Abstract
As an emerging class of optical nanomaterials, semiconducting polymer nanoparticles (SPNs) are highly photostable, optically active and versatile in chemistry; these properties make them attractive as molecular imaging agents to enable imaging of biological events and functionalities at multiple scales. More recently, a variety of SPNs have been found to exhibit high photoacoustic properties, and further empowered photoacoustic imaging for contrast enhanced in vivo molecular imaging. Target-sensitive components can be incorporated in the SPNs to create activatable imaging probes to sense and monitor the target dynamics in living objects. Intrinsically biophotonic and biocompatible, SPNs can be further engineered for multimodal imaging and for real-time imaging of drug delivery.
Graphical Abstract
Introduction
Photoacoustic (PA) imaging is a non-ionizing imaging technology that hybridizes optical excitation and ultrasonic detection, thus able to provide deep tissue penetration and high spatial resolution1, 2. Ever since its first demonstration, there has been rapid progress in both the instrumentation and the contrast agent development. Up till now, PA has been applied to visualize biological structures at the multiscale ranging from organelles, cells to organs, expanding its applications in cardiology3–5, oncology6, 7, neurology8–10, gastroenterology11, 12 and dermatology12 in both the pre-clinical and clinical investigations. Many endogenous molecules, such as hemoglobin, DNA/RNA, melanin, lipid and water, can produce photoacoustic signals13–15. Therefore, PA can be utilized to obtain the anatomical and functional information for a variety of biological structures in a label-free manner. In addition, PA is able to monitor dynamic processes in vivo such as oxygen saturation of hemoglobin, blood flow and tissue temperature change16, 17. The integration of static and dynamic PA allows for the measurements of all parameters required for quantifying oxygen metabolism, of which the level is paramount important for the diagnosis and staging of hyper-metabolic diseases16. Exogenous contrast agents expand the PA imaging beyond endogenous molecules with high sensitivity, specificity and signal-to-background ratio, and have drastically broadened the horizon of PA imaging. Over the past decade, an increasing number of exogenous contrast agents have been developed, from organic dyes2, fluorescent proteins18, 19, reporter genes20–22 to nanoparticles23–29, including gold nanoparticles, gold-coupled core-shell nanoparticles, iron oxide, carbon nanotubes, 2D graphene analogues and porphyrin-based lipidic nanostructures. Each type of materials possesses both advantages and disadvantages for PA imaging. For example, the primary advantage of gold nanoparticle is the large absorption cross-sections, which renders picomolar-level detection sensitivity. However, metallic nanoparticles usually face the issues of biocompatibility and poor photostability, and carbon nanoparticles and graphenes have relatively broad PA spectra. The development and application of the aforementioned materials has been extensively reviewed26–29.
Semiconducting polymers (SPs) are a group of organic polymers with π-conjugated structures where the electrons can move along the polymeric backbones, making them optically and electrically active30. Early applications of semiconducting polymers focus on the use in optoelectronics such as solar energy conversion31, 32 and thin-film transistors33–35 due to their excellent photovoltaic properties. Recently, many semiconducting polymers are assembled into nano-scale structures via miniemulsion and nanoprecipitation when the polymers are transferred from hydrophobic organic solvents to water36. The so-formed semiconducting polymer nanoparticles (SPNs) are highly fluorescent, non-blinking, photostable upon irradiation and non-toxic, thus have been extensively applied for cell labeling37–39, tumor-targeted imaging40 and reactive oxygen and nitrogen species detecting41. In addition to the fluorescent properties, the direct bandgap in the SPs often results in an allowed efficient absorption. Taking the advantage of this high light-harvesting capacity, we recently developed a series of bioluminescent and chemiluminescent probes for in vivo lymph node mapping42 and real-time sensing of drug-induced hepatotoxicity43. Most recently, our studies have revealed that near-infrared (NIR) light-absorbing SPNs efficiently transform photonic energy to ultrasound waves, therefore can serve as a versatile nanoplatform for PA molecular imaging44, 45. This short review will focus on the most recent research investigation of SPNs for PA imaging.
SPNs for Photoacoustic Imaging
Polymer developing strategies
Initial examples of polymeric nanoparticles for PA imaging were based on polypyrrole (PPy NPs). Dai et al. reported the formulation of polypyrrole particles using a polymerization method by mixing pyrrole monomers and a PVA-FeCl3 mixture46. The resulted particles were monodispersed at a size of around 46 nm and showed strong absorption and photoacoustic signals at 808 nm. A maximum imaging depth of 4.3 cm in the phantom study (PPy NPs at the concentration of 100 μg ml−1) was reported, which amounted to about 5 times the effective optical penetration depth. In vivo imaging results further demonstrated that PPy NPs afforded contrast-enhanced PA imaging of the brain vasculature compared to the intrinsic optical contrast from the mice. In a follow-up study, tantalum oxide was encapsulated into the PPy NPs to generate bimodal X-ray and PA imaging agents with photothermal therapeutic property47. Effective tumor ablation was observed after TaOx-PPy nanoparticles-mediated photothermal therapy under the precision guidance of CT/PA imaging, indicating the theranostic potential of this nanoconstruct.
