups, and greater than 70% of subjects in both groups were vitamin D deficient (25(OH)D < 20 ng/mL). After 1 week, plasma hepcidin concentrations decreased by 73% from baseline in those who received vitamin D3 (geometric mean ratio [GMR] = 0.27 (95% CI: 0.11-0.62); P = 0.005); there was no significant change in the placebo group (GMR = 0.73 (95% CI: 0.49-1.09); P = 0.11). Plasma cytokine and ferritin concentrations did not change significantly in either group.
Conclusions
High-dose vitamin D3 significantly reduced plasma hepcidin concentrations in healthy adults 1 week post-dosing, without a change in plasma pro-inflammatory cytokine or ferritin concentrations. These data suggest that vitamin D may have a role in regulating iron recycling by acting independently of changes in pro-inflammatory markers.
Keywords: vitamin D, hepcidin, inflammation, anemia, iron
1. Introduction
Vitamin D deficiency and anemia are both prominent nutrition-related public health concerns. In the United States, it has been reported that 32% of adults have vitamin D deficiency as defined by 25-hydroxyvitamin D [25(OH)D] concentrations < 20 ng/mL [1]. In 2010, it was estimated that nearly one third of the global population had anemia [2]. Co-existence of vitamin D deficiency and anemia is not uncommon, as poor diets and illness are contributing factors to both conditions [3, 4], and chronic diseases, including chronic kidney disease (CKD) and cardiovascular disease, incur high rates of both [5-8]. Recently, vitamin D deficiency was identified as a potential risk factor for anemia, particularly anemia of inflammation, in the general population [9, 10].
Anemia of inflammation may develop due to disturbances in iron recycling secondary to pro-inflammatory cytokine-induced increases in the hepatic production of hepcidin, the major iron-regulatory hormone [11]. Elevations in hepcidin promote iron sequestration within cells of the reticuloendothelial system, thus limiting iron availability for erythropoiesis and hemoglobin synthesis [12]. Recent in vitro studies suggest a role for vitamin D in down-regulating both pro-inflammatory cytokines and hepcidin [13]. Treatment of cultured human monocytes with vitamin D has been shown to decrease the release of pro-inflammatory cytokines interleukin-6 (IL-6) and interleukin-1β (IL-1β) and down-regulate the expression of hepcidin mRNA [14, 15]. Furthermore, the hepcidin antimicrobial peptide (HAMP) gene has been found to contain a vitamin D response element, suggesting a mechanism for transcriptional regulation of hepcidin by vitamin D [15].
Despite the strong biological plausibility for the association between vitamin D status and anemia, rigorous randomized controlled trials examining the effect of high-dose vitamin D supplementation on specific biomarkers involved in the pathophysiology of anemia of inflammation, namely pro-inflammatory cytokines and hepcidin, are lacking. It remains unclear whether vitamin D-mediated effects on iron recycling occur due to reductions in inflammation or through direct action on hepcidin expression. Moreover, the down-stream effects of vitamin D on makers of iron status have not yet been elucidated. Therefore, the purpose of this study was to examine the acute effect of high-dose vitamin D3 supplementation on plasma hepcidin, inflammatory cytokine, and ferritin concentrations in healthy adults to better understand the mechanism by which vitamin D may influence iron recycling. We hypothesized that treatment with vitamin D3 would reduce circulating hepcidin and plasma inflammatory cytokine concentrations, and increase plasma ferritin concentrations.
2. Materials and Methods
2.1. Subjects and Protocol
Subjects were participants in a double-blind, randomized, placebo-controlled trial designed to evaluate the impact of a large bolus dose of vitamin D3 given prior to winter, on 25(OH)D concentrations year round in healthy adults [16]. Briefly, adults between the ages of 18 and 65 who were healthy by self-report were recruited from the Emory University campus in Atlanta, GA between August and December 2012. Participants were excluded if they were currently pregnant or breastfeeding, had granulomatous conditions, a history of kidney or liver disease, diabetes, a history of malignancy, thyrotoxicosis, a history of calcium or bone abnormalities including hyperparathyroidism, osteoporosis, and Paget's disease, an inability to ambulate, an intake of greater than 1000 mg/day of calcium, and/or used medications including antihypertensives, barbituates, anticonvulsants, or steroids. Sex, race, height, weight, time spent outdoors, and vitamin D supplement use were collected via participant self-report. Participants were asked to refrain from taking any additional vitamin D supplementation during the course of the study. A total of 28 participants were randomized to receive a one-time oral bolus dose of 250,000 IU of vitamin D3 (Biotech Pharmacal, Fayetteville, AR) or matching placebo (Biotech Pharmacal, Fayetteville, AR). This study was approved by the Emory University Institutional Review Board, and is registered at clinicaltrials.gov (NCT01924910). All participants provided written informed consent upon enrollment. The current study uses samples drawn from participants at baseline (n=14 in the vitamin D group; n=14 in the placebo group) and approximately 1 week (5-10 days) later (n=13 in the vitamin D group; n=11 in the placebo group).
