Abstract
Pfs48/45 and Pfs25 are leading candidates for the development of Plasmodium falciparum transmission blocking vaccines (TBV). Expression of Pfs48/45 in the erythrocytic sexual stages and presentation to the immune system during infection in the human host also makes it ideal for natural boosting. However, it has been challenging to produce a fully folded, functionally active Pfs48/45, using various protein expression platforms. In this study, we demonstrate that full-length Pfs48/45 encoded by DNA plasmids is able to induce significant transmission reducing immune responses. DNA plasmids encoding Pfs48/45 based on native (WT), codon optimized (SYN), or codon optimized and mutated (MUT1 and MUT2), to prevent any asparagine (N)-linked glycosylation were compared with or without intramuscular electroporation (EP). EP significantly enhanced antibody titers and transmission blocking activity elicited by immunization with SYN Pfs48/45 DNA vaccine. Mosquito membrane feeding assays also revealed improved functional immunogenicity of SYN Pfs48/45 (N-glycosylation sites intact) as compared to MUT1 or MUT2 Pfs48/45 DNA plasmids (all N-glycosylation sites mutated). Boosting with recombinant Pfs48/45 protein after immunization with each of the different DNA vaccines resulted in significant boosting of antibody response and improved transmission reducing capabilities of all four DNA vaccines. Finally, immunization with a combination of DNA plasmids (SYN Pfs48/45 and SYN Pfs25) also provides support for the possibility of combining antigens targeting different life cycle stages in the parasite during transmission through mosquitoes.
Keywords: Malaria, DNA vaccine, Glycosylation, Combination antigens, Electroporation
1. Introduction
The death toll due to malaria was estimated at 438,000 in 2015, 90% of which occurred in African children under the age of 5 years (WHO World Malaria Report 2015). While global mortality rates are on a steady decline, it is imperative that additional intervention tools be developed and deployed to maintain the current progress and momentum. An important intervention that is widely acknowledged to play a critical role in reducing transmission i.e. malaria TBVs, has yet to become available for human use [1]. The only malaria vaccine, RTS, S based on the Plasmodium falciparum circumsporozoite protein advanced to phase III clinical trials [2], has revealed only 30–50% efficacy and it is unlikely to be effective in interrupting malaria transmission.
A central goal of vaccines aimed at reducing transmission is to target parasite stages in the mosquito vector in order to prevent transmission of sporozoites to human hosts during a mosquito blood meal [3]. Key TBV candidates in P. falciparum include gametocyte and gamete surface antigens such as Pfs48/45 and Pfs230 as well as zygote/ookinete surface antigens Pfs25 and Pfs28 [4]. Pfs48/45 belongs to a family of proteins characterized by six conserved cysteines (6-Cys family proteins) [5–7] and studies have firmly established it to be a target of transmission blocking antibodies [5, 8, 9]. Pfs48/45 plays an essential role in male gamete fertility [10, 11] and it is postulated to function alongside Pfs47 (a female-specific paralog of Pfs48/45) and Pfs230 in contributing to recognition and fertilization of male and female gametes [12], Pfs48/45 specific antibodies have been shown to reduce transmission by inhibiting gamete fertilization [13, 14]. Because of their biosynthetic origin in the circulating intraerythrocytic gametocytes [15, 16], Pfs48/45 and Pfs230 are able to induce antibody responses in naturally exposed individuals [17] allowing for the possibility of natural boosting of immunity [18].
TBV development based on Pfs48/45 has met with limited success due to its large size and difficulties in expressing properly folded protein retaining functional epitopes formed from correct pairing of 16 cysteine residues. Consequently, early attempts at protein expression in bacteria, vaccinia virus and yeast [19–21] met with limited success due to improper protein folding. In the last 5 years some progress has been made using E. coli expression of partial protein (containing 10 of the 16 C-terminal cysteine residues) that is able to induce blocking antibodies [22]. Likewise, full length Pfs48/45 expressed in E. coli after codon harmonized was found to be effective in inducing transmission-blocking antibodies in mice and olive baboons [23]. Despite these initial successes, reproducibility, stability and conformational integrity of Pfs48/45 continue to thwart vaccine development based on recombinant protein/adjuvant formulations.
The ability of DNA vaccines to induce a broad range of immune responses along with ease of manufacturing, stability, storage and transport make them ideal for diseases such as malaria [24]. With respect to malaria TBV, considerable progress has been made with Pfs25 based DNA vaccines, evaluated in mice and nonhuman primates [25–30]. While the initial studies reported suboptimal immunogenicity of Pfs25 DNA vaccine in various animals [26], significant advances were made with the use of in vivo electroporation (EP) delivery [28, 29]. EP delivery involves applying brief electrical pulses at the site of injection (typically muscle or skin) [31]. Heterologous prime-boost strategy, i.e. priming with DNA vaccine followed by boosting using either recombinant protein or a different expression vector [32], have also been shown to improve immunogenic outcomes of DNA vaccines. Additional efforts to optimize immunogenicity has focused on codon optimization of the coding sequence to improve expression in mammalian cells after immunization [33], and assessing the impact of protein glycosylation [34]. The latter has the potential to be critical especially since Plasmodium proteins do not undergo modification by N-linked glycosylation [35–37], and proteins encoded by DNA plasmids are expected to undergo glycosylation when expressed in mammalian cells. A few studies have examined the impact of N-linked glycosylation on the immunogenicity of P. falciparum asexual stage antigens MSP-1 and AMA-1 [38–40]. A recent study has also looked at the role of N-linked glycosylation in Pfs48/45 expressed in plants [41], suggesting a preference for the absence of N-glycosylation for vaccine development.
The goal of the present study was to systematically examine Pfs48/45 using a DNA vaccine platform for the first time. In order to develop a viable vaccine candidate and advance it for future pre-clinical studies in nonhuman primates and eventual clinical trials, we also assessed the impact of codon optimization, EP, mutations affecting N-linked glycosylation, and a heterologous prime-boost delivery on various immunogenicity parameters. In addition to evaluation of DNA vaccines encoding Pfs48/45, we also evaluated a combination DNA vaccines based on codon optimized Pfs25 and Pfs48/45 with the intention of eliciting antibodies targeting different stages of development of parasites in the mosquitoes.
2. Material and Methods
2.1. DNA plasmids
DNA vector VR1020 (Vical Inc. San Diego, CA) was used to prepare four different plasmid constructs, each encoding Pfs48/45 (minus N-terminal signal and C-terminal anchor sequences). The first DNA plasmid contained the native coding sequence of Pfs48/45 (WT), the second contained a codon optimized version of Pfs48/45 for enhanced expression in mammalian cells (SYN Pfs48/45), and the third and fourth plasmids contained SYN sequences in which all 7 codons for putative glycosylation sites were altered to prevent N-linked glycosylation. The third and fourth plasmids differed in the mutational approaches utilized to alter the putative glycosylation sties. In the case of third plasmid construct (MUT1 Pfs48/45), sequence modifications included N50→D, N131→D, T192→A, N204→T, N254→K, S301→A, N303→D in all NXS/T sites. The fourth plasmid was constructed by changing all S/T of the NXS/T site to A (MUT2 Pfs48/45). Plasmid DNA (<30 EU/mg) was produced and supplied at 2.5 mg/mL (Aldevron, Fargo, ND). For combination immunization, VR1020 encoding SYN Pfs25 (minus N-terminal signal and C-terminal anchor sequences and codon optimized for optimal expression in mammalian cells) was employed [30]. Combination immunization were conducted by mixing SYN Pfs48/45 and SYN Pfs25 DNA together as described below.
