Abstract
Major Depressive Disorder (MDD) is a highly prevalent psychiatric diagnosis that is associated with a high degree of morbidity and mortality. This debilitating disorder is currently one of the leading causes of disability nationwide and is predicted to be the leading cause of disease burden by the year 2030. A large body of previous research has theorized that serotonergic dysfunction, specifically of the serotonin (5-HT) 1A receptor, plays a key role in the development of MDD. The purpose of this review is to describe the evolution of our current understanding of the serotonin 1A (5-HT1A) receptor and its role in the pathophysiology MDD through the discussion of animal, post-mortem, positron emission tomography (PET), pharmacologic and genetic studies.
Keywords: 5-HT1A, Depression, Review
1. Introduction
Major Depressive Disorder (MDD) is a highly prevalent and recurrent disorder that is predicted to be the leading cause of disease burden in the United States by the year 2030; as measured via Disability Adjusted Life Years (DALYs) (Mathers and Loncar, 2006). MDD is a type of mood disorder that involves a distinct change of mood that affects one's ability to function day-to-day and is characterized by sadness or irritability along with other psychophysiological items including changes in: sleep, appetite, and sexual desire. There may also be other symptoms present such as: anhedonia, which is the loss of ability to experience pleasure in previously enjoyed activities, suicidal thoughts or slowing of speech and movement. Moreover, cognitive functions including learning, memory and attention are also disrupted in MDD (Ogren et al., 2008). Within the elderly population, symptoms of dementia/neurodegenerative change may actually be attributable to MDD (pseudo-dementia) and cognitive function can return to baseline when the depression is effectively treated (Raskin et al., 2007). These changes must last a minimum of two weeks and cause significant functional impairment in work and family relations (Belmaker and Agam, 2008). Results from the National Comorbidity Survey Replication (NCS-R) indicate that lifetime incidence of MDD in the United States is 16.2% (32.6–35.1 million US adults) with 12% in men and 20% in women (Kessler et al., 2003).
Recently, however, a rationale for the differing prevalence rates of MDD between sexes has been the more closely examined. For several decades, epidemiological studies have reported that women are approximately twice as likely as men to develop MDD (Jovanovic et al., 2008; Parker and Brotchie, 2010). However, these prevalence rates depend on the reliability of current diagnostic classifications and structured diagnostic interviews (Karlsson et al., 2010). Since men and women can experience depression differently in terms of symptomatology, the criteria currently used to diagnose clinical depression may have led to an underdiagnosis of MDD in males. It has been shown that sex discordance exists within depression symptomatology such that men exhibit significantly more anger, irritability and symptomatic substance intake while women often suffer more from hypersomnia, withdrawal from friends and heaviness of the limbs (Winkler et al., 2005). Consistent with this view, a 2013 study reported that when changes in case criteria of MDD were implemented in a way that account for higher rates of anger, aggression and substance abuse in men, MDD prevalence estimates between sexes are eliminated (Martin et al., 2013). It is therefore possible that the significant difference in epidemiological reporting of MDD prevalence between sexes lies in the current diagnostic interview instruments currently used clinically (Angst and Dobler-Mikola, 1984). Although studies have presented a positive correlation between anger and depression, it is important to note, however, the difficulty in assessing the symptom of anger. This is due to the multidimensional nature of the symptom itself, in which hostility (the predisposition to become angry), anger (the emotion itself) and the ability both experience anger while not necessarily expressing anger (anger suppression) leads to difficulty in assessing and reporting of the symptom accurately (Riley et al., 1989; Winkler et al., 2005). Whether these differences in symptomatology are related to differences in psychopathology is still under investigation. However, whether due to genetic, environmental, or physiological differences, characterization of the etiology of sex differences in a psychopathology as prevalent as MDD is of utmost import for the field of psychiatry (Kaufman et al., 2015).
There are currently several theories that seek to explain the underlying pathophysiology of MDD. One such theory, known as the monoamine deficiency hypothesis of depression, proposes that a deficiency in monoanmine neurotransmission (serotonin, norepinephrine and dopamine) in the central nervous system (CNS) underlies the development of MDD (Belmaker and Agam, 2008; Schildkraut, 1965). A study finding that helped to initially link serotonergic abnormalities to MDD reported that patients who had remitted from a depressive episode could be induced into another depressive episode after having their tryptophan stores depleted via fasting. L-tryptophan is an amino acid necessary for serotonin synthesis (Lovenberg et al., 1967; Stockmeier, 2003). Several other studies have looked into how the end metabolite of serotonin, 5-HIAA, supports a role for serotonin in depressive illness (Åsberg et al., 1984; Dencker et al., 1966; Mann, 1999; Mendels et al., 1972; Murphy et al., 1978). However, a study in 1981 by Träskman et al. examined a cohort of subjects who had attempted suicide and compared monoamine metabolite levels to controls. The main finding of this study was that subjects who had attempted suicide had significantly lower levels of CSF 5-HIAA than controls, with a more pronounced reduction in the metabolite if the suicide attempt was violent. It was also found that subjects who had comorbid depression had significantly lower CSF 5-HIAA and homovanillic acid (HVA), another monoamine metabolite, levels when compared to controls (Traskman et al., 1981). As such, suicidality may represent a confounding factor in these analyses. It is important to note that although this review focuses on 5-HT, other monoamines i.e. norepinephrine and dopamine have been postulated to be abnormally regulated in patients with MDD. Decreased norepinephrine metabolism, increased tyrosine hydroxylase activity and reduced dopamine transporter activity have also been implicated within the monoamine deficiency hypothesis (Hasler, 2010; Schildkraut, 1965). Further credence for the monoamine deficiency hypothesis is provided by the fact that the three classes of antidepressants currently used to treat MDD i.e. monoamine oxidase inhibitors, tricyclic antidepressants and selective serotonin reuptake inhibitors (MAO-I, TCA and SSRI, respectively) all act by increasing the amount of serotonin present in the synaptic cleft by either inhibiting its synaptic reuptake or by inhibiting its metabolism (Gray et al., 2013). Moreover, a large body of previous research has theorized that serotonergic dysfunction, specifically of the serotonin 1A (5-HT1A) receptor, plays a vital role in the pathophysiology of MDD (Albert and Lemonde, 2004; Arango et al., 2001; Boldrini et al., 2008; Hesselgrave and Parsey, 2013; Miller et al., 2009; Miller et al., 2013; Parsey et al., 2006; Savitz et al., 2009; Stockmeier et al., 1998). As such, this review will describe the evolution of our current understanding of the serotonin 1A (5-HT1A) receptor and its role in the pathophysiology of the most common affective disorder: MDD, as established by the work cited above and other studies implicating serotonergic dysfunction in MDD (Agren, 1980; Asberg et al., 1976; Mann et al., 1992; Miller et al., 2009; Parsey et al., 2010; Parsey et al., 2006; Placidi et al., 2001; Stockmeier et al., 1998)
2. The serotonin molecule
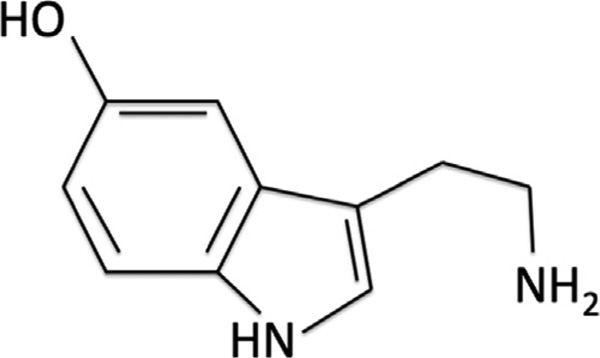
5-Hydroxytryptamine (5-HT), more commonly known as serotonin (Figure 1), is a monoamine neurotransmitter that is found throughout the human body (Jonnakuty and Gragnoli, 2008). 5-HT is synthesized in a small population of neurons from the raphe nuclei, in the midbrain, that express the enzyme tryptophan hydroxylase (Banerjee et al., 2007). However, serotonin synthesis is not restricted to the CNS as the enzyme tryptophan hydroxylase is also found in enterochromaffin cells within the gastrointestinal tract (Fuller and Wong, 1990; Furness and Costa, 1982). In fact, it is important to note that the majority of serotonin (5-HT) in the human body is produced by enterochromaffin cells within the gastrointestinal tract (Manocha and Khan, 2012). Serotonin binds to over 14 receptor proteins, most of which are G protein-coupled receptors (Banerjee et al., 2007). This single molecule is thought to be involved in several physiological and cognitive systems throughout the body including: emotions, memory, sleep, and thermal regulation (Jacobs and Azmitia, 1992).