2.2. Analytical procedures
Plasma 25(OH)D concentrations were determined using an automated chemiluminescent technique (IDS-iSYS automated machine, Immunodiagnostic Systems, Inc., Fountain Hills, AZ), as previously described [16]. Plasma pro-inflammatory cytokines, interleukin (IL)-1β, IL-6, and IL-8, were measured using a high-sensitivity magnetic bead-based Luminex Performance Assay multiplex kit (R&D Systems, Minneapolis, MN) with a Bioplex analyzer (Bio-Rad, Hercules, CA). Plasma monocyte chemoattractant protein-1 (MCP-1) concentrations were assayed using a bead-based Luminex Performance assay kit (R&D Systems, Minneapolis, MN) on a Bioplex analyzer (Bio-Rad, Hercules, CA). Plasma ferritin concentrations were determined via ELISA (ab108698 – Ferritin Human ELISA kit, Abcam Inc., Cambridge, MA) following manufacturer instructions; the intra-assay CV was 2.48%. A ferritin cut-off value of < 12 ng/mL was used to define low iron stores [17].
Plasma hepcidin concentrations were determined using an electrochemiluminescence immunoassay as previously described [14, 18, 19]. Briefly, streptavidin-coated and blocked 96-well plates were incubated with 25 μL of biotin-labeled capture antibody (4 μg/mL) for 1 hour. Plasma samples were diluted 1:50 in assay buffer, added to their respective washed wells, and incubated at room temperature for 1 hour. Captured plasma hepcidin was detected with 25 μL of 0.1 μg/mL ruthenium-labeled conjugate hepcidin-specific detection antibody, and hepcidin concentrations were interpolated against a standard curve of reference standard hepcidin (Eli Lilly and Company, Indianapolis, IN, USA) [18, 19].
2.3. Statistical Analyses
Descriptive statistics were performed for all variables and reported as mean ± SD or geometric mean (95% confidence interval (CI)) for continuous variables, and number (%) for categorical variables. Variables which were we not normally distributed were transformed to the natural logarithmic scale; in the case of variables with values of zero (IL-1β and IL-6), a constant of 0.01 was added to all non-missing values prior to log-transformation. Baseline comparisons between the vitamin D and placebo groups were examined using two sample t-tests for continuous variables, and χ2 or Fisher's exact test for categorical variables. Between- and within-group differences in plasma 25(OH)D, cytokine, ferritin, and hepcidin concentrations from baseline to 1 week were evaluated using two sample independent t-tests, and paired t-tests, respectively. For variables requiring log-transformation, the results were back-transformed so as to be expressed in the original unit of measurement, as geometric means and their corresponding 95% CI. The mean differences between groups and between time points of the log-transformed data were exponentiated (back-transformed) to generate geometric mean ratios (GMR). A GMR of 1 indicates no treatment effect. All analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, NC) using a two-sided P-value of 0.05 to define statistical significance.
3. Results
3.1. Participant characteristics
Baseline demographic and biochemical characteristics for this study population are shown in Table 1. This was a young and predominantly female cohort. The mean body mass index (BMI) was within the normal range for both groups. Most participants were Caucasian and reported spending less than 10 hours outdoors per week. Very few participants (n=5 total) reported regular intake of vitamin D supplements prior to the start of the study. Baseline demographic and health status characteristics did not differ significantly between vitamin D and placebo groups. As previously reported [16], geometric mean baseline plasma 25(OH)D concentrations for the vitamin D and placebo groups were in the vitamin D deficient range with greater than 70% of participants in either group having a baseline 25(OH)D concentration < 20 ng/mL. Plasma 25(OH)D concentrations increased 150% relative to baseline after 1 week in the group that received high-dose vitamin D3, compared to no significant change in the placebo group [GMR = 2.5 (95% CI: 2.0-3.0), P < 0.001 for the vitamin D group vs. GMR = 1.1 (95% CI: 0.9-1.1), P = 0.38 for the placebo group]. Baseline geometric mean ferritin concentrations in both groups were greater than the cut-off value of 12 ng/mL used to define low iron stores. Plasma pro-inflammatory cytokine, hepcidin, and ferritin concentrations did not differ significantly between vitamin D and placebo groups at baseline.