2.2. Mammalian cell transfection
DNA plasmids (WT, SYN, MUT1 and MUT2 Pfs48/45) were used to transfect mammalian HEK293T cells using Megatran 1.0 (OriGene Technologies, Rockville MD) transfection reagent as per the product protocol. Cultured cells (24 well) were transfected with 2.5 ug DNA and the culture medium was changed three hours post transfection. Cells were maintained without or with tunicamycin (2.5 ug/mL) (Sigma-Aldrich, St. Louis, MO), an N-glycosylation blocker [42]. After 48 hours of treatment, supernatant (culture media) was harvested and cells were lysed with SDS-PAGE sample buffer for evaluation of protein expression by Western blotting under non-reducing and reducing SDS-PAGE conditions.
2.3. Immunization dose and schedule
Five to seven week old female Balb/c mice (NCI, Bethesda) were used for immunization studies. Each plasmid DNA, administered in 20 ul PBS was tested in individual groups of mice (n=5 per group). Group 1 received 50 ug DNA/mouse without electroporation (no EP), group 2 received 50 ug/mouse with EP, group 3 received 25 ug/mouse with EP and group 4 received 5 ug/mouse with EP. Two additional groups were included in the studies, one received SYN Pfs25 DNA at 50 ug/mouse with EP and the other one received a mixture of SYN Pfs48/45 and SYN Pfs25, 50 ug of each plasmid DNA / mouse. For combination immunization, 50ug of SYN Pfs48/45 and 50 ug SYN Pfs25 were resuspended in a total volume of 20ul PBS for each mouse. All groups received 3 intramuscular DNA doses given at 4-week intervals with bleeds collected prior to and one month after each dose. Mice immunized with 25 ug and 5 ug of the 4 Pfs48/45 DNA constructs also received a final boost using 10 ug E. coli produced recombinant Pfs48/45 protein adsorbed with alum [23]. EP was performed using ICHOR pulse generator and TriGrid Electrode Array (Ichor Medical Systems Inc. San Diego, CA). DNA was injected using 0.3 ml U-100 Insulin syringes (BD Biosciences, NJ) and followed approximately 10 seconds later by an electrical pulse at an amplitude of 250 V/cm of electrode spacing (2.5 mm spacing used). Mice were anaesthetized for immunization using Isoflurane and received the immunizations and electroporation in the anterior-tibialis muscle of the right leg.
2.4. Antibody analysis
Sera obtained after three DNA immunization and a final protein boost were analyzed by ELISA to determine (i) percentage of responder mice after primary immunization, (ii) end point titers, (iii) avidity of binding and (iv) isotypes. ELISAs were conducted using 96-well Immulon 4HBX plates coated with rPfs48/45 [23] or rPfs25 [43] expressed in E. coli (100 ul/well of 1.5 ug/mL in carbonate buffer, pH 9.6). In the case of combined immunization with SYN Pfs48/45 and SYN Pfs25, antibody responses to each antigen (rPfs48/45 and rPfs25) were determined individually. To determine avidity of antibodies, plates were incubated for 15 mins with NaSCN (0, 1, 2, 4, 8 M) after primary antibody incubation and washed prior to incubation with secondary antibody and remaining ELISA steps. Binding of antibody to antigen after NaSCN treatment was expressed as a percent of total binding in the absence of NaSCN. For antibody isotype analysis, mouse sera were tested at 1:250 (no EP group) and 1:4000 (EP groups) dilutions using peroxidase-conjugated goat anti-mouse IgM, IgG1, IgG2a, IgG2b and IgG3 (Invitrogen, Carlsbad, CA) as secondary antibodies.
2.5. Membrane feeding assays (MFAs)
Total IgG was purified using protein A-Sepharose beads from pooled sera at terminal bleeds after 3 DNA immunization (50 ug DNA; WT, SYN and MUT1 and MUT2 DNA groups) as described and stored as sterile solutions in PBS [30]. IgG from no-EP immunization groups (WT, SYN, MUT1 and MUT2) were tested at 1 mg/mL and 0.5 mg/mL and from EP immunized groups at 1 mg/mL, 0.5 mg/mL and 0.25 mg/mL. For MFA, mature stage V gametocytes of P. falciparum (NF54) produced in vitro were mixed with IgG (0.25 to 1.0 mg/mL) and human RBCs to a final 50% hematocrit (using aliquots of normal human sera that have not undergone heat inactivation and stored at −20°C) and 0.3% gametocytemia and fed to female An. gambiae (Keele strain) mosquitoes (4–5 day old) using water jacketed glass feeders maintained at 37°C. All MFAs included negative controls of IgG from pre-immune normal mice sera (NMS) and normal human sera (NHS). Percent transmission reduction activity is defined as percent reduction in the number of oocysts and was calculated using the following formula: percentage oocyst reduction = 100 – [(geometric mean number of oocysts with test sera / geometric mean number of oocysts with NMS) × 100].
2.6. Statistical analysis
Antibody end point titer was defined as reciprocal serum dilution with absorbance higher than the average absorbance of pre-immune sera+3 standard deviations (SD). The percent inhibition of oocyst development and mosquito infectivity differences were analyzed as described [30]. Statistical analysis was performed using the GraphPad Prism software package.