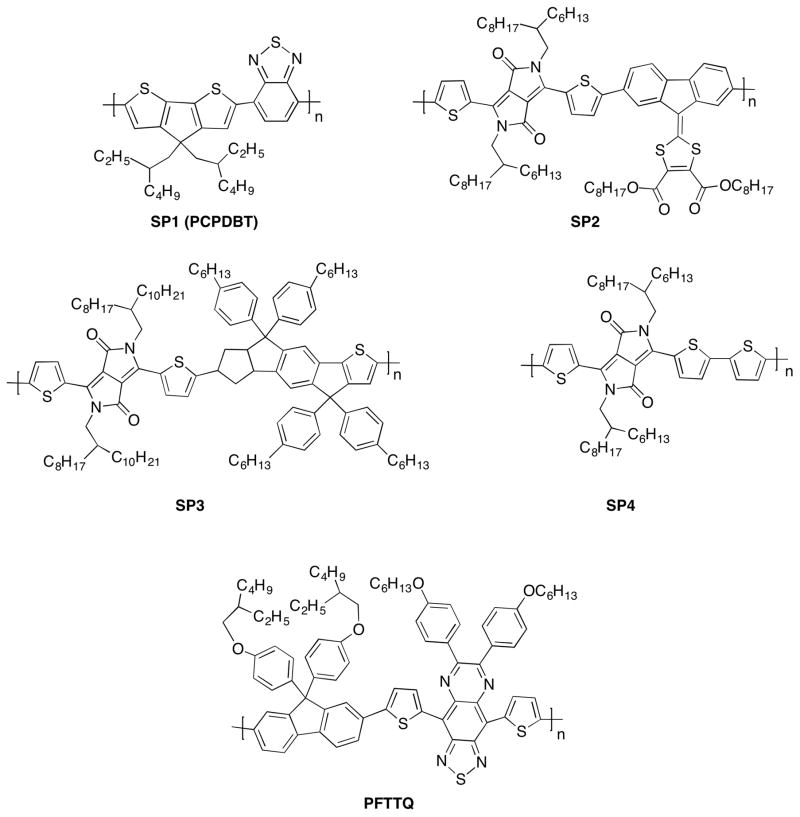
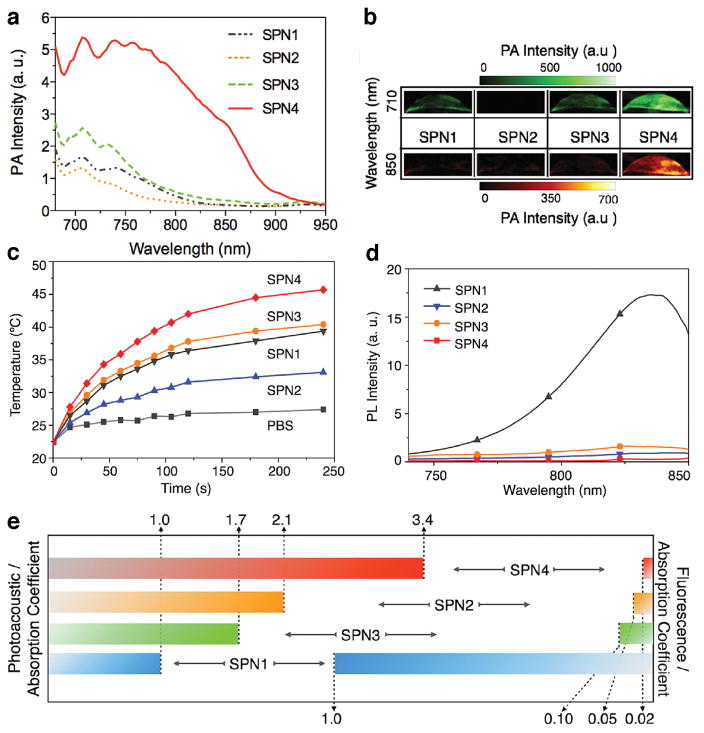
To achieve polymeric nanoparticles with enhanced versatility and PA capacity, a strategy to build backbones where electron-rich and electron-deficient moieties were combined to form a donor-acceptor structure was proposed (Figure 1). Low bandgap poly(cyclopentadithiophene-alt-benzothiadiazole) (SP1)48 , diketopyrrolopyrrole (DPP)-based SPs (SP2-4)45 and poly[9,9-bis(4-(2-ethylhexyl)phenyl)fluorene-alt-co-6,7-bis(4-(hexyloxy)phenyl)-4,9-di-(thiophen-2-yl)thiadiazolo-quinoxaline] (PFTTQ)49 were constructed into nanoparticles by coprecipitation with lipids when added to water under sonication (Figure 2). The so-formed SPNs were monodispersed spherical nanoparticles sizing around 40 nm (PDI < 0.2) and the addition of lipid excipients resulted in SPNs with high stability under storage and excellent compatibility at physiologically relevant conditions44, 45. The optical properties of these SPs can be exquisitely tuned via co-polymerizing the electron-deficient units with different electron donators, which subsequently affected the photoacoustic properties. For chromophores, after the absorption of energy, the excited species typically dissipate the energy through three pathways: (i) dissipating the energy in the form of fluorescence and measured by the fluorescence quantum yield, (ii) stored by long-living entities (e.g. phosphorescence) via intersystem crossing, and (iii) heat release. The fraction of the energy from pathway (iii) gives rise to thermoplastic expansion, which generates sound waves, as of the mechanism of photoacoustic effect. As the aforementioned SPs are not phosphorescent, their photoacoustic properties rely on the light-absorbing capacity and energy dissipation through (i) and (iii). In the study comparing the fluorescent and photoacoustic properties of SP1-4 (Figure 3)45, it was shown that DPP-based SP3 and SP4 exhibited relatively stronger photothermal effect and photoacoustic signals upon laser irradiation than SP1, in an order of SP4>SP3>SP1>SP2 (Figure 3a–c). On the contrary, the fluorescence intensities showed a reversed trend comparing to the photoacoustic intensities when the SPs were irradiated at the same wavelength with mass excitation coefficients normalized at this wavelength (Figure 3d, e). This result reveals an insight into the relations between chemical structures and optical properties of SPs. Among the three DPP-based SPs, SP4 had the strongest electron donor-acceptor backbone structure and the narrowest bandgap, which explained why pathway (iii) is favoured in the energy dissipation. This study has indicated that the PA properties of SPs are strongly associated with their molecular structures and can be finely tuned with structural modification through chemical synthesis. In addition, the lipid components in the formulation had no effect on the PA brightness and spectrum of the particle, suggesting that optimization of the formulation to achieve favourable physiological stability and pharmacokinetic profiles would not compromise the PA properties.
Figure 1.