Table 1. Baseline characteristics of study population by treatment group.
| Vitamin D (n = 14) | Placebo (n = 14) | P-valuea | |
|---|---|---|---|
| Age (years), mean ± SD | 28.2 ± 6.7 | 26.5 ± 5.2 | 0.44 |
| Female, n (%) | 12 (85.7) | 10 (71.4) | 0.65 |
| Caucasian, n (%) | 9 (64.3) | 9 (64.3) | 1.00 |
| BMI (kg/m2b), mean ± SD | 23.7 ± 2.9 | 22.3 ± 2.2 | 0.17 |
| Hours spent outdoors per weekb, mean ± SD | 9.0 ± 5.2 | 7.0 ± 5.4 | 0.32 |
| Vitamin D supplementation prior to trialc (yes), n (%) | 4 (28.6) | 1 (7.1) | 0.33 |
| Plasma 25-hydroxvitamin D [25(OH)D] (ng/mL)d | 16.6 (13.6-20.2) | 16.5 (13.4-20.4) | 1.00 |
| Plasma 25(OH)D < 20 ng/mL, n (%) | 10 (71.4) | 11 (78.6) | 1.00 |
| Plasma ferritin (ng/mL)d,e | 24.5 (12.9-46.4) | 36.1 (19.8-65.8) | 0.35 |
| Plasma IL-1β (pg/mL)d | 0.70 (0.41-1.18) | 1.05 (0.63-1.75) | 0.24 |
| Plasma IL-6 (pg/mL)d | 1.93 (1.29-2.90) | 1.87 (0.71-4.95) | 0.95 |
| Plasma IL-8 (pg/mL)d | 1.96 (1.63-2.36) | 2.01 (1.53-2.65) | 0.88 |
| Plasma MCP-1 (pg/mL)d | 84.9 (72.2-99.9) | 82.0 (69.1-97.5) | 0.75 |
| Plasma hepcidin (ng/mL)d | 9.3 (4.1-20.8) | 12.8 (7.8-21.1) | 0.47 |
Abbreviations: IL-1β: interleukin-1β; IL-6: interleukin-6; IL-8: interleukin-8; MCP-1: monocyte chemoattractant protein-1
Two-sample t-test for continuous variables, χ2 or Fisher's exact test for categorical variables
n=13 for vitamin D group
reported dosages ranged from 400-1000 IU/day
geometric mean (95% confidence interval)
n=13 for vitamin D group, n=11 for placebo group
3.2. Effect of high-dose vitamin D on pro-inflammatory cytokine concentrations
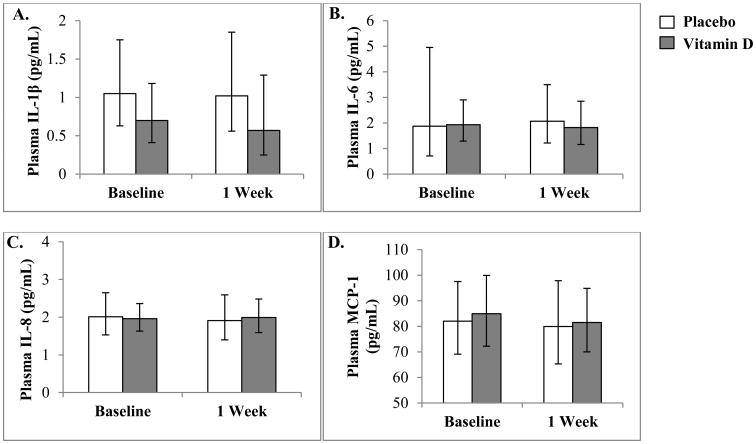
Geometric means of plasma IL-6, IL-1β, IL-8, and MCP-1 concentrations with their corresponding 95% confidence intervals at baseline and approximately 1 week later are shown in Fig. 1. For any of the plasma cytokines, geometric means did not differ significantly between vitamin D and placebo groups 1 week after dosing (P = 0.23-0.87). Likewise, there were no significant differences in plasma cytokines within groups from baseline to 1 week (P = 0.14-0.99).