3. Results
3.1. Evidence for N-linked glycosylation of Pfs48/45 in mammalian cells
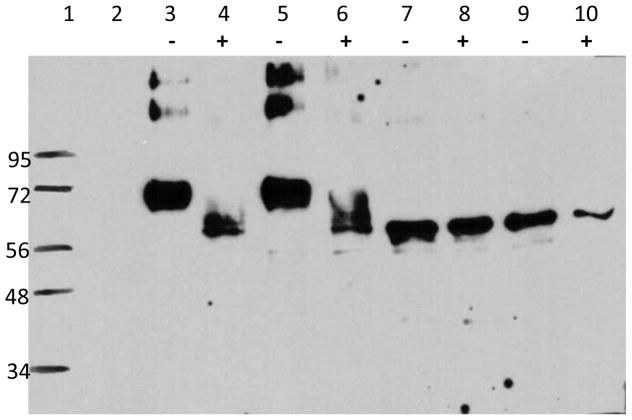
To evaluate protein expression and N-linked glycosylation, mammalian (HEK293T) cells were transfected with WT, SYN, MUT1 and MUT2 Pfs48/45 DNA and cultured in the presence or absence of tunicamycin for 48 hrs. Cell lysates and culture supernatants were analyzed by western blotting using pooled, polyclonal sera from mice immunized with SYN Pfs48/45 DNA vaccines as primary antibody. Pfs48/45 was detected only in the cell lysate, suggesting lack of secretion of the expressed protein (Fig. 1). Moreover, a protein band could only be detected under reducing SDS-PAGE conditions, suggesting that the protein produced in mammalian cells was highly aggregated. In multiple repeat transfection experiments, WT and SYN Pfs48/45 DNA showed higher protein expression on a per cell basis in comparison to MUT1 and MUT2. Transfection experiments were also repeated with different cell lines (HEK293T, CHO, COS and 3T3) in order to circumvent the heavy aggregation seen with HEK293T cells but the yield was much lower. Eventually, reduction with β-mercaptoethanol resulted in clean sharp bands that revealed differences due to glycosylation as seen in Fig. 1. The Western blot images were analyzed using ImageJ software (http://rsbweb.nih.gov/ij/) and the relative proportions of band intensities were 1: 1.17: 0.85: 0.59 for WT, SYN, MUT1 and MUT 2, respectively. Additionally, the size of Pfs48/45 protein expressed by MUT1 and MUT2 DNA (~56kDa) was smaller (by ~20 to 25% as compared to ~72 kDa apparent size) expressed using WT and SYN DNA. We attribute this to the presence of N-linked glycosylation in the latter two groups. Further evidence for N-linked glycosylation was provided by the analysis of cells treated with tunicamycin (TN) revealing a shift in the size of expressed protein from WT and SYN plasmids comparable to that from MUT1 and MUT2 plasmids. As expected, TN treatment had no effect on the observed molecular size of expressed protein from MUT1 and MUT2 plasmids (Fig. 1).
Fig. 1. Evidence for Pfs48/45 N-linked glycosylation.
Mammalian (HEK293T) cells were transfected with Pfs48/45 DNA plasmids and cultured in the presence or absence of tunicamycin for 48 hours. Cell lysates were analyzed by SDS-PAGE under reducing conditions. Lane 1 shows molecular weight standards, lane 2 shows results with un-transfected cells (no DNA). Results with cells transfected with WT (lanes 3, 4), SYN (lanes 5, 6), MUT1 DNA (lanes 7, 8) and MUT2 DNA (lanes 9, 10) are shown in cells cultured in the absence (-) and presence of tunicamycin (+). Equal number of cells lysed in SDS-PAGE sample buffer were loaded in each lane.
3.2. Antibody responses in WT, SYN, MUT1 and MUT2 Pfs48/45 DNA-immunized groups
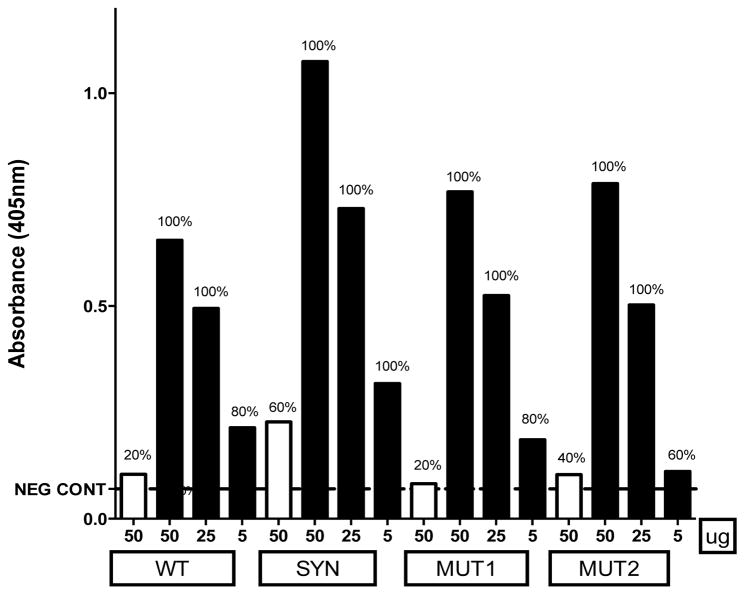
Immune sera were examined after the first DNA immunization to determine primary antibody responses as well as to assess any difference in sero-conversion between EP and no-EP modes of immunization (Fig. 2). When tested at 50 ug and 25 ug dose by EP, all four vaccine constructs revealed 100% of the mice responding by sero-conversion after a single vaccine dose. Even at 5ug with EP, 60–100% mice were responsive, whereas in comparison, immunization with 50 ug in the no-EP group demonstrated only 20–60% sero positivity. Additionally, immune reactivity (as determined by OD reading over negative control + 3SD) was much lower for the no-EP groups and showed a dose-dependent trend for EP groups (Fig. 2).
Fig. 2. Percent mice responding after primary immunization.
Immune sera obtained after primary immunization with indicated Pfs48/45 DNA vaccine plasmids doses (mg) were analyzed by ELISA for anti-rPfs48/45 antibodies at 1:100 dilution. Empty bars indicate no-EP groups; filled bars are EP groups at indicated doses of various Pfs48/45 DNA vaccines. Percent responding mice (indicated as percentages above each bar) were determined based on positive ELISA absorbance reading over pre-immune negative control absorbance + 3SD (indicated by the horizontal line).
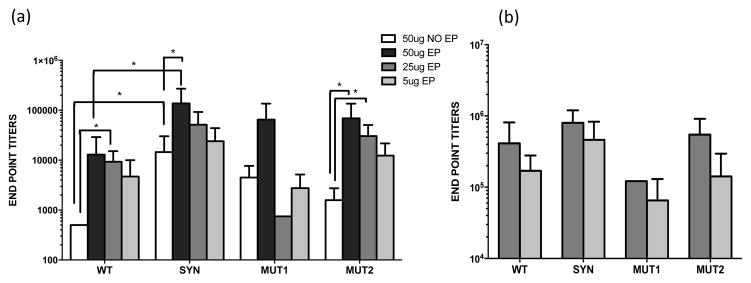
Antibody end-point titers against rPfs48/45, after third DNA immunizations are shown in Fig. 3a. For each of the four Pfs48/45 DNA vaccines (WT, SYN, MUT1 and MUT2) titers for EP groups at 50 ug and 25 ug doses were higher than no-EP at 50 ug dose. Data analysis also revealed that the titers in the 5 ug EP groups, a 10-fold lower DNA dose were either comparable to or higher than 50 ug no-EP for all DNA vaccine groups. Comparing titers between different constructs revealed that SYN DNA at 50 ug, with EP and no-EP, had significantly higher titers than WT DNA. Titers for both MUT1 and MUT2 were lower but not significantly different compared to SYN DNA and matched the levels of WT group. Mice immunized with 25ug and 5ug DNA doses with EP also received a heterologous protein boost with rPfs48/45 in alum [23]. The end point titers after a protein boost increased ~10-fold, however no differences were observed in the titers among the four DNA constructs (Fig. 3b).