Figure 1.
Structure of the serotonin molecule.
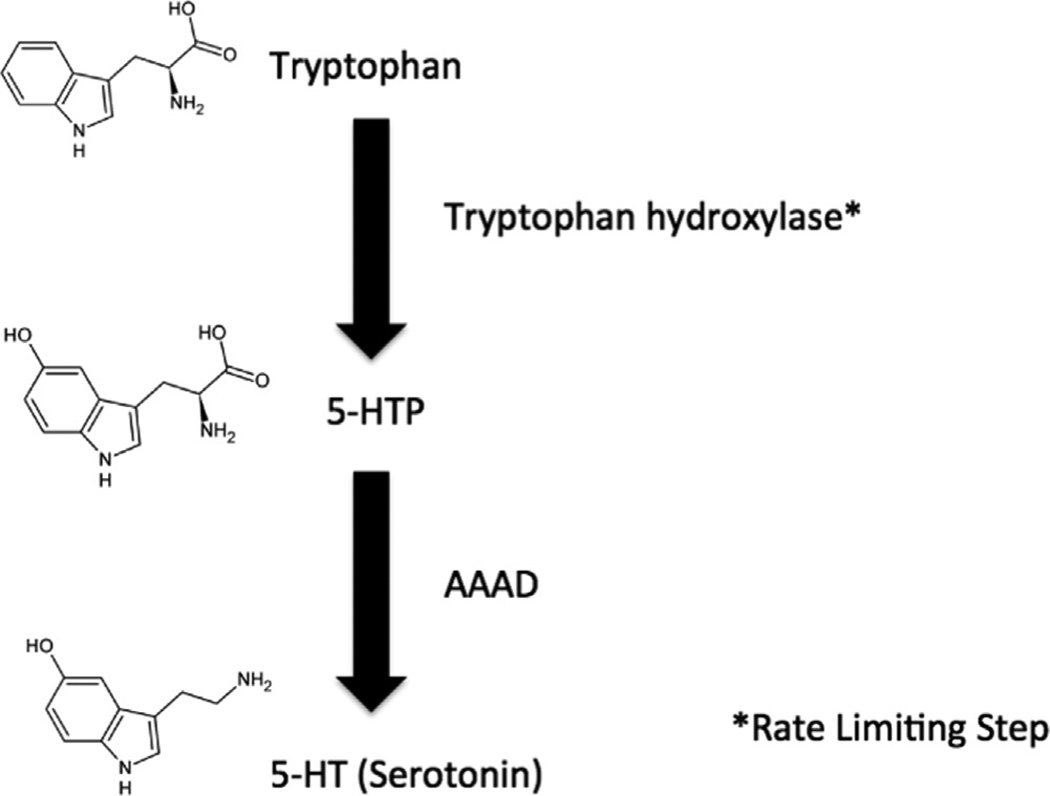
Serotonin is synthesized in the body from the essential amino acid L-tryptophan (Shrestha et al., 2012). Once ingested from diet, L-tryptophan is then converted to serotonin via a series of reactions (Figure 2). First, L-tryptophan is hydroxylated to 5-hydroxy-L-tyrptophan (5-HTP) by the enzyme tryptophan hydroxylase (TPH) using oxygen and tetrahydropteridine as cofactors. There are two isoforms of TPH which can mediate this reaction: TPH1 expressed mainly in the periphery, although with some expression in the brain during development; and TPH2, expressed in brain only, and as such most pertinent to this discussion (Bach-Mizrachi et al., 2008; Walther et al., 2003). This TPH reaction is the rate-limiting step in serotonin synthesis. This 5-HTP intermediate is then converted to serotonin via the enzyme L-amino acid decarboxylase, also known as aromatic amino acid decarboxylase (AAAD) (Diksic and Young, 2001).
Figure 2.
Pathway of serotonin (5-HT) synthesis.
Due to its acid dissociation constant, serotonin is charged at physiological pH and thus, will not cross the blood brain barrier (BBB) (Diksic and Young, 2001). Therefore, the amount of serotonin present within the CNS depends on the amount of L-tryptophan, not serotonin, that is peripherally available. L-tryptophan present in systemic circulation is actively transported across the BBB, into the CNS, using a carrier protein where it is then converted into serotonin (Figure 2). Once synthesized in the central nervous system, serotonin is stored in secretory granules where it remains until neuronal depolarization induces its release into the synaptic cleft and post-synaptic binding can occur. Upon release into the synapse, the serotonin molecules are eventually taken up via the serotonin transporter (5-HTT), located on the presynaptic axonal membrane. Once this reuptake occurs, the serotonin molecules are then metabolized by the enzyme monoamine oxidase (MAO) to its metabolite, 5-hydroxyindole acetic acid (5-HIAA) (Jonnakuty and Gragnoli, 2008). More specifically, there are two isoforms of MAO that exist in neurons (MAO-A and MAO-B) and both break down serotonin via the process of oxidative deamination (Molinoff and Axelrod, 1971). Once serotonin is metabolized to 5-HIAA, the metabolite is actively transported from the CNS into systemic circulation where it is then excreted in urine (Jonnakuty and Gragnoli, 2008).
3. 5-HT1A receptor function and distribution
5-HT neurons originate in the raphe nuclei, of the pons and upper medulla, from which they have both ascending and descending projections. The ascending projections originate in both the median and dorsal raphe nuceli, and project to: forebrain, hippocampus, limbic system, basal ganglia and hypothalamus (Kundu et al., 2012). The descending projections originate in the caudal raphe nuclei, raphe magnus nucleus and nuclei raphe pallidus, and project to the caudal brain stem/spinal cord, dorsal horn and ventral horn/intermediate zone/intermediolateral cell column, respectively (Billard et al., 2014; Kundu et al., 2012).
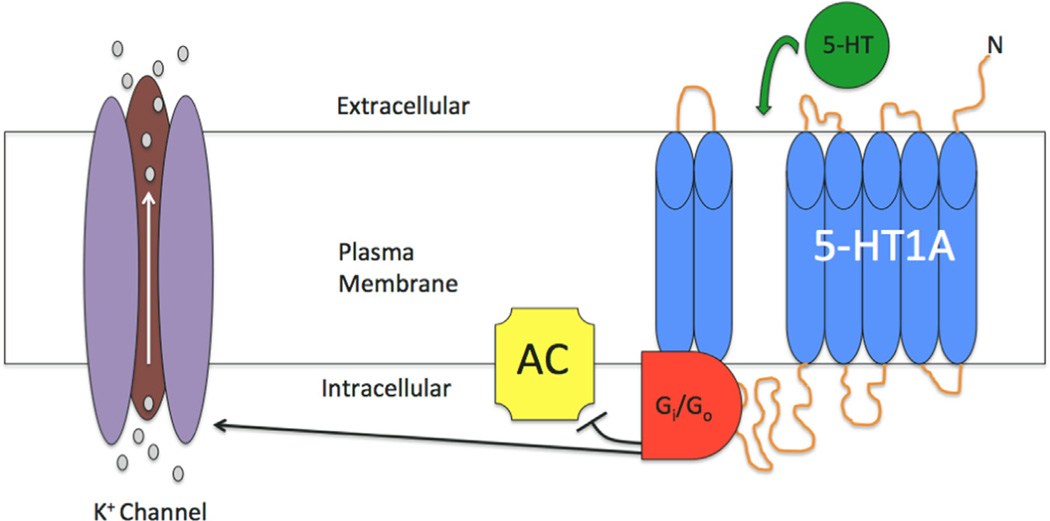
The 5-HT1A receptor is one of the most abundant serotonin receptors in the brain. G-protein-coupled, the major biophysical effect of the serotonin binding to the 5-HT1A receptor is to induce activation of hyperpolarizing K+ channels (Figure 3) (Banerjee et al., 2007). This receptor is involved in multiple other molecular cascades, which include: regulation of phospholipase-C activity, cAMP accumulation inhibition and reducing calcium currents (Claustre et al., 1991; Raymond et al., 2001). In terms of distribution, the 5-HT1A receptor in the brain has been extensively mapped using both receptor autoradiography and, recently, positron emission tomography (PET). Initial autoradiographic studies using the murine model reported elevated 5-HT1A receptor levels in: infralimbic cortex, hippocampus (specifically within CA1), cingulate cortex and raphe nuclei (Hall et al., 1997; Marcinkiewicz et al., 1984; Rasmuson et al., 1998). In the neocortex, high densities are seen in layers I-II with less but detectable binding in layers V–VI (Arango et al., 1995; Schmitz et al., 1995). In contrast, both the basal ganglia and cerebellum, except for some detectable receptors in the cerebellar vermis, are virtually devoid of 5-HT1A (Arango et al., 2001; Barnes and Sharp, 1999; Hall et al., 1997).