Fig. 1.
Geometric means and 95% confidence intervals for plasma IL-1β, IL-6, IL-8, and MCP-1 concentrations at baseline and 1 week in the placebo- and vitamin D-treated subjects. Concentrations of IL-1β (A), IL-6 (B), IL-8 (C), and MCP-1 (D) did not differ between vitamin D and placebo groups at baseline or 1 week, and there were no significant changes in concentrations of any of the cytokines from baseline to 1 week in either treatment group. Specific results for each cytokine are list below. IL-1β (A), difference between groups at 1 week: GMR = 0.56 (95% CI: 0.21-1.50), P = 0.23; within group difference from baseline to 1 week: GMR = 0.86 (95% CI: 0.55-1.33), P = 0.46; GMR = 0.92 (95% CI: 0.69-1.23), P = 0.53, for the vitamin D and placebo groups, respectively. IL-6 (B), difference between groups at 1 week: GMR = 0.88 (95% CI: 0.46-1.68), P = 0.69; within group difference from baseline to 1 week: GMR = 0.93 (95% CI: 0.77-1.12), P = 0.40; GMR = 0.80 (95% CI: 0.58-1.09), P = 0.14, for vitamin D and placebo groups, respectively. IL-8 (C), difference between groups at 1 week: GMR = 1.04 (95% CI: 0.74-1.48), P = 0.80; within group difference from baseline to 1 week: GMR = 1.05 (95% CI: 0.79-1.39), P = 0.73; GMR = 0.94 (95% CI: 0.75-1.17), P = 0.52, for vitamin D and placebo, respectively. MCP-1 (D), difference between groups at 1 week: GMR = 1.02 (95% CI: 0.81-1.29), P = 0.87; within group difference from baseline to 1 week: GMR = 1.00 (95% CI: 0.89-1.12), P = 0.99; GMR = 0.96 (95% CI: 0.89-1.05), P = 0.34, for vitamin D and placebo groups, respectively.
3.3. Effect of high-dose vitamin D on plasma hepcidin concentrations
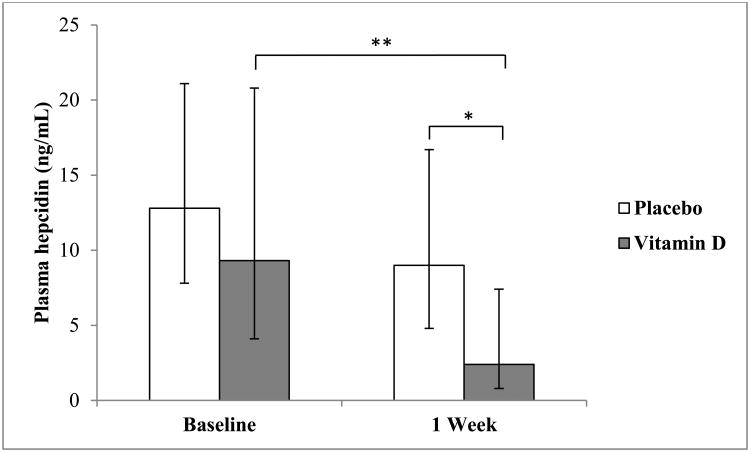
Plasma hepcidin concentrations are shown in Fig. 2. There were no significant differences between groups at baseline [GMR = 0.72 (95% CI: 0.29, 1.80), P = 0.47). By the 1 week time point plasma hepcidin concentrations were 73% lower in the vitamin D group relative to the placebo group [GMR = 0.27 (95% CI: 0.08-0.96), P = 0.04]. The within-group difference was also statistically significant in the vitamin D group such that hepcidin decreased by 73% from baseline to 1 week [GMR = 0.27 (95% CI: 0.11-0.62); P = 0.005]. There was no significant change in hepcidin from baseline to 1 week in the placebo group [GMR = 0.73 (95% CI: 0.49-1.09), P = 0.11].
Fig. 2.
