Highlights
-
•
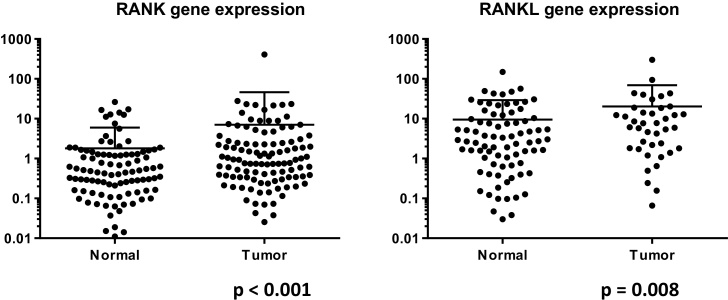
Expression of RANK and RANKL genes in prostate cancer is higher than non-neoplastic prostate.
-
•
RANK/RANKL expression is not related to pathological features.
-
•
There is no significant correlation of RANK/RANKL expression with biochemical recurrence after radical prostatectomy.
Abbreviations: RANK, receptor activator of nuclear factor kappa B; RANKL, receptor activator of nuclear factor kappa B ligand
Keywords: RANK/RANKL, Prostate cancer
Bone is the most common metastatic site in patients with prostate cancer. Skeletal-related events consisting of pathological fracture, spinal cord compression, and intractable pain are causes of a reduced quality of life for patients [1], [2]. Denosumab (XGEVA), a human monoclonal antibody against receptor activator of nuclear factor kappa B ligand (RANKL), was found to be a new therapeutic option for bone metastasis [3], [4], [5].
Prostate cancer cells penetrate the bone marrow and induce osteoblasts to produce cytokines that promote the secretion of RANKL. RANKL expression acceleration induces osteoclast hyperplasia and facilitates bone resorption [1]. Denosumab controls these mechanisms by inhibiting RANK. A previous study demonstrated that primary prostate cancer cells expressed the RANK and RANKL genes, which was further elevated in bone metastasis lesions [6], [7], [8], [9], [10]. Therefore, denosumab is expected to exert its antitumor effect by inhibiting RANKL.
cDNA was extracted from a total of 115 radical prostatectomy specimens obtained at Yokohama City University Hospital. RANK/RANKL gene expression was examined by real-time qPCR, using RANK and RANKL primers (Applied Biosystems, Foster City, CA, USA), as we previously described [11]. Overall, the expression levels of RANK (p < 0.001) and RANKL (p = 0.008) genes in prostate cancer tissues were significantly higher than those in non-cancerous tissues [Fig. 1]. Specifically, the levels of RANK and RANKL expression in tumor were higher than those in paired normal tissue in 70.9% and 78.1%, respectively. However, no correlations were observed between RANK or RANKL gene expression and Gleason score, pT stage, or lymph node metastasis. The median PSA recurrence free survival was 4517 days in lower RANK expression group and 3782 days in higher RANK expression group, but not significantly (p = 0.172). In RANKL expression, the medican PSA revurrence free survival was 3702 in lower RANKL expression group and 2265 days in higher RANKL expression group (p = 0.205).
Fig. 1.
RANK/RANKL gene expression in normal tissue vs. tumor tissue.
A correlation between RANKL gene expression and pathological features has been shown in renal cell carcinoma [12], [13]. In the present study, we found overexpression of RANK and RANKL genes in prostate cancer compared with non-neoplastic prostate. However, there were no correlations between RANK/RANKL expression and pathological features, including pT stage and lymph node metastasis. Given that all of the specimens used in this study were obtained by radical prostatectomy, their similar characteristics might have obscured any differences.
A few studies have reported potential correlations between RANK/RANKL expression and oncologic outcomes. For instance, Ferreira et al. showed that RANK expression predicted disease-free survival and overall survival in breast cancer patients [14]. To the best of our knowledge, there have been no attempts to link between RANKL expression and the prognosis of prostate cancer patients. We found no significant correlation of RANK or RANKL expression with biochemical recurrence after radical prostatectomy.
Our study showed the increased expression of RANK and RANKL genes in prostate cancer, but its role was not proven. Further studies including a larger patient cohort are needed to validate the current results.