Figure 3.
Functional activity of the 5-HT1A receptor. Binding of serotonin (5-HT) to the 5-HT1A receptor, both pre-synaptic and post-synaptic subtypes, signals the opening of a hyperpolarizing potassium (K+) channel and inhibition of adenylyl cyclase (AC). These actions, mediated by G proteins (Gi/Go), serve to hyperpolarize the cell and reduce its firing rate. Gray circles associated with K+ channel represent K+ ions moving into extracellular space.
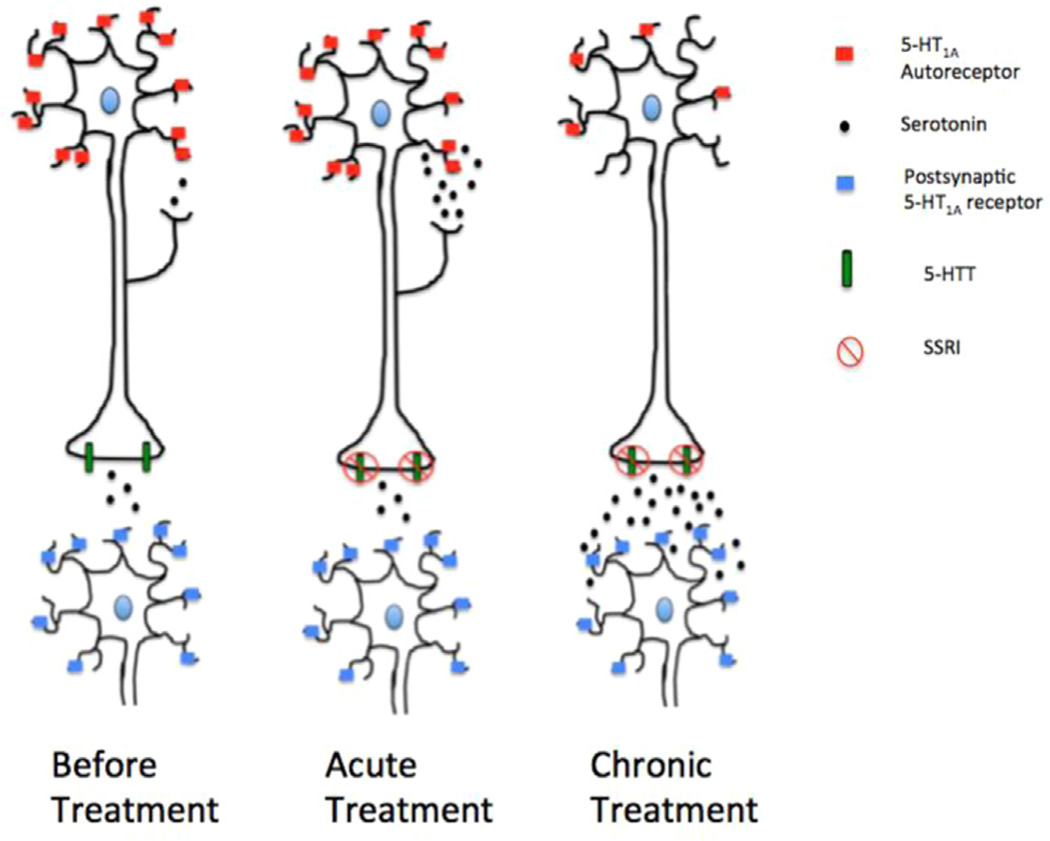
In the CNS, there exist two distinct populations of 5-HT1A receptors: pre-synaptic (autoreceptors) and post-synaptic (hetero-receptors). Although the binding of serotonin to both receptor sub-types induces neuronal hyperpolarization, the effect of sustained receptor stimulation differs between the pre-synaptic and post-synaptic 5-HT1A receptor subtypes. Studies have further shown that 5-HT1A autoreceptors in the raphe do not affect cAMP levels, while postsynaptic 5-HT1A receptors induce increased adenylyl cyclase activity (Billard et al., 2014). Moreover, the way in which each of these two receptor subtypes responds to sustained serotonin stimulation provides insight into the mechanism of action of Selective Serotonin Reuptake Inhibitors (SSRIs); the first line treatment of MDD. Essentially, 5-HT1A autoreceptors act as a negative feedback mechanism in which rapid increases in serotonin release from serotonergic neurons can be reduced and brought back to equilibrium. When SSRIs are administered, the inhibition of presynaptic serotonin transporters (5-HTT) causes less serotonin to be transported from the synapse into pre-synaptic neurons. This, in turn, increases the amount of serotonin present in the synapse. Initially, the increased serotonin binds to pre-synaptic autoreceptors found on the soma of raphe neurons and, through hyperpolarization, is able to cause inhibition of action potential firing from these neurons and thus, causes a decrease in serotonin release (Figure 4). However, chronic administration of 5-HT1A agonists or SSRIs induces 5-HT1A autoreceptor desensitization in the raphe nucleus of the midbrain, but not of the post-synaptic hetero-receptors found in the hippocampus (Banerjee et al., 2007; de Montigny and Blier, 1984; Pineyro and Blier, 1999). Once this receptor desensitization occurs, the lack of autoreceptor inhibition allows increased serotonin to be released and bind post-synaptically to 5-HT1A hetero-receptors, therefore inducing the anxiolytic and anti-depressive effects of SSRIs (Banerjee et al., 2007). In addition, newer approaches to treat depression, such as combined serotonin/norepinephrine reuptake inhibitors (SNRIs) and deep brain stimulation (DBS) have been shown to modulate 5-HT (Howland et al., 2008; Sartorius and Henn, 2007).
Figure 4.