Geometric means and 95% confidence intervals of plasma hepcidin concentrations at baseline and 1 week in the placebo- and vitamin D-treated subjects. Plasma hepcidin concentrations did not differ between groups at baseline [GMR = 0.72 (95% CI: 0.29, 1.80), P = 0.47). Plasma concentrations of hepcidin in the vitamin D and placebo groups differed significantly at 1 week [2.4 ng/mL (95% CI: 0.8-7.4) for the vitamin D group and 9.0 ng/mL (95% CI: 4.8-16.7) for placebo; GMR =0.27 (95% CI: 0.08-0.96), P = 0.04]. Plasma hepcidin concentrations in the vitamin D group decreased significantly from baseline values after 1 week [GMR = 0.27 (95% CI: 0.11-0.62), P = 0.005]; there was no significant change from baseline in the placebo group [GMR = 0.73 (95% CI: 0.49-1.09), P = 0.11]. **P <0.01, *P < 0.05.
3.4. Effect of high-dose vitamin D on plasma ferritin concentrations
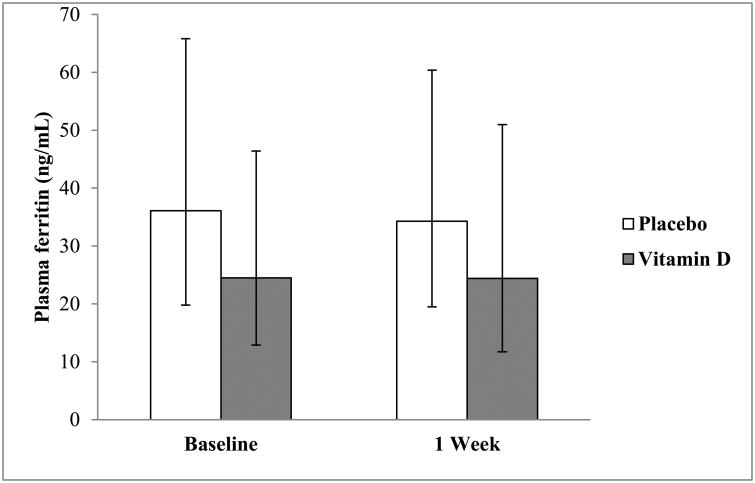
Plasma ferritin concentrations are shown in Fig. 3. Baseline and 1 week ferritin concentrations did not differ significantly between the vitamin D and placebo groups (P = 0.35 and P = 0.44, respectively). Neither group had significant changes in plasma ferritin concentrations from baseline to 1 week (P = 0.95; P = 0.55, for vitamin D and placebo groups, respectively).
Fig. 3.
Geometric mean plasma ferritin concentrations with their 95% confidence intervals at baseline and 1 week in the placebo- and vitamin D-treated subjects. Vitamin D and placebo groups did not differ in plasma ferritin concentrations at baseline [GMR = 0.68 (95% CI: 0.29-1.57), P = 0.35] or 1 week post-dosing [GMR = 0.71 (95% CI: 0.29-1.75), P = 0.44]. Neither group had significant changes in plasma ferritin concentrations from baseline to 1 week [GMR = 0.99 (95% CI: 0.83-1.19), P = 0.95; GMR = 0.95 (95% CI: 0.80-1.14), P = 0.55, for vitamin D and placebo groups, respectively].
4. Discussion
In this cohort of healthy adults with a high prevalence of vitamin D deficiency, treatment with a single large dose of vitamin D3 significantly reduced plasma hepcidin concentrations after 1 week, but did not have a statistically significant effect on plasma concentrations of pro-inflammatory cytokines or ferritin. To our knowledge, this study is the first to evaluate the effect of high-dose vitamin D supplementation on markers involved in the pathophysiology of anemia of inflammation in healthy adults in the context of a randomized controlled trial. Mechanisms by which vitamin D has been proposed to influence iron recycling and anemia are summarized in Fig. 4. Our findings suggest that even in the absence of inflammatory conditions, vitamin D may act directly on hepcidin, independent of inflammatory cytokines.
Fig. 4.