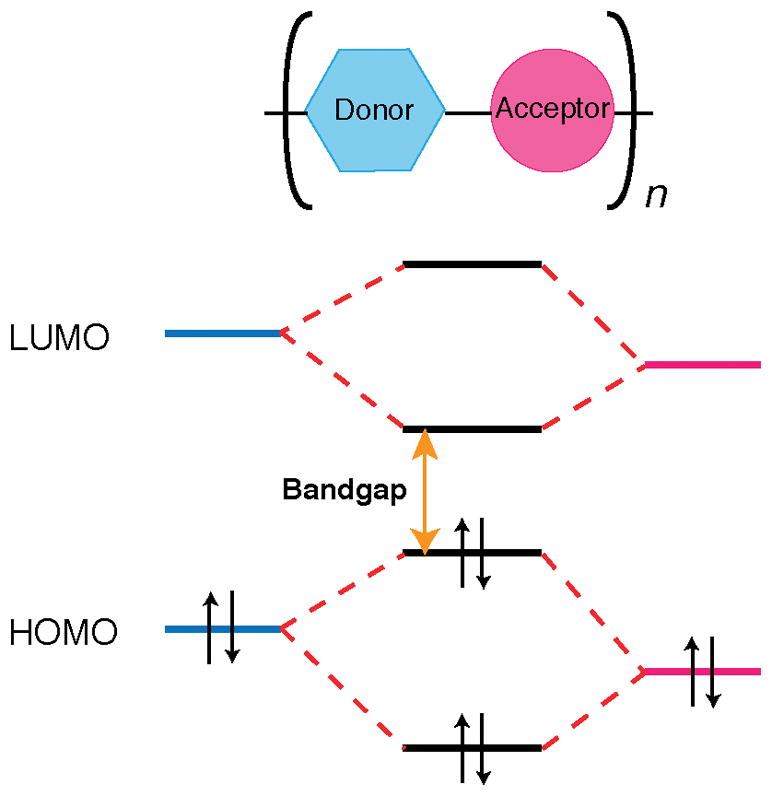
Simplified electronic structure of donor-acceptor co-polymer chain with low bandgap.
Figure 2.
Chemical structures of low bandgap semiconducting polymers used for nanoparticle formulations and photoacoustic imaging.
Figure 3.
Photoacoustic, photothermal and fluorescence profiles of SPN1-4. (a)PA spectra of SPN1-4 solutions (in 1×PBS, pH 7.4) (b) Representative PA images of SPN1-4 solutions ([SPN]= 2 μg mL−1) excited by pulsed laser at 710 and 850 nm, respectively. (c) Photothermal properties of SPN1-4 solutions, showing the temperature of the SPN solutions ([SPN]= 20 μg mL−1 in 1×PBS, pH 7.4) as a function of laser irradiating time. (d) Fluorescence spectra of SPN1-4 solutions (in 1×PBS, pH 7.4). PL: photoluminescence. (e) Normalized PA and fluorescence intensities based on the same mass extinction coefficients at 710 nm of SPNs solutions. Reproduced from Ref [39]. Copyright John Wiley & Sons, Inc.
Advantages of SPNs as PA agents
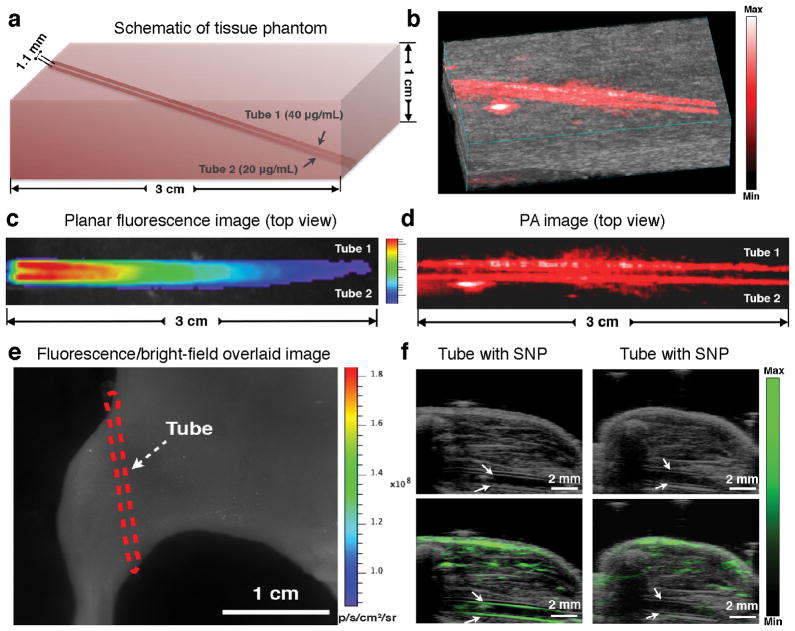
The conversion of light energy to ultrasonic energy in the photoacoustic effect allows it to overcome the high photon scattering in biological tissues and generate a much deeper penetration with better spatial resolution. As PA contrast agents, SPNs exhibited a centimetre-level imaging depth in both phantom and in vivo imaging studies44, 46. Being both fluorescent and photoacoustic, SPN1 enabled a direct comparison between fluorescence imaging and PA imaging. As expected, PA imaging with SPN1 exhibited much better spatial resolution and deeper penetration than fluorescence in both tissue-mimicking phantoms (Figure 4a–d) and mouse leg embedment imaging (Figure 4e, f), proving the merits of SPNs as PA agents.
Figure 4.
(a) Schematic of the tissue-mimicking phantom containing hemoglobin and intralipid implanted with two polyethylene tubes filled with SPN1 solution (Tube 1: [SPN]= 40 μg mL−1, Tube 2: [SPN]= 20 μg mL−1). (b) PA/Ultrasound co-registered 3D image of the tissue-mimicking phantom. (c) Fluorescence/bright-field overlaid top-view image of the tissue-mimicking phantom. (d) (d) 2D PA top-view image of the tissue-mimicking phantom. (e) Representative fluorescence/bright-field overlaid image of a mouse with the SPN1-filled PE tube implanted subcutaneously at the dorsal aspect of the leg (Red dash line indicates the location of the tube). (f) Representative ultrasound (top) and PA/ultrasound (bottom) images of the mouse leg implanted with tubes filled with the nanoparticle solution (left, [SPN]= 20 μg mL−1) or water (right), respectively. White arrows indicate the sidewalls of tubes. Reproduced from Ref [41]. Copyright Nature Publishing Group.
SPNs have several advantages that distinguish them from the most well established PA nanocontrasts in the literature. In the study comparing the photoacoustic profiles of SPNs to two high-performing PA agents, single-walled carbon nanotubes (SWNTs) and gold nanorods (GNRs), Pu et al. showed that the photoacoustic amplitude of SPN1s (molar extinction coefficient: 5.0 × 107 M−1cm−1) was 5.2- and 7.1-times higher than that of SWNTs (molar extinction coefficient: 6.0 × 106 M−1cm−1) and GNRs (molar extinction coefficient: 3.0 × 109 M−1cm−1), respectively, at the same mass concentration44. When administered in vivo, the detection limit of SPN1 was as low as 2 μg ml−1 (3.8 nM), whereas neither SWNT nor GNR was traceable at that concentration. Moreover, SPN1s were able to generate stable PA signals with minimal change in the amplitude after exposure to 2.4 × 104 laser pulses at a fluence of 9 mJ cm−2, while GNRs decreased by 30% under the same condition. Liu et al. also showed that under the same laser fluence of 15 mJ cm−2, no significant change in the absorption spectrum and particle morphology of the PFTTQ NPs was observed, whereas gold nanoparticles rapidly degraded upon continuous laser irradiation49. These results revealed that SPNs could not only generate strong PA signals, but also maintain high performance over long laser exposure, which is critical for photoacoustic imaging agents.