Fig. 3. Analysis of anti-Pfs48/45 titers by ELISA in mice (n=5) immunized with different Pfs48/45 DNA vaccines.
(a) shows average end-point titers after 3 DNA immunizations and (b) shows average end-point titers after a r-Pfs48/45 protein boost in the groups that received 25 and 5 ug plasmid DNA doses. End-point titers were defined as reciprocal of serum dilutions giving an absorbance (405nm) higher than that with pre-immune sera + 3SD. Statistically significant differences between groups were determined by student t-test; p < 0.001 – 0.05, indicated by (*). The error bars indicate SD.
3.3. Antibody responses in the Pfs48/45 and Pfs25 co-immunization groups
Fig. 4 shows comparison of antigen-specific antibody end point titers in mice immunized with SYN Pfs48/45 and SYN Pfs25, either alone or with a combination of the two. The Pfs48/45 antibody titers in mice immunized with SYN Pfs48/45 were not significantly different when compared with antibody titers in mice immunized with a combination of SYN Pfs48/45 and SYN Pfs25. Similarly no significant differences in Pfs25-specific antibody end point titers were detected when comparing mice immunized either with SYN Pfs25 or a combination of SYN Pfs25 and SYN Pfs48/45 (Fig. 4).
Fig. 4. Comparison of antibody titers by ELISA in mice (n=5) immunized individually or a combination of SYN Pfs48/45 and SYN Pfs25 DNA vaccines.
White bars show antibody titers against rPfs25 and grey shaded bars show antibody titers against rPfs48/45. The immunization groups are indicated along the X-axis. The end-point titers were defined as reciprocal serum dilutions giving an absorbance (405nm) higher than that with pre-immune sera + 3SD. The error bars indicate SD.
3.4. Antibody avidity
We compared the avidity of antibodies elicited by all four Pfs48/45 DNA vaccine constructs (50 ug by EP) after 3 DNA immunizations using NaSCN dissociation procedure. NaSCN molar concentration inducing 50% dissociation of bound antibody (a measure of antibody avidity) for SYN DNA (1.87 M) was found to be marginally higher than for antibodies elicited in mice immunized with WT, MUT1 and MUT2 (0.87, 1.1, 1.59 M, respectively). Additionally, sera from 5 ug EP groups after the protein boost were also compared, and a similar trend persisted: 2.1 M for SYN DNA as compared to 1.37 M (WT DNA), 1.9 M (MUT1 DNA) and 1.33 M (MUT 2 DNA). However, these differences among all immunization groups, before and after the protein boost, were not statistically significant.
3.5. Antibody isotypes
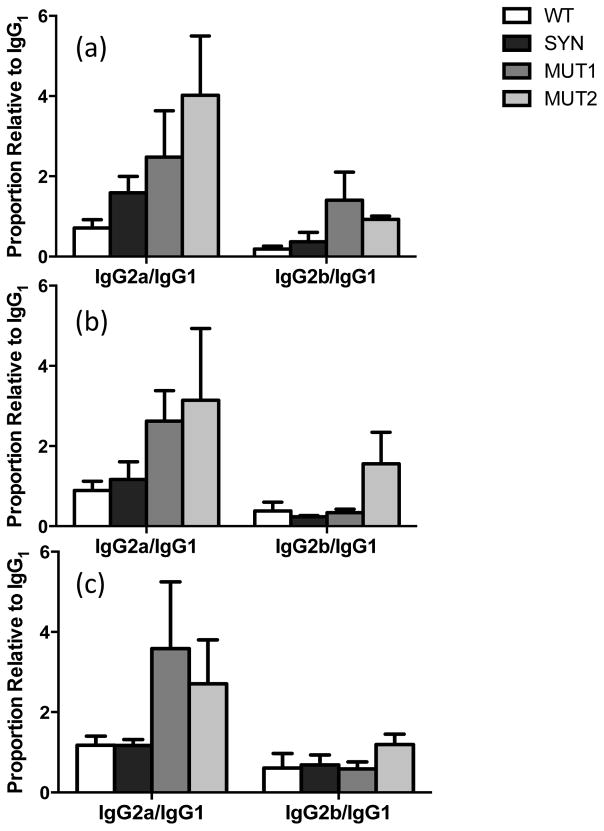
Immunoglobulin isotypes were determined for antibodies elicited by glycosylated Pfs48/45 (encoded by WT and SYN plasmids) and unglycosylated Pfs48/45 (encoded by either N-glycosylation site mutants, MUT1 and MUT2). It was hypothesized that the presence of N-glycan sidechains might result in conformational changes, which in turn might lead to alterations in epitope processing and presentation. Results in Fig. 5 indicate a balanced IgG2a/IgG1 response for WT and SYN DNA groups but a skewing towards elevated IgG2a was observed for both mutant groups. This isotype distribution was consistent for no-EP and EP groups and persisted even after the protein boost.
Fig. 5. Evaluation of IgG isotypes after Pfs48/45 DNA immunization.
Sera from mice immunized three times with WT, SYN, MUT1 and MUT2 Pfs48/45 DNA vaccines were analyzed for IgM, IgG1, IgG2a, IgG2b and IgG3 isotypes of Pfs48/45 reactive antibodies. Results are expressed as a ratio of IgG2a/IgG1 and IgG2b/IgG1. (a), and (b) show results for mice immunized with 50ug DNA without and with EP, respectively. (c) shows antibody isotypes in mice immunized with 5ug DNA (with EP) and boosted with rPfs48/45. The error bars indicate SD.