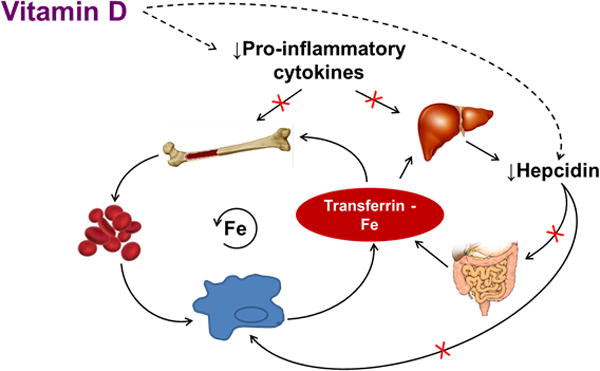
Proposed Role of Vitamin D in Enhancing Iron Recycling. Iron recycling, under non-pathologic conditions, involves transferrin-bound iron in circulation traveling to the bone marrow to support erythropoiesis. Upon senescence, red blood cells (RBCs) are engulfed by macrophages and iron is recycled back into circulation to support further erythropoiesis. Dietary iron may also enter the circulating pool from absorption in the duodenum based on the body's needs. Elevations in pro-inflammatory cytokines suppress erythropoiesis in the bone marrow and shorten RBC lifespan due to increased macrophage activation and erythrophagocytosis. Pro-inflammatory cytokines IL-6 and IL-1β also stimulate the liver to up-regulate the expression of hepcidin antimicrobial peptide (HAMP). Hepcidin inhibits iron egress from cells of the reticuloendothelial system, including enterocytes and macrophages, by binding to and inducing the degradation of the cellular iron exporter, ferroportin, resulting in decreased iron absorption from the small intestine, and increased iron sequestration within the macrophage. Vitamin D has been shown to promote erythropoiesis and iron recycling by increasing erythroid progenitor proliferation, decreasing pro-inflammatory cytokines, and suppressing hepcidin expression. The results from the current study demonstrate that treatment with vitamin D may directly reduce circulating hepcidin concentrations independent of changes in inflammatory cytokines. Decreases in pro-inflammatory cytokines and hepcidin may increase iron bioavailability for erythropoiesis and hemoglobin synthesis by preventing iron sequestration in macrophages, and removing impairments on iron absorption, thus restoring iron recycling.
Figure adapted with permission from [13] Smith EM and Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes 2015, 22:432–38.
While few studies have explored the association of vitamin D with hepcidin, our findings are consistent with the data of Bacchetta et al. [15] in which seven healthy volunteers given 100,000 IU vitamin D2 had a 33% reduction in hepcidin concentrations 72 hours post-dosing. Similarly, in patients with CKD, treatment with vitamin D has been associated with reductions in hepcidin mRNA expression in peritoneal macrophages [20] and circulating hepcidin concentrations [14]. Results from observational studies have been mixed. In a study of pregnant adolescents, circulating 25(OH)D concentrations were not associated with hepcidin concentrations [21], while a study in CKD patients showed that lower levels of 1,25(OH)2D were associated with increased hepcidin concentrations [22]. Taken together, our data and the previous reports provide evidence for hepcidin-lowering by vitamin D as a potential mechanism by which vitamin D may influence iron recycling. The recent discovery of a vitamin D response element on the HAMP gene also lends strong biological plausibility to our results [15]. Further long-term studies are needed to establish the clinical implications of inflammation-independent changes in circulating hepcidin concentrations.
Concentrations of pro-inflammatory cytokines did not change significantly in response to high-dose vitamin D supplementation in this healthy cohort. This is in contrast to epidemiologic studies, which have reported increased odds of anemia of inflammation with lower vitamin D status [9, 10]. Vitamin D has also been shown to have anti-inflammatory effects in various in vitro studies, observational studies, and clinical trials [14, 23]. However, these studies have largely been conducted in populations with chronic conditions or infections, where elevations in pro-inflammatory cytokines are more likely to be observed. In utilizing healthy volunteers for this study, we were able to demonstrate that vitamin D may affect hepcidin concentrations even without altering inflammatory cytokine concentrations.
Despite the significant reduction in hepcidin, circulating ferritin concentrations were unchanged in response to high-dose vitamin D3 supplementation. However, given the physiological response to iron recycling in the presence of elevated hepcidin concentrations, this is not entirely unexpected. Any effects of hepcidin reduction on iron egress from cells and iron bioavailability may have been better captured as transport iron, using markers such as serum iron, total iron binding capacity, and transferrin saturation as opposed to ferritin, the storage form of iron [12, 24]. In a previous observational study, we found that 25(OH)D status was significantly positively associated with hemoglobin and serum iron concentrations in a cohort of generally healthy adults, while serum ferritin concentrations did not differ between participants who were vitamin D deficient and those who were not [10]. Similarly, Thomas et al. [21] found that 25(OH)D status was significantly positively associated with hemoglobin concentrations and serum iron among pregnant adolescents, but did not observe a significant association between vitamin D status and ferritin concentrations.