Applications of SPNs-enabled PA imaging
SPNs with high PA capability can be robustly used for cerebral cortex imaging. For example, with both PPy and PFTTQ SPNs, the circulation could be clearly visualized in cortical vessels while the endogenous signals from the vasculature remained fairly low at a laser wavelength over 800 nm46, 49. Nanosized particles usually exhibit preferential accumulation in malignant tumors through the enhanced permeability and retention (EPR) effect. The tumor uptake and in vivo PA imaging capability of SPNs were examined in mouse xenograft models by real-time PA imaging45. After systemic administration of SPN4s, significant higher PA signals were observed in the tumor area compared to surrounding tissues and to the tumor region of the control group. With the high spatial resolution of PA imaging, the PA signals from within and outside the tumor blood vessels can be clearly distinguished, which indicated an effective extravasation of the particles through blood vessels. The enhanced uptake of SPN4 was further confirmed in the ex vivo biodistribution study. The elevated accumulation of SPNs together with efficient blood vessel extravasation suggested that SPNs can be potentially applied for high-resolution PA imaging of tumor.
Lymph node (LN) detection and staging, especially for the tumor draining sentinel lymph node (SLN) are of paramount importance for clinical cancer diagnosis and intervention. SLN-navigated surgery without actual lymph dissection could potentiate minimal invasive surgeries thus significantly enhances the clinical outcome and prognosis. Intriguingly, nanoparticles with a diameter of 20–50 nm tend to accumulate and retain in the lymph node, which can be beneficial for non-invasive nodal tracing50. In light of this, we tested the capacity of SPNs for LN mapping, and found that SPN1s were able to pick up lymph nodes including brachial lymph node, inguinal lymph node and superficial cervical lymph node after administrated intravenously, both by in vivo fluorescence imaging and high-resolution PA44. This study has demonstrated that SPNs could be potentially used as non-invasive lymph node tracers as well.
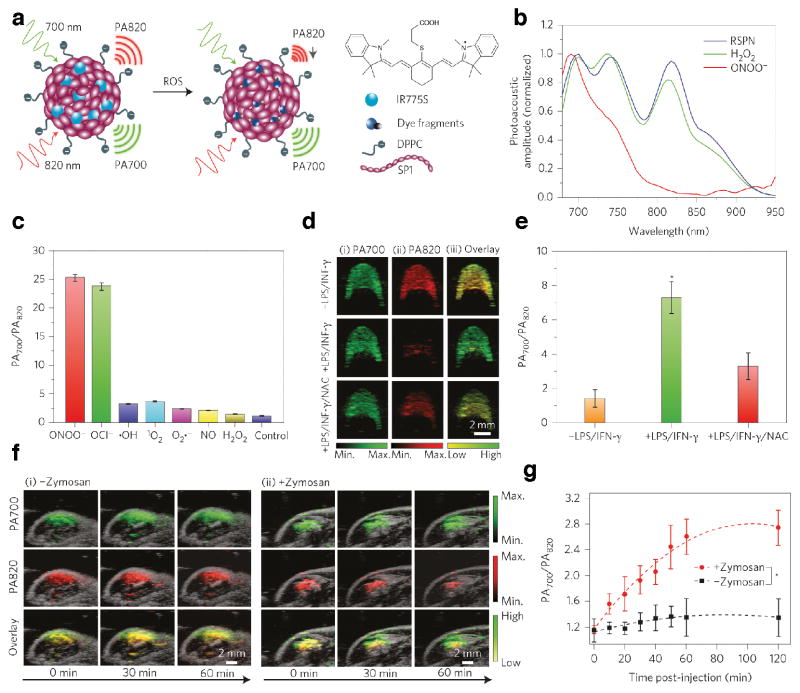
The versatility in synthetic modification of the polymer structures and nano-formulation of SPNs offers the possibility to achieve an application-oriented design of the particle. Specific receptor targeting ability can be incorporated into the nanoparticle by integrating lipids or polymers conjugated with ligands, such as, but not limited to antibody51, affibody52, RGD peptide53, folate54 and biotin55. By facile adjustment of the polymer structure, SPNs can not only be formulated to obtain desired PA spectra, to load dye molecules and cargo drugs, but also modified with various components to build smart materials for sensing applications in vivo. By doping with a reactive oxygen species (ROS)-sensitive cyanine dye derivative (IR775S), Pu et al. developed an SPN-based ratiometric photoacoustic probe (RSPN) for in vivo ROS imaging (Figure 6a)44. The resultant RSPN was able to maintain the stability and size as regular SPN; however, the spectral profiles changed due to the addition of the dye molecule, giving three maxima in the PA spectrum--at 700 nm corresponded to SP and 735 and 820 nm corresponded to IR775S (Figure 6b). IR775S was specifically sensitive to ONOO− and ClO−; In the presence of these two species in solution, the PA amplitude at 735 and 820 nm decreased dramatically due to the ROS-mediated degradation of IR775S. However, no significant spectral change occurred in the presence of other oxidative species such as •NO, •OH, O2• −, 1O2 (Figure 6c). This ROS-specific spectral response enabled a dual-peak ratiometric detection of ROS (PA700/PA820) at a large dynamic range ([ROS] from 50 nM to 5 μM).
Figure 6.
(a) Schematics of RSPN structure and ROS sensoring mechanism. (b) PA spectra of RSPN in the absence and presence of H2O2 and ONOO−. ([RSPN]= 5 μg mL−1, [ROS]= 5 μM). (c) Ratio of PA amplitude at 700 nm to that at 820 nm (PA700/PA820) of RSPN treated with various ROS ([ROS]= 5 μM). (d) Representative PA image of RAW264.7 cell pellets embedded in alga phantom with or without stimulation of LPS/INF-γ, and with NAC protection. (e) Quantification of PA700/PA820 for cell pellets with or without stimulation of LPS/INF-γ, and with NAC protection (n=4, P<0.05). (f) Real-time in vivo PA imaging of mouse acute edema induced by Zymosan after intramuscular injection of RSPN. (g) PA700/PA820 value as a function of time post-injection of RSPN. (Statistically significant difference started from 10 min post-injection, P<0.05). Reproduced from Ref [41]. Copyright Nature Publishing Group.