Effect of SSRI treatment on the 5-HT1A receptor. Efficacy of SSRI treatment putatively occurs through down-regulation of the raphe 5-HT1A autoreceptors (red squares) that is seen after chronic treatment. These autoreceptors act to inhibit 5-HT postsynaptic neuronal release. Sustained administration of 5-HT1A agonists or SSRIs induces the internalization of 5-HT1A autoreceptors in the raphe nucleus of the midbrain (note reduction of 5-HT1A autoreceptors as treatment progresses from ‘acute’ to ‘chronic’. The reduction in autorecep-tor inhibition following chronic SSRI treatment allows increased neuronal firing and thus, more serotonin to be released. It is this subsequent increase in serotonin release in which SSRIs are able to exert their anxiolytic and antidepressant effects. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Animal studies
Animal models have allowed for the understanding and visualization of 5-HT1A modulations in MDD: most notably the murine and primate paradigms. In the murine model, the tail suspension test (TST) has been used to assess behavioral withdrawal in mice. This test is based on the idea that mice subjected to the stress of being suspended by their tail will actively try to escape up until a point when they develop an immobile posture. This immobile posture is used as a corollary for a depressive-like state or learned helplessness, in which the individual is unresponsive to unpleasant stimuli (Liu and Gershenfeld, 2003; Steru et al., 1985). There exist other animal tests that are used to model MDD and identify antidepressant efficacy. The behavior despair test (BDT), also known as the forced swim test (FST) is considering a measure of acute depression and is based on placing a rodent in a cylinder filled with water and observing how much time is required for the rodent to become immobile after multiple escape attempts. It has been shown that antidepressant treatment decreases the duration of immobility exhibited by the rodents (Castagne et al., 2009; Porsolt et al., 1977). A 2002 post-mortem autoradiography study found that mice bred to expressive helpless and more depressive behavior exhibited significantly higher levels of 5-HT1A, measured via the 5-HT1A agonist 3H-8-OH-DPAT (Table 1), than non-helpless wild type mice in several cortical and limbic brain regions. These regions included: medial prefrontal cortex, CA3 field of the hippocampus, dentate gyrus, baso-medial amygdala and the medial raphe nuclei of the midbrain (Naudon et al., 2002). Furthermore, the same group also treated both helpless and wild type mice with the SSRI fluoxetine for 3 weeks and reassessed the mice for depressive behavior. It was found that the fluoxetine treatment significantly reduced depressive affect in the helpless mice as assessed by TST scores, while leaving the behavior of the wild type mice unaltered. Finally, the elevated 3H-8-OH-DPAT binding densities initially seen in the helpless mice were no longer observed in any of the aforementioned brain regions after SSRI treatment. Thus, it was concluded that treatment with fluoxetine was able to mitigate helplessness, which is used as a surrogate marker of depression, in these mice and that the drug was able to decrease the over-expression of 5-HT1A receptors seen initially in the helpless cohort (Naudon et al., 2002). In 2010, a study was published that used genetically modified mice as an elegant model to show the relationship between 5-HT1A autoreceptor levels, vulnerability to stress and anti-depressant response. By modulating 5-HT1A autoreceptor levels, it was shown that mice with higher 5-HT1A autoreceptor levels had decreased resilience to stress, increased depressive phenotype (Richardson-Jones et al., 2010). As such, causality was established between firing rate, activity of 5-HT1A autoreceptors and development of the depressive phenotype, rather than de novo levels of serotonin in the brain.
Table 1.
5-HT1A PET tracers.
| Antagonist | Agonist |
|---|---|
| [3H]WAY100635a | [3H]8-OH-DPATa |
| [carbonyl-11C]WAY100635 | [11C]MPT |
| [carbonyl-11C]DWAY | |
| [11C](R)-RWAY | |
| [18F](cis)-FCWAY | |
| [18F]MPPF | |
| [18F]MeFWAY |
This table is adapted from Kumar and Mann (2014).
Used in autoradiography.
A 2003 review article reported that between 1966 and 2003, a MEDLINE search showed that 24 case reports had been published which describe the onset of depressive symptoms occurring in human patients after treatment with the dermatological acne medication Accutane (Hull and D'Arcy, 2003). It was later shown that 13-cis-retinoic acid, the active ingredient in Accutane, is able to induce depression-related behaviors in adolescent male mice and that the drug can modulate serotonergic neurotransmission in vitro (O'Reilly et al., 2007). Using the RN46A-B14 cell line derived from rat embryonic raphe nuclei, cells exposed to 13-cis-retinoic acid showed increased 5-HT1A and serotonin transporter protein levels, measured via Western Blot analysis, as compared to the unexposed, control cell line. Given the roles of 5-HT1A autoreceptors and the serotonin transporter in serotonin signaling, increasing levels of both structures would cause decreased serotonin release from serotonergic raphe neurons, reduce serotonin availability in the synapse and thereby, induce to the development of the depressive symptoms which were seen in both the humans and mice exposed to 13-cis-retinoic acid (O'Reilly et al., 2007).
5. Sex differences
The female ovarian sex hormones, estrogen and progesterone, have also been shown to be connected to modulation of mood (Pecins-Thompson and Bethea, 1999). The decline in these sex steroids that occur during childbirth and menopause have been correlated with the development of depression (Gitlin and Pasnau, 1989). Similarly hormone replacement therapy, such as transdermal estrogen, has been shown to alleviate the depression in some of these subjects (Gregoire et al., 1996). It has been hypothesized that the depression observed following changes in female sex hormone levels is due to parallel changes within the serotonin system that are caused by the changes in sex hormone levels (Eriksson et al., 1995; Halbreich and Tworek, 1993; Parry, 1997; Su et al., 1997). Animal literature has shown that 5-HT1A binding is modulated by estrogen levels in females (Flugge et al., 1999; Frankfurt et al., 1994; Maswood et al., 1995; Pecins-Thompson and Bethea, 1999; Zhang et al., 1999). An early rat study was able to show that sex hormones, specifically estrogen and progesterone, are able to modulate 5-HT1A autoreceptors throughout the course of the menstrual cycle (Maswood et al., 1995). It has also been reported that 5-HT1A autoreceptors located in the dorsal raphe nucleus, whose main function is to release serotonin in the brain, are negatively regulated by female sex hormones and that in rhesus macaques, both estrogen and progesterone induce down-regulation of 5-HT1A autoreceptor mRNA (Maswood et al., 1995; Pecins-Thompson and Bethea, 1999). These findings have been replicated in rats by showing that estradiol treatment induces a decrease in 5-HT1A mRNA expression within the dorsal raphe nucleus (Birzniece et al., 2001). The ability of female sex hormones to modulate raphe 5-HT1A levels is extremely interesting due to its aforementioned role in the development of MDD (Kishi et al., 2013; Miller et al., 2009; Miller et al., 2013; Parsey et al., 2010; Parsey et al., 2006; Stockmeier et al., 1998). Of note, it is important to realize that since female sex hormones have been shown to reduce 5-HT1A RNA in raphe, it would be predicted that 5-HT neurotransmission would be enhanced. However, this has not been reported. This inconsistency was addressed by a 2015 study, which showed that “withdrawal” from female sex hormones i.e. acute decline in estradiol levels, induced depressive symptoms. This randomized, double blind, placebo controlled study used a cohort of perimenopausal women who had been prescribed estradiol as hormone replacement therapy and then were randomized to a parallel design to receive either continued estradiol or a placebo. Interestingly, depressive symptoms only occurred in women with a previous history of perimenopausal depression who had been randomized to the placebo cohort, suggesting that there are a subset of the population who are inherently predisposed to developing MDD and who are more susceptible to fluctuations in sex hormone levels (Schmidt et al., 2015). These study findings have added much support to the probable connection between female sex hormones and regulation of mood. For this reason, studies of serotonin in females may need to adjust for the current phase of the menstrual cycle.
6. Post-mortem studies
Initial whole brain post-mortem studies of MDD examining 5-HT1A found modulation of 5-HT1A receptor levels was restricted to subcortical regions. In a cohort of depressed suicide victims, a lower density of 5-HT1A receptors in the hippocampus and amygdala were observed (Cheetham et al., 1990). Similarly, several other post-mortem studies using depressed, non-suicide cohorts have shown reduced receptor density in cortical regions such as the ventral prefrontal cortex (Bowen et al., 1989), a region involved in behavioral inhibition (Bechara et al., 2000), and temporal polar cortex in depressed subjects as compared to healthy control subjects. One interesting point to note regarding the aforementioned findings is that a study using post-mortem tissue from depressed suicide victims found increased 5-HT1A binding in the ventrolateral prefrontal cortex (Arango et al., 2001). However, further inquiry showed that in this particular study, some of the subjects had co-morbid alcoholism and that the reported decrease in 5-HT1A binding between MDD and control subjects could be attributed to the effects of chronic alcohol use on the subjects’ serotonergic neurochemistry (Savitz et al., 2009).
Due to its role in the serotonergic system, some studies restricted their examination specifically to the role of raphe 5-HT1A autoreceptors in MDD. Using autoradiography, it was reported that the caudal aspect of the dorsal raphe nucleus (DRN) exhibited lower 5-HT1A binding in depressed suicide victims as compared with healthy controls, while the opposite effect i.e. increased binding, was seen in the rostral aspect of the DRN (Boldrini et al., 2008; Stockmeier et al., 1998). It is thought that increased autoreceptor binding in the rostral aspect of the DRN may be responsible for the decreased serotonin release previously observed within the ventromedial prefrontal cortices of depressed subjects, as compared to healthy controls (Boldrini et al., 2008).