Conflict of interest
We declare no conflicts of interest.
Source of funding
KAKENHI grants (16K20152) from the Ministry of Education, Culture, Sports, Science and Technology of Japan were provided to T.K.
Authors contribution
KS and TK wrote the manuscript.
KS, TK, YH, ST, DT, TM, YH, JT, YM performed the operation.
YY, MY, HU wrote and checked the manuscript.
Ethical approval
Institutional review board of Yokohama City University Medical Center approved this study (D1507018).
Consent
We obtained written informed consent for publication. Institutional review board of Yokohama City University Medical Center approved this study (D1507018).
Guarantor
Takashi Kawahara.
Contributor Information
Mari Ohtaka, Email: marimo633@yahoo.co.jp.
Takashi Kawahara, Email: takashi_tk2001@yahoo.co.jp.
Taku Mochizuki, Email: apotaku24@yahoo.co.jp.
Daiji Takamoto, Email: daiji_niigata@yahoo.co.jp.
Yusuke Hattori, Email: hatu98@yokohama-cu.ac.jp.
Jun-ichi Teranishi, Email: jteran@yokohama-cu.ac.jp.
Yasuhide Miyoshi, Email: miyoyasu@yokohama-cu.ac.jp.
Yasushi Yumura, Email: yumura@yokohama-cu.ac.jp.
Hisashi Hasumi, Email: hisahasu@gmail.com.
Yumiko Yokomizo, Email: yumiko1@yokohama-cu.ac.jp.
Narihiko Hayashi, Email: twnary@yahoo.co.jp.
Keiichi Kondo, Email: kkurouro@urahp.yokohama-cu.ac.jp.
Masahiro Yao, Email: masayao@yokohama-cu.ac.jp.
Hiroshi Miyamoto, Email: hmiyamo1@jhmi.edu.
Hiroji Uemura, Email: hu0428@yokohama-cu.ac.jp.
References
- 1.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl. 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 3.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., Chin J.L., Vinholes J.J., Goas J.A., Chen B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 4.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., Chin J.L., Vinholes J.J., Goas J.A., Zheng M. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl. Cancer Inst. 2004;96(11):879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 5.Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odero-Marah V.A., Wang R., Chu G., Zayzafoon M., Xu J., Shi C., Marshall F.F., Zhau H.E., Chung L.W. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18(8):858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Suarez E., Jacob A.P., Jones J., Miller R., Roudier-Meyer M.P., Erwert R., Pinkas J., Branstetter D., Dougall W.C. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 8.Schramek D., Leibbrandt A., Sigl V., Kenner L., Pospisilik J.A., Lee H.J., Hanada R., Joshi P.A., Aliprantis A., Glimcher L. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan W., Zhang W., Strasner A., Grivennikov S., Cheng J.Q., Hoffman R.M., Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G., Sircar K., Aprikian A., Potti A., Goltzman D., Rabbani S.A. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107(2):289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara T., Kashiwagi E., Ide H., Li Y., Zheng Y., Ishiguro H., Miyamoto H. The role of NFATc1 in prostate cancer progression: cyclosporine A and tacrolimus inhibit cell proliferation, migration, and invasion. Prostate. 2015;75(6):573–584. doi: 10.1002/pros.22937. [DOI] [PubMed] [Google Scholar]
- 12.Jones D.H., Nakashima T., Sanchez O.H., Kozieradzki I., Komarova S.V., Sarosi I., Morony S., Rubin E., Sarao R., Hojilla C.V. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 13.Mikami S., Katsube K., Oya M., Ishida M., Kosaka T., Mizuno R., Mochizuki S., Ikeda T., Mukai M., Okada Y. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J. Pathol. 2009;218(4):530–539. doi: 10.1002/path.2567. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira A., Alho I., Vendrell I., Melo M., Bras R., Costa A.L., Sousa A.R., Mansinho A., Abreu C., Pulido C. The prognostic role of RANK SNP rs34945627 in breast cancer patients with bone metastases. Oncotarget. 2016;7(July (27)):41380–41389. doi: 10.18632/oncotarget.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]