The sensitivity and specificity of RSPN were first examined in vitro to detect the endogenously generated ROS in macrophage RAW264.7 cells. For cells in the resting state, the cell pellets showed both PA peaks at 700 nm and 820 nm with intensity ratios ~1.4. Upon stimulation with lipopolysaccharide (LPS)/interferon-γ (INF-γ) to mimic in inflammatory condition, the ratio of PA700/PA820 increased by 5-fold. This ROS-induced ratio increase can be effectively inhibited by the addition of N-acetylcysteine (NAC), a free-radical scavenger, indicating the specificity of the ROS sensing (Figure 6d, e).
RSPNs were further applied in vivo for the PA imaging of acute edema. Zymosan, a structural polysaccharide of the cell wall of Saccharomyces cerevisiae, was injected intramuscularly into mouse thigh to set up acute edema models and RSPNs were injected to the same location for the inflammatory ROS imaging during edema. Real-time PA acquisition at 700 nm and 820 nm clearly depicted the dynamics of particle as well as the ROS detection (Figure 6f): the diffusion of the particles around the area was seen by the gradual increase of PA700 and the ROS generated during the edema was presented by the continuous increase in the value of PA700/PA820 (Figure 6g). This result highlights the potential of SPNs as activatable PA sensors for reactive species generated during pathological processes, thus offers insights on the aetiology of the disease and contributes in the process of clinical translation of photoacoustic imaging modality.
Biocompatibility
Toxicity is one of the general concerns that stem the translation of nanomedicine into clinical practice56. Consisting of all-organic components, SPNs are considered highly biocompatible, comparing to most metallic PA nanoagents, and will not cause significant decrease in the cell viability after incubation. However, very few toxicity studies have been performed to systematically evaluate the potential toxic effect of SPNs after entering the body. We have conducted acute toxicity study SPNs, and firstly evaluated the systematic toxicity of SPNs following intravenous injection into mice57. Blood chemistry, liver and kidney function was assessed by analysis of sampled blood at Day 1, 4 and 14 after intravenous injection of SPNs. Comparing to the control animals injected with saline, mice undertook single administration of 0.8 mg of SPNs appeared to be well tolerated of the dose. No unusual behaviors or differences, including vocalizations, labored breathing, difficulties in moving, hunching or unusual interactions with cage mates, was observed before and after injection between each group. For the blood test results, temporary lowering of the mean corpuscular volume (MCV) was observed at early stage, but the corpuscular hemoglobin (MCH) values did not change significantly over time, indicating that SPN did not cause significant impacts on hematopoiesis. White blood cell (WBC) differential counts showed a fluctuation at day 1, suggesting there was a transient inflammatory response to the presence of foreign materials, but the counts returned to normal level at Day 4 and WBC maintained normal after that in the study. Overall SPNs did not show acute or chronic toxicity effect up to 14 days after intravenous administration at a most widely used preclinical dose43. However, thorough studies on the immuno-response and long-term toxicological effect of SPNs must be carried out before clinical translation of these nanoparticles.
Beyond PA imaging
As a versatile nanoplatform, SPNs can be modified for other imaging modalities in addition to PA imaging. By integrating bioluminescence resonance energy transfer (BRET) and fluorescence resonance energy transfer (FRET), we have developed a self-luminescent NIR imaging system that can effectively by-pass tissue autofluorescence and provide high tumor-to-background contrast imaging42. Chemiluminescence resonance energy transfer (CRET) can be similarly incorporated into SPNs for real-time imaging of oxidative and nitrosative stress in liver induced by drug toxicity, which can provide critical parameters for drug safety evaluation43. Further extending its application in molecular imaging, SPNs can readily combine multiple imaging modalities either by loading contrast agents or inserting functional groups through structure modification to provide complementary information for disease diagnosis and staging. For example, iodinated contrast agents were loaded to provide CT imaging capacity58, 59 and metal chelators were introduced into the polymer structures to provide PET or SPECT imaging ability60, 61. By formulating with gadolinium-containing phospholipids, Hashim et al. synthesized a colloidally stable gadolinium-containing conjugated polymer nanoparticle with fluorescence/MRI bimodal imaging capacity62. Moreover, considerable efforts have been put into the cargo loading of SPNs. As stable carriers, SPNs were found to deliver and release hydrophobic drugs and genes at the diseased area under the guidance of fluorescence63–65.
Conclusions
Although introduced only recently for PA imaging, SPNs offer a number of advantages over many traditional contrast agents. SPNs can be robustly formulated and reproduced through an easy nanoprecipitation method. The spectral property of SPNs is solely dependent on the constitutive SPs but not on particle dimensions, suggesting that SPNs with different optical properties can be produced while maintaining similar pharmacokinetics and that SPNs could be ameliorated for better in vivo distribution and clearance profile without compromising their photoacoustic features. SPNs are highly photostable and have been shown to outperform SWNTs and GNRs, two most widely applied nanoparticles, on a per mass basis. Importantly, they can be transformed into SPN-based sensors by complexing with sensing molecules such as reactive species-responsive dye derivatives to actively depict the molecular target, as exampled by RSPN43. Comparing to fluorescence imaging66, 67, SPN-enabled PA imaging has the advantages of high spatial resolution and deep tissue penetration, which is preferable for in vivo imaging.
It has always been attempting to abrogate the tradeoff between fine resolution and image depth for optical imaging. The conversion from photons to ultrasonic waves in PA imaging has led to a major breakthrough in the optical diffusion limit. In conjunction with elevating laser energy and further improvement of light penetration, contrast agents responsive to irradiation of light at longer wavelengths may further advance deep-penetrating PA. It has been demonstrated that an imaging depth of up to 3 mm can be achieved using fluorophores in NIR II region (1.0–1.7 μm) with micro-scale resolution for epifluorescence imaging, which is unattainable with traditional NIR I fluorophores (depth of ~ 0.2 mm)68, 69. Conjugated polymers have exhibited great potential as NIR II optical agents due to the tunable low bandgap and versatility in chemical modification. By exquisitely adjusting the bandgap, many conjugated polymers with absorption over 1000 nm (Eg < 1.15 eV) have been synthesized and applied in the optoelectronic field70–75. And donor/acceptor conjugated polymeric nanoparticles have shown tunable emission wavelength (1050–1350 nm) in the NIR II region and been applied to high time- and space-resolution fluorescence imaging76. It is foreseeable that SPNs can be engineered and applied to PA imaging at the NIR II wavelength, providing for further improved imaging depth and resolution for deep inner organ registrations.