Our findings may have potential implications for vitamin D as a therapy in combatting anemia. As hepcidin concentrations have been inversely associated with hemoglobin concentrations and positively associated with risk for anemia [25], reducing hepcidin levels in the blood may be a target for anemia therapies. However, very few trials have directly evaluated the effect of vitamin D treatment on hemoglobin concentrations or anemia. Among those that have, the results have been mixed, likely due to the differences in dosage and form of vitamin D administered, and the population studied [26-28]. In studies of patients with CKD, vitamin D or its analogues have been shown to increase hemoglobin concentrations [27, 29-31]. Larger studies examining the effect of vitamin D administration on circulating hepcidin concentrations, and the subsequent impact on hemoglobin concentrations or anemia in both healthy and diseased populations at risk for anemia are necessary.
A major strength of this study was the double-blind, randomized, placebo-controlled clinical trial design. Also, the healthy, young study population allowed us to ascertain the effects of vitamin D on iron recycling in the absence of potentially confounding conditions. However, we were limited in that we were unable to measure hemoglobin concentrations in our study participants to determine the influence of vitamin D treatment on hemoglobin and anemia status. Given the high prevalence of vitamin D deficiency in this population, the generalizability of our results to other populations with a lower prevalence of vitamin D deficiency may be limited. However, as a pilot efficacy study, the high prevalence of vitamin D deficiency in this population may have been advantageous in allowing us to ascertain the impact of vitamin D therapy on our outcomes. Other limitations of this study include the lack of measures of iron status other than ferritin. With our relatively short duration of observation and the small sample size we were possibly underpowered to detect changes in inflammatory markers and ferritin, and the generalizability of our findings may be limited. However, the fact that we observed a statistically significant reduction in hepcidin concentrations in response to vitamin D supplementation in spite of the short duration and small sample size suggests a robust effect of vitamin D on hepcidin concentrations, and lends merit to our findings.
In summary, this pilot study addresses a gap in the literature related to the mechanism underlying the association between vitamin D status and anemia observed in several epidemiologic studies. Supplementation with high-dose vitamin D3 significantly reduced circulating hepcidin concentrations after 1 week among healthy adults without chronic or inflammatory disease and independent of circulating cytokine markers of inflammation. The down-stream effects of vitamin D on markers of iron status and anemia require further examination in larger studies of longer duration.
Supplementary Material
Acknowledgments
Funding sources: This work was supported, in part, by National Institutes of Health grants T32 DK007734 (EMS), K01 DK102851 (JAA), K24 DK096574 (TRZ), and UL1 TR000454 (Atlanta Clinical and Translational Science Institute). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design, analysis or writing of this article.
Abbreviations
- 1
25(OH)2D, 1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CKD
chronic kidney disease
- GMR
geometric mean ratio
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-8
interleukin-8
- MCP-1
monocyte chemoattractant protein-1
Footnotes
Statement of authorship: Contributions of authors to the manuscript: EMS, MDK, and VT designed the study; EMS, JAA, MDK, JHS, RJK, LH, SMZ, and VT collected the data and conducted the analytical procedures; EMS and VT analyzed the data; EMS, JAA, SMZ, TRZ, and VT interpreted the data; EMS wrote the draft of the manuscript; all authors critically reviewed the manuscript, contributed to revisions, and read and approved the final manuscript.