As described, post-mortem studies have reported opposing findings. It important to note that confounding variables such as effect of psychiatric and non-psychiatric medications on brain neurochemistry, sex differences, co-morbid psychiatric diagnosis, substance abuse, and post-mortem interval may contribute to the lack of consensus on many psychiatric studies (Hafeman et al., 2012; Phillips et al., 2008).
7. Positron emission tomography (PET)
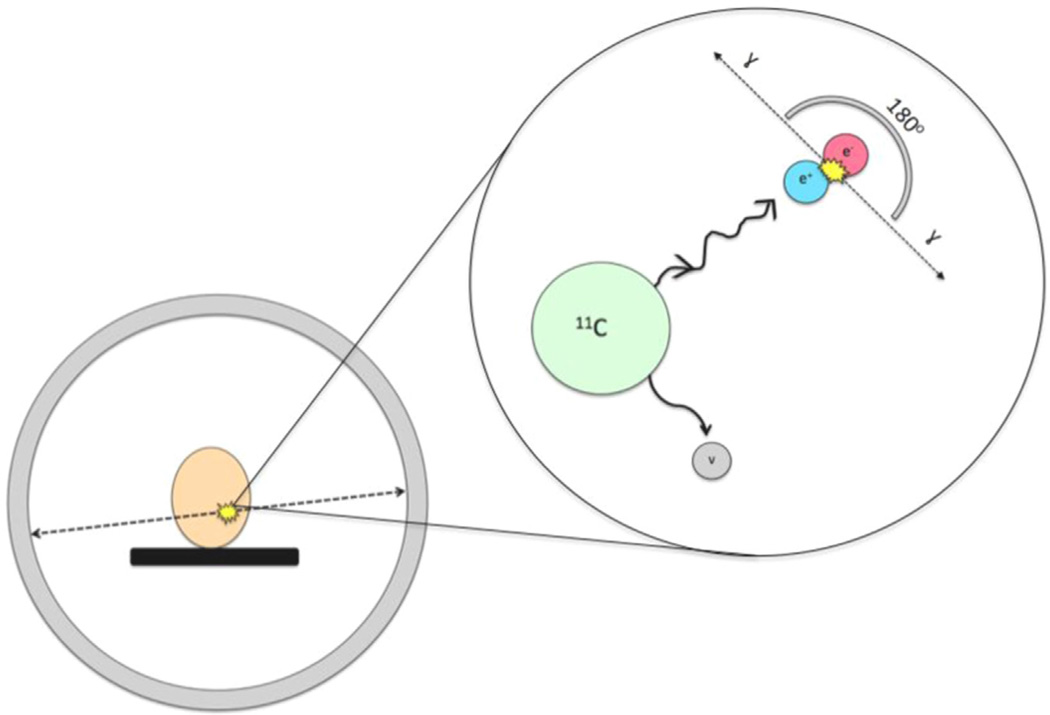
With positron emission tomography (PET), physiological functions including metabolism, neurotransmitter systems and drug occupancy, can be assessed, in vivo, with minimum disturbance to a subject's physiological homeostasis (Berger, 2003). PET involves several fundamental physical principles. Using a type of particle accelerator known as a cyclotron, charged particles are accelerating at extremely high speeds. These accelerated particles are then used to bombard stable atoms in order to produce a positronemitting isotope. In the example of the radiotracer [carbonyl-11C]WAY-100635, a selective 5-HT1A receptor antagonist that allows for the in vivo visualization of the 5-HT1A receptor (Table 1), 11C is produced using this principle. The radioactive 11C is then incorporated into the WAY-100635 precursor molecule, thus creating [carbonyl-11C] WAY-100635 (Gunn et al., 1998). Once injected into the subject's peripheral vein, usually an antecubital vein, the tracer travels to the brain, and throughout the body, where it can either bind specifically to the 5-HT1A receptors, non-specifically to other proteins or exist freely in tissue. Because the nucleus of the 11C undergoes radioactive decay, it periodically emits positrons. Once a positron is emitted, the particle will travel until it collides with an electron, usually in nearby tissue, and in doing so will cause an annihilation reaction to occur that converts mass, in the form of a positron and electron, into energy, in the form of two photons (Berger, 2003). Importantly, these photons are emitted at an angle of exactly 180 degrees and are then detected via scintillation crystals within the PET camera that surrounds the subject (Figure 5). Scintillation is the process in which ionizing radiation is able to induce luminescence i.e. the crystals are able to absorb the emitted photons and produce light than can then be converted into an interpretable electrical signal (Berger, 2003). Neuroimaging studies using the radiotracer [carbonyl-11C]WAY-100635 are thus able to examine the in vivo binding of the 5-HT1A receptor in subjects with MDD, allowing for an improved understanding of the connection between the 5-HT1A receptor and the pathophysiology of MDD itself (Drevets et al., 1999).
Figure 5.
Mechanism of PET camera. Magnified area shows annihilation reaction that occurs when a positron, emitted from the radioactive PET tracer, collides with an electron, usually in nearby tissue. This reaction is able to convert mass, in the form of a positron and electron, into energy, in the form of two photons. Importantly, these photons are emitted at an angle of exactly 180° and are then detected via scintillation crystals within the PET camera that surrounds the subject’s head.
PET has been used clinically as a qualitative measure. For research purposes, quantification of radiotracer uptake is required. The quantity we are trying to measure is the total number of available target (Bmax), in this case the 5-HT1A receptor. The relative concentration of specifically bound tracer is termed binding potential. Various forms of the binding potential can be calculated, depending on the tracer concentration used for comparison (Innis et al., 2007). In 2007, a collaboration review paper was published that defined and standardized the nomenclature for these various types of binding potentials (Table 2). For example, the term BPF refers to the ratio, at equilibrium, of the concentration of specifically bound tracer in tissue to the concentration of free tracer in plasma. BPND, refers to the ratio of specifically bound tracer to the concentration of non-displaceable tracer in tissue. Bmax refers to the maximum number of binding sites. Non-displaceable tracer in tissue is defined as free tracer present in tissue as well as tracer that is nonspecifically bound. This non-displaceable concentration is usually estimated from tracer uptake in a region of the brain that is assumed to be devoid of the tracer target (a reference region, (Innis et al., 2007). Like with any experimental methodology, each outcome measure has both advantages and disadvantages. Although BPF is closest to in vitro estimates of Bmax, the disadvantage of this outcome is that it requires blood sampling during the experiment and thus, makes the study more invasive. Although an advantage of BPND is that it may be calculated without blood sampling, the disadvantage of this outcome measure is that is requires an assumption to be made that the reference region is virtually devoid of the receptor of interest (Innis et al., 2007). If the reference region being used does not have a negligible receptor density, then the measured BPND values will be less accurate.
Table 2.
In vivo Binding potential definitions
| Outcome measure: | In vitro equation | What specific tracer binding is compared to: |
Units | Requirement of blood sampling |
|---|---|---|---|---|
| BPF | =Bavail/KD | Free plasma concentration | mL cm−3 | Yes |
| BPND | =fND*Bavail/KD | Nondisplacable uptake | Unitless | No |
Bavail is the concentration of unoccupied receptors, KD is the tracer dissociation constant, fp is the free fraction of tracer in the nondisplacable compartment. This table is adapted from Innis et al. (2007).
The introduction of PET imaging facilitated great strides in understanding the involvement of the 5-HT1A receptor in MDD. Early studies reported that subjects with MDD exhibit reduced 5-HT1A binding in several ROIs including: the medial temporal cortex and hippocampus as well as the midbrain's raphe nuclei (Drevets et al., 2000; Drevets et al., 1999). These findings were then replicated by several groups that also reported binding reductions MDD subjects within the frontal, temporal and limbic cortices of both medicated and unmedicated individuals (Bhagwagar et al., 2004; Sargent et al., 2000). However, a 2006 study reported higher rather than lower 5-HT1A binding in MDD subjects, as compared to healthy controls, across 13 cortical and subcortical ROIs (Parsey et al., 2006). The difference in binding polarity was initially attributed to differences either in study population or the study's methodology (Parsey et al., 2010). Moreover, it was found that the increase in 5-HT1A binding between controls and MDD subjects, reported in the 2006 study, was only present when the MDD sample was comprised of those individuals who had never been exposed to antidepressant medications (labeled as antidepressant naïve or AN).