The advancement in polymer chemistry, particle formulation, and better understanding in their pharmacokinetics, will further motivate the integration of the SPN platform with the biosensor development, the combination of PA imaging with other modalities to obtain complementary biological information, the utilization of the SPNs as a carrier to enable real-time imaging of drug delivery, and even the clinical translation for disease imaging and treatment. Progress made on these fronts, together with continuous advances in the photoacoustic imaging instrumentation, will enable SPNs for a broader regimen of biological and biomedical applications.
Figure 5.
In vivo photoacoustic imaging of tumor and LN. Representative in vivo PA imaging of mouse subcutaneous tumors pre-injection and at 2 h after intravenous injection of SPN4, shown as maximum intensity projection image (a) and 3D reconstructed image (b). (c) Representative ultrasound (top) and PA/ultrasound co-registered (bottom) images of the mouse lymph nodes at 24 h after intravenous injection of SPN1 (50 μg). Reproduced from Ref [39] and [41]. Copyright John Wiley & Sons, Inc., Nature Publishing Group.
References
- 1.Kim C, Favazza C, Wang LV. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem Rev. 2010;110:2756–2782. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen K, van Soest G, van der Steen AF. Intravascular photoacoustic imaging: a new tool for vulnerable plaque identification. Ultrasound Med Biol. 2014;40:1037–1048. doi: 10.1016/j.ultrasmedbio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Kruizinga P, van der Steen AF, de Jong N, Springeling G, Robertus JL, van der Lugt A, van Soest G. Photoacoustic imaging of carotid artery atherosclerosis. J Biomed Opt. 2014;19:110504. doi: 10.1117/1.JBO.19.11.110504. [DOI] [PubMed] [Google Scholar]
- 5.Wu M, Jansen K, van der Steen AF, van Soest G. Specific imaging of atherosclerotic plaque lipids with two-wavelength intravascular photoacoustics. Biomed Opt Express. 2015;6:3276–3286. doi: 10.1364/BOE.6.003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taruttis A, van Dam GM, Ntziachristos V. Mesoscopic and macroscopic optoacoustic imaging of cancer. Cancer Res. 2015;75:1548–1559. doi: 10.1158/0008-5472.CAN-14-2522. [DOI] [PubMed] [Google Scholar]
- 7.Wang LV, Gao L. Photoacoustic microscopy and computed tomography: from bench to bedside. Annu Rev Biomed Eng. 2014;16:155–185. doi: 10.1146/annurev-bioeng-071813-104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat Biotechnol. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 9.Matthews TP, Zhang C, Yao DK, Maslov K, Wang LV. Label-free photoacoustic microscopy of peripheral nerves. J Biomed Opt. 2014;19:16004. doi: 10.1117/1.JBO.19.1.016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang L, Ji L, Zhang T, Wang B, Yang J, Zhang Q, Jiang MS, Zhou J, Carney PR, Jiang H. Noninvasive real time tomographic imaging of epileptic foci and networks. Neuroimage. 2013;66:240–248. doi: 10.1016/j.neuroimage.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, Shi S, Barnhart TE, Alexandridis P, Huizinga JD, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol. 2014;9:631–638. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JM, Favazza C, Chen R, Yao J, Cai X, Maslov K, Zhou Q, Shung KK, Wang LV. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat Med. 2012;18:1297–1302. doi: 10.1038/nm.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Li C, Wang LV. Photoacoustic tomography of water in phantoms and tissue. J Biomed Opt. 2010;15:036019. doi: 10.1117/1.3443793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HF, Maslov K, Stoica G, Wang LV. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat Biotechnol. 2006;24:848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 15.Yao DK, Maslov K, Shung KK, Zhou Q, Wang LV. In vivo label-free photoacoustic microscopy of cell nuclei by excitation of DNA and RNA. Opt Lett. 2010;35:4139–4141. doi: 10.1364/OL.35.004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao J, Maslov KI, Zhang Y, Xia Y, Wang LV. Label-free oxygen-metabolic photoacoustic microscopy in vivo. J Biomed Opt. 2011;16:076003. doi: 10.1117/1.3594786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao J, Maslov K, Shi Y, Taber L, Wang LV. In vivo photoacoustic imaging of transverse blood flow by using Doppler broadening of bandwidth. Opt Lett. 2010;35:1419–1221. doi: 10.1364/OL.35.001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumholz A, Shcherbakova DM, Xia J, Wang LV, Verkhusha VV. Multicontrast photoacoustic in vivo imaging using near-infrared fluorescent proteins. Sci Rep. 2014;4:3939. doi: 10.1038/srep03939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filonov GS, Krumholz A, Xia J, Yao J, Wang LV, Verkhusha VV. Deep-tissue photoacoustic tomography of a genetically encoded near-infrared fluorescent probe. Angew Chem Int Ed Engl. 2012;51:1448–1451. doi: 10.1002/anie.201107026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Zemp RJ, Lungu G, Stoica G, Wang LV. Photoacoustic imaging of lacZ gene expression in vivo. J Biomed Opt. 2007;12:020504. doi: 10.1117/1.2717531. [DOI] [PubMed] [Google Scholar]
- 21.Qin C, Cheng K, Chen K, Hu X, Liu Y, Lan X, Zhang Y, Liu H, Xu Y, Bu L. Tyrosinase as a multifunctional reporter gene for Photoacoustic/MRI/PET triple modality molecular imaging. Scientific reports. 2013:3. doi: 10.1038/srep01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jathoul AP, Laufer J, Ogunlade O, Treeby B, Cox B, Zhang E, Johnson P, Pizzey AR, Philip B, Marafioti T, et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat Photon. 2015 advance online publication. [Google Scholar]