Conflict of interest: JHS and RJK are employed by Eli Lilly and Company and performed the hepcidin assay. Eli Lilly and Company played no role in the study design or decision to publish.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142:498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ, Murray CJ. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Camaschella C. Iron-Deficiency Anemia. N Engl J Med. 2015;372:1832–43. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504–10. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]
- 6.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–33. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–27. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–4. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117:2800–6. doi: 10.1182/blood-2010-09-309708. [DOI] [PubMed] [Google Scholar]
- 10.Smith EM, Alvarez JA, Martin GS, Zughaier SM, Ziegler TR, Tangpricha V. Vitamin D deficiency is associated with anaemia among African Americans in a US cohort. Br J Nutr. 2015;113:1732–40. doi: 10.1017/S0007114515000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–81. vi. doi: 10.1016/j.hoc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–41. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 13.Smith EM, Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes. 2015;22:432–8. doi: 10.1097/MED.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zughaier SM, Alvarez JA, Sloan JH, Konrad RJ, Tangpricha V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J Clin Transl Endocrinol. 2014;1:19–25. doi: 10.1016/j.jcte.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, Nayak A, Wesseling-Perry K, Westerman M, Hollis BW, Salusky IB, Hewison M. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25:564–72. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearns MD, Binongo JN, Watson D, Alvarez JA, Lodin D, Ziegler TR, Tangpricha V. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2015;69:193–7. doi: 10.1038/ejcn.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–6. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield AM, Luan P, Witcher DR, Manetta J, Murphy AT, Wroblewski VJ, Konrad RJ. A dual-monoclonal sandwich ELISA specific for hepcidin-25. Clin Chem. 2010;56:1725–32. doi: 10.1373/clinchem.2010.151522. [DOI] [PubMed] [Google Scholar]
- 19.Troutt JS, Rudling M, Persson L, Stahle L, Angelin B, Butterfield AM, Schade AE, Cao G, Konrad RJ. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin Chem. 2012;58:1225–32. doi: 10.1373/clinchem.2012.186866. [DOI] [PubMed] [Google Scholar]
- 20.Bacchetta J, Chun RF, Gales B, Zaritsky JJ, Leroy S, Wesseling-Perry K, Boregaard N, Rastogi A, Salusky IB, Hewison M. Antibacterial responses by peritoneal macrophages are enhanced following vitamin D supplementation. PLoS One. 2014;9:e116530. doi: 10.1371/journal.pone.0116530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas CE, Guillet R, Queenan RA, Cooper EM, Kent TR, Pressman EK, Vermeylen FM, Roberson MS, O'Brien KO. Vitamin D status is inversely associated with anemia and serum erythropoietin during pregnancy. Am J Clin Nutr. 2015;102:1088–95. doi: 10.3945/ajcn.115.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho C, Isakova T, Collerone G, Olbina G, Wolf M, Westerman M, Gutierrez OM. Hepcidin and disordered mineral metabolism in chronic kidney disease. Clin Nephrol. 2011;76:90–8. doi: 10.5414/cn107018. [DOI] [PubMed] [Google Scholar]
- 23.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Drygalski A, Adamson JW. Iron metabolism in man. J Parenter Enteral Nutr. 2013;37:599–606. doi: 10.1177/0148607112459648. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson MA, Kim JY, Roy CN, Warady BA, White CT, Furth SL. Hepcidin and risk of anemia in CKD: a cross-sectional and longitudinal analysis in the CKiD cohort. Pediatr Nephrol. 2015;30:635–43. doi: 10.1007/s00467-014-2991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst JB, Tomaschitz A, Grubler MR, Gaksch M, Kienreich K, Verheyen N, Marz W, Pilz S, Zittermann A. Vitamin D Supplementation and Hemoglobin Levels in Hypertensive Patients: A Randomized Controlled Trial. Int J Endocrinol. 2016;2016:6836402. doi: 10.1155/2016/6836402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riccio E, Sabbatini M, Bruzzese D, Capuano I, Migliaccio S, Andreucci M, Pisani A. Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial. PLoS One. 2015;10:e0118174. doi: 10.1371/journal.pone.0118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sooragonda B, Bhadada SK, Shah VN, Malhotra P, Ahluwalia J, Sachdeva N. Effect of vitamin D replacement on hemoglobin concentration in subjects with concurrent iron-deficiency anemia and vitamin D deficiency: a randomized, single-blinded, placebo-controlled trial. Acta Haematol. 2015;133:31–5. doi: 10.1159/000357104. [DOI] [PubMed] [Google Scholar]
- 29.Albitar S, Genin R, Fen-Chong M, Serveaux MO, Schohn D, Chuet C. High-dose alfacalcidol improves anaemia in patients on haemodialysis. Nephrol Dial Transplant. 1997;12:514–8. doi: 10.1093/ndt/12.3.514. [DOI] [PubMed] [Google Scholar]
- 30.Goicoechea M, Vazquez MI, Ruiz MA, Gomez-Campdera F, Perez-Garcia R, Valderrabano F. Intravenous calcitriol improves anaemia and reduces the need for erythropoietin in haemodialysis patients. Nephron. 1998;78:23–7. doi: 10.1159/000044877. [DOI] [PubMed] [Google Scholar]
- 31.Lin CL, Hung CC, Yang CT, Huang CC. Improved anemia and reduced erythropoietin need by medical or surgical intervention of secondary hyperparathyroidism in hemodialysis patients. Ren Fail. 2004;26:289–95. doi: 10.1081/jdi-120039528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.