3.6. Membrane Feeding Assays (MFAs)
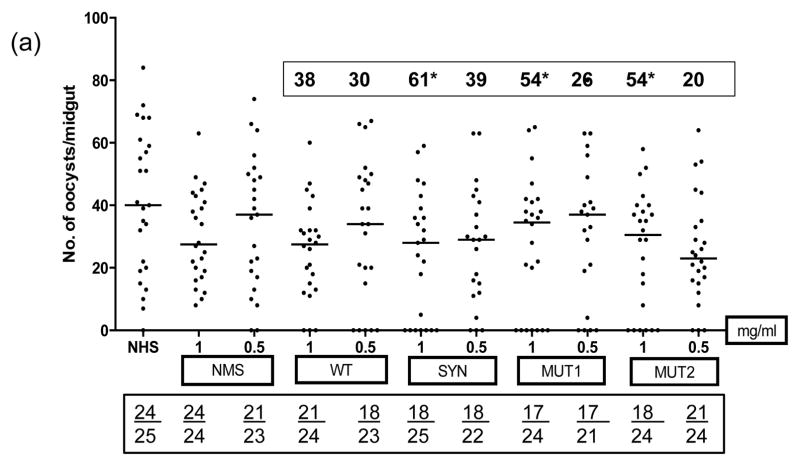
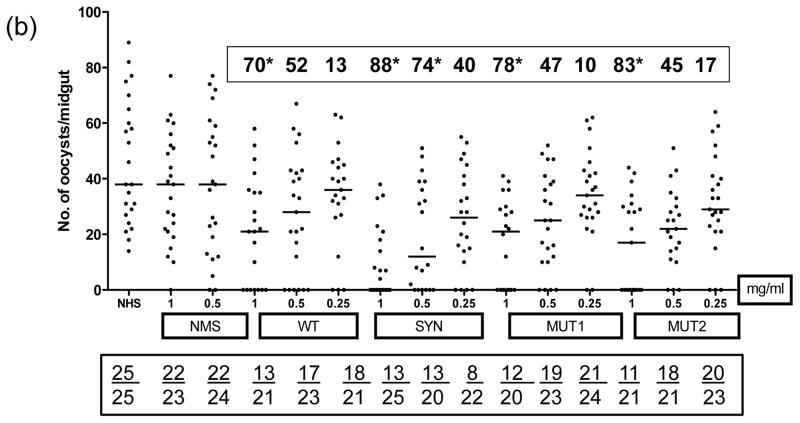
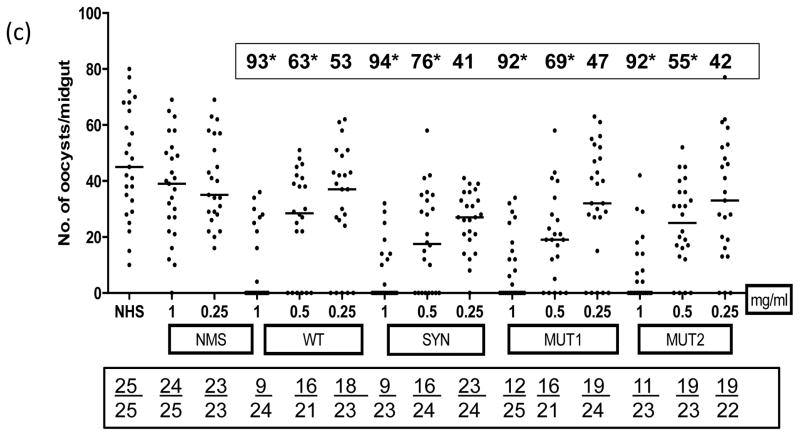
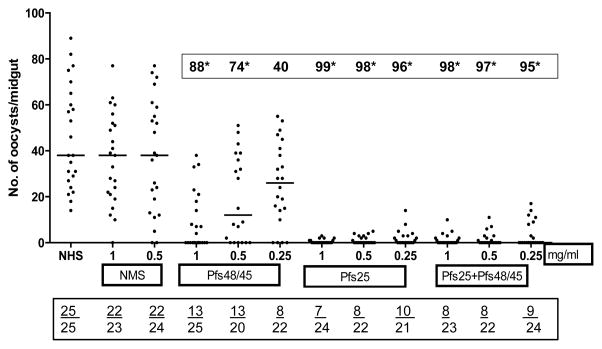
MFAs were conducted to determine the transmission reducing activity of purified antibodies in the immune sera from mice immunized with WT, SYN, MUT1 and MUT2 DNA plasmids (50 ug given three times with EP or no-EP) and 5 ug DNA groups after the protein boost (Fig. 6). All MFAs were conducted in the presence of normal human serum without any heat inactivation. Immunization with various plasmids without electroporation resulted in only partially effective antibodies (20 to 61% transmission reduction, Fig. 6a) and IgG (1 mg/mL) from SYN Pfs48/45 group exhibiting 60% transmission reduction as compared to 54%, 54% and 38% with MUT1, MUT2 and WT Pfs48/45 groups, respectively. On the other hand, IgG from mice immunized with SYN Pfs48/45 plasmid DNA by EP exhibited much higher transmission reducing activity (88%, 74% and 40% at 1.0, 0.5 and 0.25 mg/mL IgG concentrations, respectively) as compared to mice immunized with WT, MUT1 and MUT2 Pfs48/45 plasmids (Fig. 6b). Importantly, a protein boost in mice previously immunized with 10-fold lower dose (5 ug by EP) with all four vaccine constructs exhibited >90% transmission reduction suggesting a potentiating role for heterologous prime-boost in effective immunogenicity of Pfs48/45 DNA vaccine (Fig. 6c). Assuming average IgG concentration of 5–10 mg/mL in mice, these results demonstrate potent transmission reducing activity at 1:5 to 1:10 dilution equivalent of various immune sera.
Fig 6 (a–c). Membrane Feeding Assays.
Transmission blocking activity of purified IgG from pooled immune sera obtained after immunization with WT, SYN, MUT1 and MUT2 Pfs48/45 DNA vaccines was determined by membrane feeding assays. (Fig. 6a) shows results for no-EP immunization groups tested at 1 and 0.5 mg/mL IgG, (Fig. 6b) shows results for EP immunization groups tested at 1, 0.5 and 0.25 mg/mL IgG, and (Fig. 6c) shows results for 5 ug DNA immunization (with EP) and rPfs48/45 protein boost groups tested at 1, 0.5 and 0.25 mg/mL IgG. Results of NHS (normal human serum) or NMS (IgG purified from normal mouse sera) controls are also shown. Scatter plots represent the number of oocysts counted in the midgut of individual mosquitoes and the median oocyst counts by the horizontal bars. Geometric mean oocyst counts were used to calculate percent transmission reducing activity shown by numbers within a box above each scatter plot. Numbers within the rectangular box below show the total number of mosquitoes infected / total number of mosquitoes dissected for each group. Statistical differences were analyzed by Mann Whitney U test and are indicated by an asterisk (*).
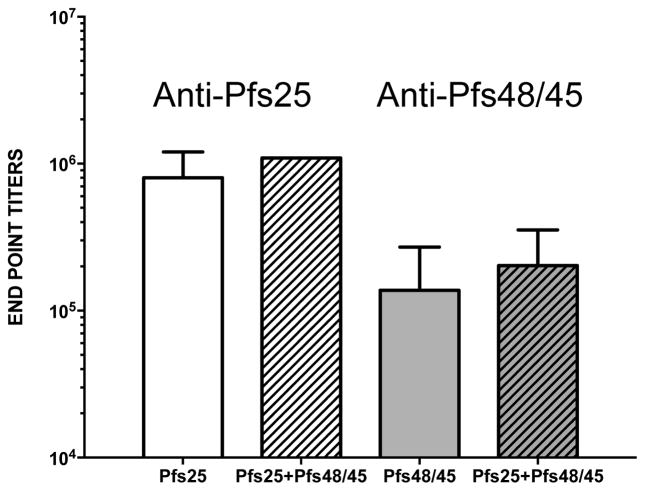
Results of MFA using purified IgG from mice immunized (3 DNA immunizing doses) with a combination of SYN Pfs25 and SYN Pfs48/45 at 1, 0.5 and 0.25 mg/mL IgG concentrations are shown in Fig. 7. IgG from mice immunized with 50 ug SYN Pfs25 showed 96–99% transmission reduction and a similar level of reduction was revealed by IgG from the SYN Pfs25 and SYN Pfs48/45 combination group.