This debate was ultimately resolved when findings of increased 5-HT1A binding in MDD, as compared to controls, were duplicated and then triplicated (Miller et al., 2013; Parsey et al., 2010): showing that when BPF is used, 5-HT1A binding is elevated in MDD when compared to controls. The rationale behind the discrepant findings regarding the direction of 5-HT1A modulation between MDD and healthy control subjects lies in the methodology used and reference region specific binding. The calculation of BPF, the closest metric to in vitro binding potential, does not involve dividing by reference region binding, whereas calculation of BPND does (Innis et al., 2007). A 2013 study was able to show that, using the same data set, there is elevated 5-HT1A binding when BPF is calculated and decreased 5-HT1A binding when BPND is used. This is due to a low, but detectable level of 5-HT1A binding in the reference region which is elevated in the depressed population (Hesselgrave and Parsey, 2013). With regard to increased raphe 5-HT1A receptors in suicide, a recent PET paper showed the association of an increase in raphe 5-HT1A binding with depressed violent vs. non-violent or non-suicide attempters (Sullivan et al., 2015).
8. C(−1019)G promoter polymorphism in depression
In humans, the gene coding for the 5-HT1A receptor is located on chromosome 5q11.2–13 (HTR1A) (Albert and Lemonde, 2004). Because MDD has been associated with altered 5-HT1A levels, specifically higher levels of 5-HT1A autoreceptors, it was thought that abnormal regulation of 5-HT1A mRNA transcription may underlie the development of psychopathologies such as MDD. An initial polymerase chain reaction (PCR) study, used in this case for DNA sequencing, was performed on the repressor region of the 5-HT1A gene in order to identify possible polymorphisms causing up-regulation of the 5-HT1A autoreceptor and thus predisposing an individual to mental illnesses such as MDD (Lemonde et al., 2003). A single nucleotide polymorphism (SNP) showing a C/G change was found at –1019 of the repressor region of the 5-HT1A gene. Because of this polymorphism's location in a transcriptional regulatory region of the 5-HT1A gene, the next step to was identify whether transcriptional factors found in the cells of the raphe, the origin of serotonergic neurons, could bind to the C(−1019) allele, thereby influencing the level of 5-HT1A autoreceptor synthesis. In these initial experiments, two transcriptional factors were identified: NUDR and Hes5. Note that NUDR is also currently known as nuclear deformed epidermal auto-regulatory factor (NUDR/DEAF-1/Deaf-1) (Szewczyk et al., 2009). Using immunocytochemistry in rat brains, it was found that NUDR/DEAF-1 was mainly found within serotonin neurons of the raphe, along with postsynaptic neurons in the frontal cortex and hippocampus (Lemonde et al., 2003). Furthermore, although Hes5 was found to be expressed in the same regions as NUDR/DEAF-1, expression of Hes5 is seen mainly during early development (Albert and Lemonde, 2004). In terms of ability to modulate transcriptional activity, both of these transcription factors are able to bind to and repress the transcriptional activity of the C(−1019) allele. However, this transcriptional repression exhibited by both NUDR/DEAF-1 and Hes5 is greatly reduced in the presence of the G(−1019) allele (Albert and Lemonde, 2004). From this, it was theorized that the single nucleotide C(−1019)G polymorphism is able to regulate the expression of the 5-HT1A gene in neurons of the raphe (Albert and Lemonde, 2004) via the level of repression of the 5-HT1A gene transcriptional promoter site. In terms of translational implication, it was thought that individuals expressing the homozygous G(−1019) allele would be at increased risk for developing MDD relative to those individuals who are homozygous for the C(−1019) allele. Since the G(−1019) allele is unable to bind NUDR/DEAF-1, the subsequent lack of NUDR/DEAF-1 transcriptional repression would lead to over-expression of 5-HT1A autoreceptors in the raphe, as compared to those individuals homozygous for the C(−1019) allele, and thus, decrease serotonergic firing (Figure 6). Moreover, a recent in vivo study using Deaf-1 knock-out mice further illustrated that 5-HT1A autoreceptors receptors are up-regulated specifically in raphe (Czesak et al., 2012). As previously described, this increase in 5-HT1A autoreceptor expression in the raphe has been associated with the development of the MDD phenotype (Albert and Lemonde, 2004; Arango et al., 2001; Boldrini et al., 2008; Miller et al., 2009; Miller et al., 2013; Parsey et al., 2010; Parsey et al., 2006).
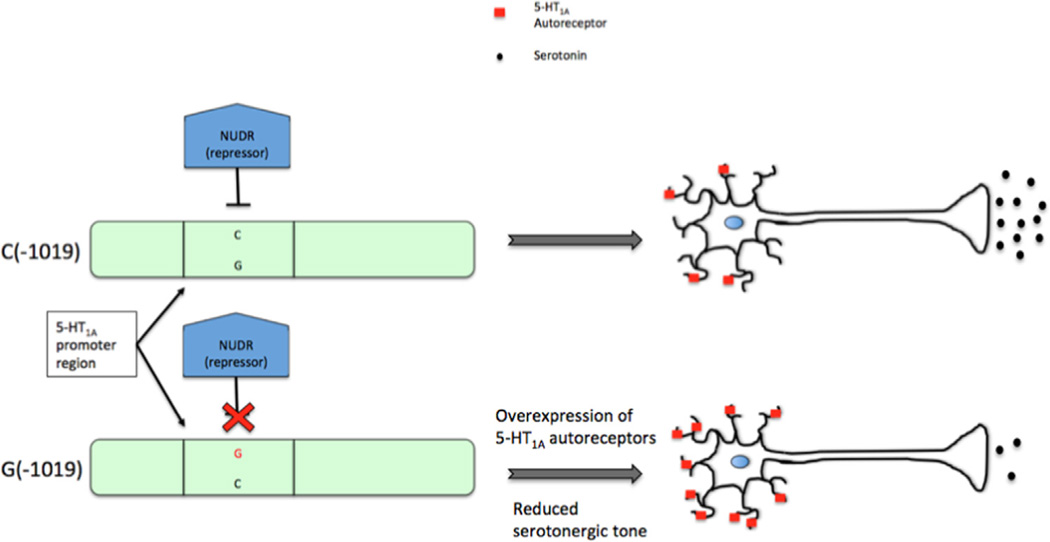
Figure 6.