- 23.Jin Y, Jia C, Huang S-W, O'Donnell M, Gao X. Multifunctional nanoparticles as coupled contrast agents. Nat Commun. 2010;1:41. doi: 10.1038/ncomms1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanzha EI, Shashkov EV, Kelly T, Kim J-W, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi L, Grobmyer SR, Zhou G, Qian W, Yang L, Jiang H. Molecular photoacoustic tomography of breast cancer using receptor targeted magnetic iron oxide nanoparticles as contrast agents. J Biophotonics. 2014;7:401–409. doi: 10.1002/jbio.201200155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong H, Peng R, Liu Z. Carbon nanotubes for biomedical imaging: the recent advances. Adv Drug Deliv Rev. 2013;65:1951–1963. doi: 10.1016/j.addr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Pan D, Kim B, Wang LV, Lanza GM. A brief account of nanoparticle contrast agents for photoacoustic imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:517–543. doi: 10.1002/wnan.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng KK, Zheng G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem Rev. 2015;115:11012–11042. doi: 10.1021/acs.chemrev.5b00140. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Chiu DT. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew Chem Int Ed Engl. 2013;52:3086–3109. doi: 10.1002/anie.201205133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu G, Gao J, Hummelen JC, Wudl F, Heeger AJ. Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science-AAAS-Weekly Paper Edition. 1995;270:1789–1790. [Google Scholar]
- 32.Günes S, Neugebauer H, Sariciftci NS. Conjugated polymer-based organic solar cells. Chemical reviews. 2007;107:1324–1338. doi: 10.1021/cr050149z. [DOI] [PubMed] [Google Scholar]
- 33.Burroughes JH, Jones CA, Friend RH. New semiconductor device physics in polymer diodes and transistors. Nature. 1988;335:137–141. [Google Scholar]
- 34.Yang Y, Heeger AJ. A new architecture for polymer transistors. Nature. 1994;372:344–346. [Google Scholar]
- 35.Yan H, Chen Z, Zheng Y, Newman C, Quinn JR, Dötz F, Kastler M, Facchetti A. A high-mobility electron-transporting polymer for printed transistors. Nature. 2009;457:679–686. doi: 10.1038/nature07727. [DOI] [PubMed] [Google Scholar]
- 36.Zhu C, Liu L, Yang Q, Lv F, Wang S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem Rev. 2012;112:4687–4735. doi: 10.1021/cr200263w. [DOI] [PubMed] [Google Scholar]
- 37.Howes P, Green M, Levitt J, Suhling K, Hughes M. Phospholipid encapsulated semiconducting polymer nanoparticles: their use in cell imaging and protein attachment. J Am Chem Soc. 2010;132:3989–3996. doi: 10.1021/ja1002179. [DOI] [PubMed] [Google Scholar]
- 38.Ye F, Wu C, Jin Y, Chan YH, Zhang X, Chiu DT. Ratiometric temperature sensing with semiconducting polymer dots. J Am Chem Soc. 2011;133:8146–8149. doi: 10.1021/ja202945g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu IC, Yu J, Ye F, Rong Y, Gallina ME, Fujimoto BS, Zhang Y, Chan YH, Sun W, Zhou XH, et al. Squaraine-based polymer dots with narrow, bright near-infrared fluorescence for biological applications. J Am Chem Soc. 2015;137:173–178. doi: 10.1021/ja5123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu C, Hansen SJ, Hou Q, Yu J, Zeigler M, Jin Y, Burnham DR, McNeill JD, Olson JM, Chiu DT. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew Chem Int Ed Engl. 2011;50:3430–3434. doi: 10.1002/anie.201007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pu K, Shuhendler AJ, Rao J. Semiconducting polymer nanoprobe for in vivo imaging of reactive oxygen and nitrogen species. Angew Chem Int Ed Engl. 2013;52:10325–10329. doi: 10.1002/anie.201303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong L, Shuhendler AJ, Rao J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shuhendler AJ, Pu K, Cui L, Uetrecht JP, Rao J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol. 2014;32:373–380. doi: 10.1038/nbt.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z, Rao J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat Nanotechnol. 2014;9:233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pu K, Mei J, Jokerst JV, Hong G, Antaris AL, Chattopadhyay N, Shuhendler AJ, Kurosawa T, Zhou Y, Gambhir SS, et al. Diketopyrrolopyrrole-Based Semiconducting Polymer Nanoparticles for In Vivo Photoacoustic Imaging. Adv Mater. 2015;27:5184–5190. doi: 10.1002/adma.201502285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zha Z, Deng Z, Li Y, Li C, Wang J, Wang S, Qu E, Dai Z. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale. 2013;5:4462–4467. doi: 10.1039/c3nr00627a. [DOI] [PubMed] [Google Scholar]
- 47.Jin Y, Li Y, Ma X, Zha Z, Shi L, Tian J, Dai Z. Encapsulating tantalum oxide into polypyrrole nanoparticles for X-ray CT/photoacoustic bimodal imaging-guided photothermal ablation of cancer. Biomaterials. 2014;35:5795–5804. doi: 10.1016/j.biomaterials.2014.03.086. [DOI] [PubMed] [Google Scholar]
- 48.Mühlbacher D, Scharber M, Morana M, Zhu Z, Waller D, Gaudiana R, Brabec C. High photovoltaic performance of a low-bandgap polymer. Adv Mater. 2006;18:2884–2889. [Google Scholar]
- 49.Liu J, Geng J, Liao L-D, Thakor N, Gao X, BL Conjugated polymer nanoparticles for photoacoustic vascular imaging. Polym Chem. 2014;5:2854–2862. [Google Scholar]
- 50.Nakajima M, Takeda M, Kobayashi M, Suzuki S, Ohuchi N. Nano-sized fluorescent particles as new tracers for sentinel node detection: Experimental model for decision of appropriate size and wavelength. Cancer science. 2005;96:353–356. doi: 10.1111/j.1349-7006.2005.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li K, Liu Y, Pu K-Y, Feng S-S, Zhan R, Liu B. Polyhedral Oligomeric Silsesquioxanes-Containing Conjugated Polymer Loaded PLGA Nanoparticles with Trastuzumab (Herceptin) Functionalization for HER2-Positive Cancer Cell Detection. Adv Funct Mater. 2011;21:287–294. [Google Scholar]
- 52.Liu J, Feng G, Ding D, Liu B. Bright far-red/near-infrared fluorescent conjugated polymer nanoparticles for targeted imaging of HER2-positive cancer cells. Polym Chem. 2013;4:4326–4334. [Google Scholar]