Fig. 7. Membrane Feeding Assays.
Transmission blocking activity of purified IgG from pooled immune sera from mice immunized with SYN Pfs25 and SYN Pfs48/45 DNA vaccines, individually or with a combination (with EP) was determined using MFA. Scatter plots represent the number of oocysts counted in individual mosquitoes and the median oocyst counts by the horizontal bars. Geometric mean oocyst counts were used to calculate percent transmission reducing activity shown by numbers within a box above each scatter plot. Numbers within the rectangular box below show the total number of mosquitoes infected / total number of mosquitoes dissected for each group. These MFAs were done at the same time as those in Fig. 6b and SYN Pfs48/45 data is included here for comparison. Statistical significance was analyzed by Mann Whitney U test and are indicated by an asterisk (*).
4. Discussion
Pfs48/45, previously identified using specific monoclonal antibodies with transmission blocking activity [5, 44] is an important target of malaria transmission - blocking immunity. Pfs48/45 belongs to 6-Cys family of proteins and target epitopes of blocking antibodies are reduction-sensitive, conformational in nature [9, 44]. Various technical limitations have restricted progress towards the development of TBV vaccine based on Pfs48/45. It has been difficult to express Pfs48/45 in appropriate conformation in recombinant expression systems and reduced and alkylated form of Pfs48/45 has been shown to be poorly immunogenic [45]. While epitope analysis of Pfs48/45 has revealed surface targets of transmission blocking monoclonal antibodies [46], lack of any structural information and knowledge of conformational target epitopes has resulted in limited success in the development of recombinant TBV based on Pfs48/45. Computational modeling [6] and experimental structure determination [47, 48] approaches have been used to predict structure of Pfs48/45. However, reproducible production of correctly folded recombinant protein remains a challenge and the immunization studies thus far have revealed efficacies not ideal for vaccine development. DNA vaccines provide a platform that allows in vivo expression of encoded antigen employing host’s transcription and translation machinery after introduction of plasmid DNA [24, 49]. While this platform has been extensively explored for Pfs25 [25–30] this is the first time Pfs48/45 DNA vaccines have been examined in pre-clinical studies. In a recent study, immunization with DNA plasmid encoding Pvs48/45 formulated with Vaxfectin in mice elicited antibodies with transmission reducing activity in the presence of complement, however no reduction was seen in heat-inactivated serum (complement negative) [50]. The goal of studies presented here was to investigate the vaccine potential of Pfs48/45 using a DNA vaccination approach. In particular we wished to evaluate the contributions of codon optimization [51, 52], EP [31, 53], heterologous prime-boost regimen [32], and the role of N-linked glycosylation [35] on the immunogenicity of Pfs48/45 DNA vaccines in pre-clinical studies in mice. Considering the need for developing multivalent TBVs targeting different lifecycle stages, we also endeavored to determine the outcome of co-immunization with Pfs25 and Pfs48/45 DNA vaccines.
Our findings from in vitro cell transfection studies demonstrated that Pfs48/45 encoded by DNA plasmid undergoes N-linked glycosylation in mammalian cells using host translational machinery. Such a post-translational modification of Pfs48/45 encoded by WT and SYN DNA was confirmed by two approaches. First, mutating all seven putative N-glycosylation sites in Pfs48/45 (Pfs48/45 MUT1 and MUT2) prevented the addition of N-glycan side chains as demonstrated by a smaller size protein band (20–25%) by Western blotting as compared to protein product produced by WT and SYN plasmid DNA. Second, tunicamycin treatment of cells transfected with WT or SYN plasmid resulted in a protein product comparable in size to MUT1 and MUT2 protein product suggesting modification of polypeptide by N-linked glycosylation of Pfs48/45.
Further, we demonstrate here the value of two different approaches to generate N-glycosylation mutants. Our rationale was to create N-glycosylation mutants which would have least impact on the structural and functional integrity of encoded protein while allowing immunogenicity comparison between glycosylated and unglycosylated forms of the immunogen. Gene sequence of Pfs48/45 has 7 putative N-glycosylation sites (N50,131,190,204,254,299,303 XS/T) and particular mutational substitutions were chosen based on the natural polymorphism at these sites as revealed by comparison of P48/45 sequences in other Plasmodium spp. (alignments from OrthoMCL 5 [54] via PlasmoDB [55]). Because N190 and N299 residues were found to be conserved in various sequences analyzed, N50,131,303 residues were altered to D, N204 to T, N254 to K and switching S/T residues at the third position of N190 and N299 sites to A. In MUT2, the S/T residues at the third position of all the seven consensus NXS/T motif were altered to A. A similar design strategy was recently used, for example, to study N-glycan dependent epitopes of HIV gp120 [56].
Results of our antibody end point titer and avidity analyses revealed no significant differences between glycosylated and unglycosylated forms of the antigens encoded by DNA vaccines. However mice immunized with WT and SYN Pfs48/45 DNA presenting glycosylated Pfs48/45 had a balanced IgG1/IgG2a response whereas mice immunized with MUT1 and MUT2 DNA presenting unglycosylated Pfs48/45 showed skewing towards elevated IgG2a isotypes. Further studies are needed to understand the mechanisms for the observed isotype skewing. MFA data revealed slightly improved transmission reducing potency of SYN Pfs48/45 DNA compared with MUT1 or MUT2 Pfs48/45 DNA, even when the antibody titers were not significantly different among them. It is possible that this difference in functional responses may be attributed to the difference in IgG isotypes. Similar studies examining the role of N-linked glycosylation in Pfs25 DNA vaccines did not show a significant difference in functional immune responses between SYN and MUT DNA [30]. It is interesting to note that in the studies with Pfs25, the antibody isotypes were comparable between SYN and MUT DNA groups [30]. These differences highlight the importance of examining vaccine optimization approaches on an individual antigen basis. Studies reported also reinforce the value of codon optimization as the SYN DNA elicited higher antibody titers and transmission reducing antibodies as compared to WT DNA.
Consistent with the previous findings and observations [31, 57–59], in vivo EP significantly enhanced antibody end point titers as well as transmission reducing activity for WT, SYN, MUT1 and MUT2 Pfs48/45 evaluated in this study. Currently, numerous DNA vaccine based clinical trials are underway worldwide [51, 60–62] and EP remains the most promising method of DNA vaccine delivery until an alternative DNA delivery method capable of matching immunogenicity enhancements is developed. Significant boosting of antibody titers as well as functional responses in all DNA immunization groups by E. coli produced recombinant Pfs48/45 [23] also support a heterologous prime-boost regimen for Pfs48/45 DNA vaccine delivery.