Effect of of the C(−1019)G 5-HT1A functional polymorphism. In subjects possessing the C allele, NUDR/DEAF-1 (blue block) acts to repress the expression of 5-HT1A autoreceptors (red squares) to a normal level. However, in subjects who possess the G allele, NUDR/DEAF-1 is unable to bind to the regulatory region, thus leading to an overexpression of 5-HT1A autoreceptors, reduced serotonergic firing, and increased predisposition to the depressive phenotype. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
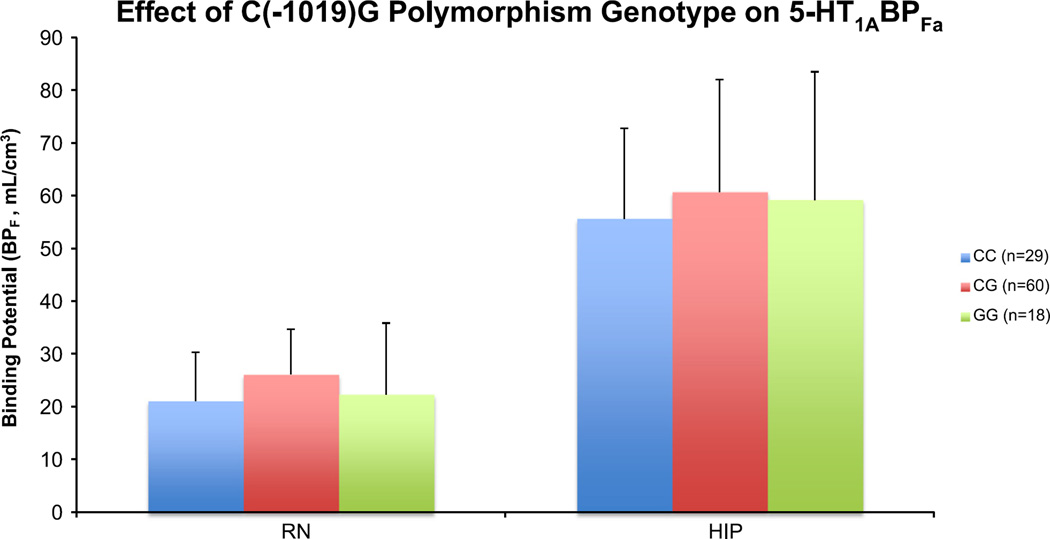
With the development of PET as a research modality, initial studies reported that the C(−1019)G polymorphism affects 5-HT1A receptor binding. It was reported that 5-HT1A BPF shows a stepwise increase in the raphe nuclei with G allele frequency in reference to the C(−1019)G 5-HT1A promoter polymorphism i.e. CC<CG<GG (Hesselgrave and Parsey, 2013; Parsey et al., 2006). However, this analysis was recently repeated with weighted means an increased sample size. Weighed means, which incorporate potential BPF errors, are more accurate since less reliable points are deweighted. In this analysis, no significant genotype effect was observed in the raphe or hippocampus for: controls (raphe: p=0.19, hippocampus: p=0.45), MDD subjects who had either never received antidepressant treatment or had not received antidepressant treatment within the previous 4 years (defined as Not Recently Medicated ‘NRM’) (raphe: p=0.62, hippocampus: p=0.60) and when all subjects are combined (raphe: p=0.22, hippocampus: p=0.39) (Figure 7) (Kaufman et al., 2015). Similarly, no genotype effect was seen in the raphe or hippocampus when genotype was coded for the presence of at least 1 G allele i.e. CC vs. CG/GG: controls (raphe: p=0.07, hippocampus: p=0.25), NRM MDD (raphe: p=0.78, hippocampus: p=0.46) and when all subjects are combined (raphe: p=0.10, hippocampus: p=0.17). Moreover, a 2007 also reported a lack of association between 5-HT1A C (−1019)G polymorphism and risk of depression (Anttila et al., 2007). This evidence suggests that genotype of the C (− 1019)G does not significantly contribute to 5-HT1A binding, as previously reported. By contrast, there have been several recent papers that suggest it may be an interaction between 5-HT1A and other gene polymorphisms that contributes to an increased risk of MDD (Kishi et al., 2009; Kishi et al., 2013). This phenomenon of a phenotype being dependent on the interaction between multiple genes is known as epistasis. It is important to keep in mind that environmental risk factors, such as life stress, may modulate the outcomes of these study findings and, perhaps, strengthen the association. A 2007 study reported that subjects carrying both the C (−1019)G polymorphism GG phenotype and at least one copy of the A allele of the brain derived neurotrophic factor (BDNF) G196A (Val66Met) polymorphism had a greater than three times higher risk of treatment resistant depression (Anttila et al., 2007). Moreover, a 2008 study was also able to provide in vivo evidence illustrating biologic epistasis between serotonergic and neurotrophic signaling, further suggesting that depression risk may be genetically determined by a combination of several polymorphism genotypes (Anttila et al., 2007; Pezawas et al., 2008).
Figure 7.
No significant C(−1019)G polymorphism genotype effect on 5-HT1A expression in the raphe and hippocampus. There is no stepwise increase in the raphe or hippocampus with G allele frequency: CC<CG<GG. The height of the bar indicates the weighted mean BPF, while the error bars represent the equivalent of standard error of each weighted mean. RN: Raphe, HIP: Hippocampus.
9. 5-HT1A binding potential as a biomarker for MDD diagnosis
Currently, there are no objective diagnostic tests one can perform to determine if an individual is depressed. The clinician must make this determination based on the patient's self-report and the clinician's judgment. This subjective system is prone to error and is also unrelated to the biological causes of depression. Another challenge in diagnosing MDD is that due to ambiguity in the diagnostic criteria, there are 945 ways to meet diagnostic criteria for a major depressive episode. Thus, two patients who share only one common symptom can be diagnosed with the same psychiatric illness. For instance, hypothetical “Patient A” may experience decreased interest/pleasure, weight gain, insomnia, sense of worthlessness and suicidality while hypothetical “Patient B” may experience decreased interest/pleasure, weight loss, hypersomnia, psychomotor retardation, and decreased concentration. According to DSM-IV criteria for Major Depressive Disorder (MDD), both “Patient A” and “Patient B” meet criteria for MDD, while only sharing one common symptom i.e. decreased interest/pleasure (APA, 1994). In the field of medicine, millions of dollars are spent on diagnostic testing for diseases such as coronary artery disease and certain types of cancers that, like MDD, have high morbidity (World Health, O, 2004). These diagnostic tests often use biomarkers to identify the presence of disease and are used to track effects of intervention. A biomarker is a characteristic that can be objectively measured and used as an indicator of either normal or pathogenic processes (Singh and Rose, 2009). Identification of a psychiatric biomarkers for MDD could help improve diagnostic classification and may aid in better classifying the great heterogeneity observed across MDD presentation into more specific sub-diagnostic categories as well as provide much needed evidence of the physiological underpinnings of this psychopathology (Singh and Rose, 2009). As pointed out by Peterson et al (Peterson and Weissman, 2011), a bio-marker for MDD could aid: in classifying the great heterogeneity observed across MDD presentation into identifiable sub-diagnostic categories and therefore allow more customized treatment strategies; the search for genetic and environmental factors; in identifying those likely to have a chronic course, be treatment resistant, or respond to medication vs. therapy; and in identifying those at increased risk for MDD.
10. The role of sex in 5-HT1A
To date, few studies have looked at the role of sex in the modulation of 5-HT1A specifically in MDD using PET in humans. However, a 2010 study showed that serotonergic differences exist in both healthy individuals and those with MDD across sexes. This study examined alpha-[(11)C]MTrp brain trapping, which is an index of serotonin synthesis (Frey et al., 2010). Sex differences in serotonin synthesis were seen in multiple regions of the prefrontal cortex and limbic system, which are involved in mood regulation. Another study looking into sex differences within the serotonergic system showed that although healthy females exhibit lower cortical trapping of alpha-[(11)C]MTrp than healthy males, females with MDD exhibit higher alpha-[(11) C]MTrp than males with MDD (Frey et al., 2010; Sakai et al., 2006). Several additional human PET studies have shown that individuals with Bipolar Depression (BD) and MDD exhibit higher 5-HT1A BPF using the radioligand [carbonyl-C-11]-WAY-100635 (Parsey et al., 2006; G. M. Sullivan et al., 2009), and that healthy females exhibit higher 5-HT1A BPF than men. Furthermore, post-hoc analysis in the BD study reported that, among the male subjects, both the main effect of diagnosis and the region by diagnosis interaction terms were statistically significant. In contrast however, the 5-HT1A BPF in female control subjects did not differ from the female BD subjects nor was the interaction term significant (G. M. Sullivan et al., 2009).
In order to elucidate sex differences in 5-HT1A binding, it was postulated that MDD and control cohorts should be stratified by sex and examined separately. In 2015, our group performed such a study using a cohort of previously published (Miller et al., 2013; Parsey et al., 2010; Parsey et al., 2006) and unpublished subjects, in order to determine whether doing so would provide insight into sex differences in MDD pathophysiology (Kaufman et al., 2015). This study reported that there was a significant difference between MDD and control subjects’ 5-HT1A BPF across 13 ROIs cortical and subcortical ROIs in males (67% higher binding in MDD compared to healthy controls). Furthermore, post hoc analysis assessing binding differences between control and MDD subjects, in each sex, showed that a region by diagnosis interaction was present in each sex such that certain regions exhibited greater binding differences between controls and MDD subjects than others. It was noted that the largest separation in binding between MDD and control subjects occurred in the raphe of the male cohort. In fact, when looking specifically at the raphe in males only, MDD subjects had 132% higher binding as compared to the controls (p=0.000) (Kaufman et al., 2015).