- 53.Li K, Zhan R, Feng SS, Liu B. Conjugated polymer loaded nanospheres with surface functionalization for simultaneous discrimination of different live cancer cells under single wavelength excitation. Anal Chem. 2011;83:2125–2132. doi: 10.1021/ac102949u. [DOI] [PubMed] [Google Scholar]
- 54.Ding D, Liu J, Feng G, Li K, Hu Y, Liu B. Bright far-red/near-infrared conjugated polymer nanoparticles for in vivo bioimaging. Small. 2013;9:3093–3102. doi: 10.1002/smll.201300171. [DOI] [PubMed] [Google Scholar]
- 55.Kandel PK, Fernando LP, Ackroyd PC, Christensen KA. Incorporating functionalized polyethylene glycol lipids into reprecipitated conjugated polymer nanoparticles for bioconjugation and targeted labeling of cells. Nanoscale. 2011;3:1037–1045. doi: 10.1039/c0nr00746c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, Meade T, Pomper M, Ptak K, Rao J, et al. Nanoparticles for cancer imaging: The good, the bad, and the promise. Nano Today. 2013;8:454–460. doi: 10.1016/j.nantod.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pu K, Chattopadhyay N, Rao J. Recent advances of semiconducting polymer nanoparticles in in vivo molecular imaging. J Control Release. 2016 doi: 10.1016/j.jconrel.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Vries A, Custers E, Lub J, van den Bosch S, Nicolay K, Grull H. Block-copolymer-stabilized iodinated emulsions for use as CT contrast agents. Biomaterials. 2010;31:6537–6544. doi: 10.1016/j.biomaterials.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 59.Yin Q, Yap FY, Yin L, Ma L, Zhou Q, Dobrucki LW, Fan TM, Gaba RC, Cheng J. Poly(iohexol) Nanoparticles As Contrast Agents for in Vivo X-ray Computed Tomography Imaging. J Am Chem Soc. 2013;135:13620–13623. doi: 10.1021/ja405196f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukukawa K, Rossin R, Hagooly A, Pressly ED, Hunt JN, Messmore BW, Wooley KL, Welch MJ, Hawker CJ. Synthesis and characterization of core-shell star copolymers for in vivo PET imaging applications. Biomacromolecules. 2008;9:1329–1339. doi: 10.1021/bm7014152. [DOI] [PubMed] [Google Scholar]
- 61.Pressly ED, Pierce RA, Connal LA, Hawker CJ, Liu Y. Nanoparticle PET/CT imaging of natriuretic peptide clearance receptor in prostate cancer. Bioconjug Chem. 2013;24:196–204. doi: 10.1021/bc300473x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashim Z, Green M, Chung PH, Suhling K, Protti A, Phinikaridou A, Botnar R, Khanbeigi RA, Thanou M, Dailey LA, et al. Gd-containing conjugated polymer nanoparticles: bimodal nanoparticles for fluorescence and MRI imaging. Nanoscale. 2014;6:8376–8386. doi: 10.1039/c4nr01491j. [DOI] [PubMed] [Google Scholar]
- 63.Ding D, Li K, Zhu Z, Pu K-Y, Hu Y, Jiang X, Liu B. Conjugated polyelectrolyte–cisplatin complex nanoparticles for simultaneous in vivo imaging and drug tracking. Nanoscale. 2011;3:1997–2002. doi: 10.1039/c0nr00950d. [DOI] [PubMed] [Google Scholar]
- 64.Feng X, Tang Y, Duan X, Liu L, Wang S. Lipid-modified conjugated polymer nanoparticles for cell imaging and transfection. J Mater Chem. 2010;20:1312–1316. [Google Scholar]
- 65.Feng X, Lv F, Liu L, Tang H, Xing C, Yang Q, Wang S. Conjugated polymer nanoparticles for drug delivery and imaging. ACS applied materials & interfaces. 2010;2:2429–2435. doi: 10.1021/am100435k. [DOI] [PubMed] [Google Scholar]
- 66.Guo H, Aleyasin H, Dickinson BC, Haskew-Layton RE, Ratan RR. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014;4:64. doi: 10.1186/2045-3701-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barzegar Amiri Olia M, Schiesser CH, Taylor MK. New reagents for detecting free radicals and oxidative stress. Org Biomol Chem. 2014;12:6757–6766. doi: 10.1039/c4ob01172d. [DOI] [PubMed] [Google Scholar]
- 68.Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, Cooke JP, Dai H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat Med. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, et al. A small-molecule dye for NIR-II imaging. Nat Mater. 2016;15:235–242. doi: 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- 70.Gong X, Tong M, Xia Y, Cai W, Moon JS, Cao Y, Yu G, Shieh CL, Nilsson B, Heeger AJ. High-detectivity polymer photodetectors with spectral response from 300 nm to 1450 nm. Science. 2009;325:1665–1667. doi: 10.1126/science.1176706. [DOI] [PubMed] [Google Scholar]
- 71.Sonar P, Singh SP, Li Y, Soh MS, Dodabalapur A. A low-bandgap diketopyrrolopyrrole-benzothiadiazole-based copolymer for high-mobility ambipolar organic thin-film transistors. Adv Mater. 2010;22:5409–5413. doi: 10.1002/adma.201002973. [DOI] [PubMed] [Google Scholar]
- 72.Chen E-C, Tseng S-R, Chao Y-C, Meng H-F, Wang C-F, Chen W-C, Hsu C-S, Horng S-F. Polymer infrared photo-detector with high sensitivity up to 1100nm. Synthetic Metals. 2011;161:1618–1622. [Google Scholar]
- 73.Fan J, Yuen JD, Wang M, Seifter J, Seo JH, Mohebbi AR, Zakhidov D, Heeger A, Wudl F. High-performance ambipolar transistors and inverters from an ultralow bandgap polymer. Adv Mater. 2012;24:2186–2190. doi: 10.1002/adma.201103836. [DOI] [PubMed] [Google Scholar]
- 74.Hu X, Dong Y, Huang F, Gong X, Cao Y. Solution-processed high-detectivity near-infrared polymer photodetectors fabricated by a novel low-bandgap semiconducting polymer. J Phys Chem C. 2013;117:6537–6543. [Google Scholar]
- 75.Steckler TT, Henriksson P, Mollinger S, Lundin A, Salleo A, Andersson MR. Very low band gap thiadiazoloquinoxaline donor-acceptor polymers as multi-tool conjugated polymers. J Am Chem Soc. 2014;136:1190–1193. doi: 10.1021/ja410527n. [DOI] [PubMed] [Google Scholar]
- 76.Hong G, Zou Y, Antaris AL, Diao S, Wu D, Cheng K, Zhang X, Chen C, Liu B, He Y, et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat Commun. 2014;5:4206. doi: 10.1038/ncomms5206. [DOI] [PubMed] [Google Scholar]