The studies reported here demonstrate that a codon optimized Pfs48/45 DNA vaccine has the potential to be developed as an alternative vaccine delivery platform. Our findings also suggest that contrary to previous studies [41], leaving N-linked glycosylation sites intact in the case of Pfs48/45 based DNA vaccine may not have a detrimental impact on functional transmission reducing immune responses. In view of the complex life cycle of Plasmodium parasites, it is generally accepted that it may be advantageous to combine multiple antigens from different lifecycle stages of the parasite. Potent immunogenicity of codon optimized Pfs25 DNA vaccine administered by in vivo EP [29, 30] and Pfs48/45 (current study) prompted us to evaluate a combination of SYN Pfs25 and SYN Pfs48/45 by EP. Our studies indicate that co-immunization with SYN Pfs25 and SYN Pfs48/45 induced comparable antigen-specific antibody titers against each individual antigen and transmission reduction in the combined group was not compromised. Our analysis does not allow us to suggest whether high blocking in the combination was due to antibodies against Pfs25 or Pfs48/45 or both. Additional studies are warranted to dissect any additive impact of combining multiple vaccines, it is reasonable to assume that targeting multiple parasite stages will ensure effective inhibition by catching any escaping parasites. The results also support the feasibility of a combination transmission blocking DNA vaccine targeting two different stages in the parasite life cycle.
Acknowledgments
These studies were supported by NIH grants AI47089 and AI101427.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaccines mCGo. A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rts S. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. The Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001:19. doi: 10.1016/s0264-410x(00)00521-1. [DOI] [PubMed] [Google Scholar]
- 4.Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007:134. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- 5.Rener J, Graves P, Carter R, Williams J, Burkot T. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. 1983;158:976–81. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerloff DL, Creasey A, Maslau S, Carter R. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci USA. 2005:102. doi: 10.1073/pnas.0502378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter R, Coulson A, Bhatti S, Taylor BJ, Elliott JF. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Molecular and Biochemical Parasitology. 1995;71:203–10. doi: 10.1016/0166-6851(94)00054-q. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal DC, Carter R, Rener J, Grotendorst CA, Miller LH, Howard RJ. Monoclonal antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitoes. J Immunol. 1983;131:2557–62. [PubMed] [Google Scholar]
- 9.Vermeulen A, Ponnudurai T, Beckers P, Verhave J, Smits M, Meuwissen J. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–76. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JAM, Dodemont HJ, et al. A Central Role for P48/45 in Malaria Parasite Male Gamete Fertility. Cell. 2001;104:153–64. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk MR, Van Schaijk BC, Khan SM, Van Dooren MW, Ramesar J, Kaczanowski S, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 2010;6:e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Schaijk BC, van Dijk MR, van de Vegte-Bolmer M, van Gemert G-J, van Dooren MW, Eksi S, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Molecular and biochemical parasitology. 2006;149:216–22. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Vermeulen AN, Roeffen WF, Henderik JB, Ponnudurai T, Beckers PJ, Meuwissen JH. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol Stand. 1985:62. [PubMed] [Google Scholar]
- 14.Roeffen W, Teelen K, Van As J, vd Vegte-Bolmer M, Eling W, Sauerwein R. Plasmodium falciparum: production and characterization of rat monoclonal antibodies specific for the sexual-stage Pfs48/45 antigen. Experimental parasitology. 2001;97:45–9. doi: 10.1006/expr.2000.4586. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, Carter R. Biosynthesis of the target antigens of antibodies blocking transmission of Plasmodium falciparum. Molecular and biochemical parasitology. 1984;13:333–42. doi: 10.1016/0166-6851(84)90124-5. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen AN, van Deursen J, Brakenhoff RH, Lensen THW, Ponnudurai T, Meuwissen JHET. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Molecular and Biochemical Parasitology. 1986;20:155–63. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]
- 17.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner J, Huang C-Y, Waisberg M, Felgner PL, Doumbo OK, Ongoiba A, et al. Plasmodium falciparum gametocyte-specific antibody profiling reveals boosting through natural infection and identifies potential markers of gametocyte exposure. Infection and immunity. 2015;83:4229–36. doi: 10.1128/IAI.00644-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milek RL, DeVries AA, Roeffen WF, Stunnenberg H, Rottier PJ, Konings RN. Plasmodium falciparum: heterologous synthesis of the transmission-blocking vaccine candidate Pfs48/45 in recombinant vaccinia virus-infected cells. Experimental parasitology. 1998;90:165–74. doi: 10.1006/expr.1998.4315. [DOI] [PubMed] [Google Scholar]
- 20.MILEK RL, ROEFFEN WF, KOCKEN CH, JANSEN J, KAAN AM, ELING W, et al. Immunological properties of recombinant proteins of the transmission blocking vaccine candidate, Pfs48/45, of the human malaria parasite Plasmodium falciparum produced in Escherichia coli. Parasite immunology. 1998;20:377–85. doi: 10.1046/j.1365-3024.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- 21.Milek RL, Stunnenberg HG, Konings RN. Assembly and expression of a synthetic gene encoding the antigen Pfs48/45 of the human malaria parasite Plasmodium falciparum in yeast. Vaccine. 2000;18:1402–11. doi: 10.1016/s0264-410x(99)00392-8. [DOI] [PubMed] [Google Scholar]
- 22.Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA. 2008:105. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury DR, Angov E, Kariuki T, Kumar N. A Potent Malaria Transmission Blocking Vaccine Based on Codon Harmonized Full Length Pfs48/45 Expressed in Escherichia coli. PLoS ONE. 2009;4:e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunological Reviews. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 25.Lobo CA, Dhar R, Kumar N. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infection and immunity. 1999;67:1688–93. doi: 10.1128/iai.67.4.1688-1693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coban C, Philipp MT, Purcell JE, Keister DB, Okulate M, Martin DS, et al. Induction of Plasmodium falciparum transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infection and immunity. 2004;72:253–9. doi: 10.1128/IAI.72.1.253-259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kongkasuriyachai D, Bartels-Andrews L, Stowers A, Collins WE, Sullivan J, Sattabongkot J, et al. Potent immunogenicity of DNA vaccines encoding Plasmodium vivax transmission-blocking vaccine candidates Pvs25 and Pvs28—evaluation of homologous and heterologous antigen-delivery prime-boost strategy. Vaccine. 2004;22:3205–13. doi: 10.1016/j.vaccine.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Nyakundi R, Kariuki T, Ozwara H, Nyamongo O, Mlambo G, et al. Functional evaluation of malaria Pfs25 DNA vaccine by in vivo electroporation in Olive baboons. Vaccine. 2013;31:3140–7. doi: 10.1016/j.vaccine.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeBlanc R, Vasquez Y, Hannaman D, Kumar N. Markedly Enhanced Immunogenicity of a Pfs25 DNA-Based Malaria Transmission-Blocking Vaccine by In Vivo Electroporation. Vaccine. 2008;26:185–92. doi: 10.1016/j.vaccine.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta D, Bansal GP, Kumar R, Ellefsen B, Hannaman D, Kumar N. Evaluation of the Impact of Codon Optimization and N-Linked Glycosylation on Functional Immunogenicity of Pfs25 DNA Vaccines Delivered by In Vivo Electroporation in Preclinical Studies in Mice. Clinical and Vaccine Immunology. 2015;22:1013–9. doi: 10.1128/CVI.00185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sardesai N, Weiner D. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramshaw IA, Ramsay AJ. The prime-boost strategy: exciting prospects for improved vaccination. Immunology today. 2000;21:163–5. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 33.Ivory C, Chadee K. DNA vaccines: designing strategies against parasitic infections. Genetic Vaccines and Therapy. 2004;2:17. doi: 10.1186/1479-0556-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisowska E. The role of glycosylation in protein antigenic properties. Cellular and Molecular Life Sciences CMLS. 2002;59:445–55. doi: 10.1007/s00018-002-8437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jortzik E, Kehr S, Becker K. Progress in Parasitology. Springer; 2011. Post-translational modifications in apicomplexan parasites; pp. 93–120. [Google Scholar]
- 36.Bushkin GG, Ratner DM, Cui J, Banerjee S, Duraisingh MT, Jennings CV, et al. Suggestive evidence for Darwinian Selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii. Eukaryotic cell. 2010;9:228–41. doi: 10.1128/EC.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Macedo CS, Schwarz RT, Todeschini AR, Previato JO, Mendonça-Previato L. Overlooked post-translational modifications of proteins in Plasmodium falciparum: N-and O-glycosylation-a review. Memórias do Instituto Oswaldo Cruz. 2010;105:949–56. doi: 10.1590/s0074-02762010000800001. [DOI] [PubMed] [Google Scholar]
- 38.Stowers AW, Chen L-h, Zhang Y, Kennedy MC, Zou L, Lambert L, et al. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proceedings of the National Academy of Sciences. 2002;99:339–44. doi: 10.1073/pnas.012590199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boes A, Spiegel H, Edgue G, Kapelski S, Scheuermayer M, Fendel R, et al. Detailed functional characterization of glycosylated and nonglycosylated variants of malaria vaccine candidate PfAMA1 produced in Nicotiana benthamiana and analysis of growth inhibitory responses in rabbits. Plant biotechnology journal. 2015;13:222–34. doi: 10.1111/pbi.12255. [DOI] [PubMed] [Google Scholar]
- 40.Giersing B, Miura K, Shimp R, Wang J, Zhou H, Orcutt A, et al. Posttranslational modification of recombinant Plasmodium falciparum apical membrane antigen 1: impact on functional immune responses to a malaria vaccine candidate. Infection and immunity. 2005;73:3963–70. doi: 10.1128/IAI.73.7.3963-3970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamedov T, Ghosh A, Jones RM, Mett V, Farrance CE, Musiychuk K, et al. Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase F. Plant biotechnology journal. 2012;10:773–82. doi: 10.1111/j.1467-7652.2012.00694.x. [DOI] [PubMed] [Google Scholar]
- 42.Elbein AD. The tunicamycins—useful tools for studies on glycoproteins. Trends in Biochemical Sciences. 1981;6:219–21. [Google Scholar]
- 43.Kumar R, Angov E, Kumar N. Potent Malaria Transmission-Blocking Antibody Responses Elicited by Plasmodium falciparum Pfs25 Expressed in Escherichia coli after Successful Protein Refolding. Infection and Immunity. 2014;82:1453–9. doi: 10.1128/IAI.01438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CARTER R, GRAVES PM, KEISTER DB, QUAKYI IA. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite immunology. 1990;12:587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 45.Merino KM, Bansal GP, Kumar N. Reduced immunogenicity of Plasmodium falciparum gamete surface antigen (Pfs48/45) in mice after disruption of disulfide bonds-evaluating effect of GILT. Immunology. 2016 doi: 10.1111/imm.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K. Epitope analysis of the malaria surface antigen pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem. 2007:282. doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]
- 47.Arredondo SA, Cai M, Takayama Y, MacDonald NJ, Anderson DE, Aravind L, et al. Structure of the Plasmodium 6-cysteine s48/45 domain. Proceedings of the National Academy of Sciences. 2012;109:6692–7. doi: 10.1073/pnas.1204363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonkin ML, Arredondo SA, Loveless BC, Serpa JJ, Makepeace KA, Sundar N. Structural and biochemical characterization of Plasmodium falciparum 12 (Pf12) reveals a unique interdomain organization and the potential for an antiparallel arrangement with Pf41. J Biol Chem. 2013:288. doi: 10.1074/jbc.M113.455667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nature Reviews Genetics. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tachibana M, Suwanabun N, Kaneko O, Iriko H, Otsuki H, Sattabongkot J. Plasmodium vivax gametocyte proteins, Pvs48/45 and Pvs47, induce transmission-reducing antibodies by DNA immunization. Vaccine. 2015:33. doi: 10.1016/j.vaccine.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Review of Vaccines. 2015:1–17. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert review of vaccines. 2012;11:189–209. doi: 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Drunen Littel-van den Hurk S, Hannaman D. Electroporation for DNA immunization: clinical application. Expert review of vaccines. 2010;9:503–17. doi: 10.1586/erv.10.42. [DOI] [PubMed] [Google Scholar]
- 54.Chen F, Mackey AJ, Stoeckert CJ, Roos DS. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic acids research. 2006;34:D363–D8. doi: 10.1093/nar/gkj123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic acids research. 2009;37:D539–D43. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doran RC, Morales JF, To B, Morin TJ, Theolis R, Jr, O’Rourke SM, et al. Characterization of a monoclonal antibody to a novel glycan-dependent epitope in the V1/V2 domain of the HIV-1 envelope protein, gp120. Molecular immunology. 2014;62:219–26. doi: 10.1016/j.molimm.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luxembourg A, Ellefsen B, Wilson D, Ubach A, Hannaman D, van den Hurk J. Electroporation-based DNA transfer enhances gene expression and immune responses to DNA vaccines in cattle. Vaccine. 2008;26:5503–9. doi: 10.1016/j.vaccine.2008.07.093. [DOI] [PubMed] [Google Scholar]
- 58.Luxembourg A, Evans CF, Hannaman D. Electroporation-based DNA immunisation: translation to the clinic. Expert opinion on biological therapy. 2007;7:1647–64. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- 59.Luxembourg A, Hannaman D, Ellefsen B, Nakamura G, Bernard R. Enhancement of immune responses to an HBV DNA vaccine by electroporation. Vaccine. 2006;24:4490–3. doi: 10.1016/j.vaccine.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, et al. Long-Term Survival of Dogs with Advanced Malignant Melanoma after DNA Vaccination with Xenogeneic Human Tyrosinase A Phase I Trial. Clinical Cancer Research. 2003;9:1284–90. [PubMed] [Google Scholar]
- 61.Liu MA, Ulmer JB. Advances in Genetics. Academic Press; 2005. Human Clinical Trials of Plasmid DNA Vaccines; pp. 25–40. [DOI] [PubMed] [Google Scholar]
- 62.Hannaman D, Dupuy LC, Ellefsen B, Schmaljohn CS. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. 2016;34:3607–12. doi: 10.1016/j.vaccine.2016.04.077. [DOI] [PubMed] [Google Scholar]