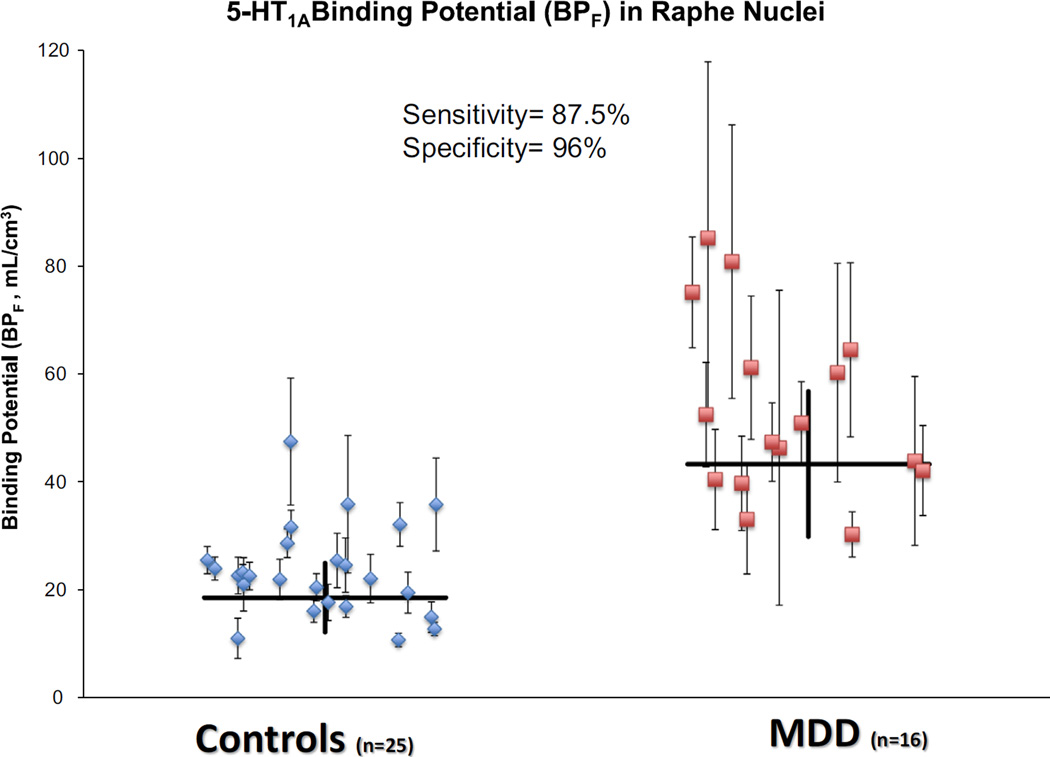
This finding led to the idea that 5-HT1A BPF values in the raphe of male subjects can be used as a biomarker to diagnose MDD. By defining a diagnostic threshold (~40 mL/cm3), it was found that 5-HT1A BPF values in the raphe of male subjects allows separation of MDD from control subjects with a diagnostic sensitivity of 87.5%, and a specificity of 96.0% (Figure 8). The findings of this particular study (Kaufman et al., 2015) may have very important clinical implications in that the ability to use elevated 5-HT1A as a biomarker or endophenotype of MDD could significantly advance our understanding of this psychopathology. The last possibility is especially important since MDD is only 31–42% genetically determined. In comparison, the genetic determination of schizophrenia is 70% (Sullivan et al., 2000). For this reason, clinicians cannot accurately predict who will develop the illness based only on family history (Sullivan et al., 2000). Being able to quantify their risk of developing MDD, would allow for preventative strategies to be taken to improve their future mental health outcomes. The current MDD diagnostic criteria do not provide insight into these questions to the extent that a biomarker would.
Figure 8.
[11C]WAY-100635 binding potential (BPF) estimates for the 5-HT1A receptor in male control and male MDD subjects in the raphe nuclei. Blue diamonds or red squares represent single measurements of raphe BPF in control and MDD subjects, respectively. Thin capped vertical error bars represent standard errors computed using a bootstrap algorithm that takes into account errors in metabolite, plasma, and image data. Weighted group mean and standard error of the weighted mean of BPF are represented by thick horizontal lines and thick vertical lines, respectively. BPF, binding potential; [11C]WAY- 100635, N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclo-hexanecarboxamide; 5-HT1A, serotonin-1A receptor.
11. Conclusion
Our knowledge regarding the role of the 5-HT1A receptor in the development of MDD has greatly evolved, established atop a foundation of preclinical animal and post-mortem literature. The 5-HT1A system is highly complex and improved neuroimaging technologies and methodologies along with innovations in genetic studies have all aided in a more complete comprehension of how modulations in the 5-HT1A receptor, whether inherited or drug/hormone induced, lead to expression of the MDD phenotype. Current paradigms suggest that subjects with MDD exhibit elevated 5-HT1A binding across several brain regions that are implicated in mood regulation. Genetic research continues to elucidate various polymorphisms, which may interact, through epistasis, and predispose the depressive phenotype. And, while there remain no biomarkers of MDD that are used clinically, future discovery of objective biomarkers and/or endophenotypes will likely drive the field. Exploring the diagnostic and prognostic potential of PET and other neuroimaging modalities will continue to advance our understanding of MDD and the role of 5-HT1A in this prevalent disease.
Acknowledgments
We acknowledge the biostatistical support from the Biostatistical Consulting Core at School of Medicine, Stony Brook University.
Role of funding source
The work was supported by the following grants: R01MH074813 (PI: Parsey), R01MH090276 (PI: Parsey), and K01MH091354 (PI: DeLorenzo) awarded by the National Institutes of Health, a Clinical and Translational Science Award (CTSA) from Columbia University, and grants from the American Foundation for the Prevention of Suicide (AFSP) and the National Alliance for Research on Schizophrenia and Depression (NARSAD).
Footnotes
Contributors
N/A.
Conflict of interest
The authors report no conflicts of interest.
References
- Agren H. Symptom patterns in unipolar and bipolar depression correlating with monoamine metabolites in the cerebrosp-inal fluid: II Suicide. Psychiatry Res. 1980;3(2):225–236. doi: 10.1016/0165-1781(80)90039-6. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10(6):575–593. doi: 10.1177/1073858404267382. http://dx.doi.org/10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Angst J, Dobler-Mikola A. Do the diagnostic criteria determine the sex ratio in depression? J. Affect. Disord. 1984;7(3–4):189–198. doi: 10.1016/0165-0327(84)90040-5. [DOI] [PubMed] [Google Scholar]
- Anttila S, Huuhka K, Huuhka M, Rontu R, Hurme M, Leinonen E, Lehtimaki T. Interaction between 5-HT1A and BDNF genotypes increases the risk of treatment-resistant depression. J. Neural Transm. 2007;114(8):1065–1068. doi: 10.1007/s00702-007-0705-9. http://dx.doi.org/10.1007/s00702-007-0705-9. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM- IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25(6):892–903. doi: 10.1016/S0893-133X(01)00310-4. http://dx.doi.org/10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688(1–2):121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Åsberg M, Bertilsson L, Martensson B, Scalia Tomba G, Thoren P, Träskman-Bendz L. CSF monoamine metabolites in melancholia. Acta Psychiatr. Scand. 1984;69:201–219. doi: 10.1111/j.1600-0447.1984.tb02488.x. [DOI] [PubMed] [Google Scholar]
- Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V. “Serotonin depression”-a biochemical subgroup within the affective disorders? Science. 1976;191:478–480. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol. Psychiatry. 2008;13(5):507–513. doi: 10.1038/sj.mp.4002143. http://dx.doi.org/10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Mehta M, Kanjilal B. The 5-HT1A receptor: a signaling hub linked to emotional balance. In: Chattopadhyay A, editor. Serotonin Receptors in Neurobiology. Boca Raton (FL): CRC Press; 2007. [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl. J. Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. http://dx.doi.org/10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berger A. How does it work? Positron emission tomography. BMJ. 2003;326(7404):1449. doi: 10.1136/bmj.326.7404.1449. http://dx.doi.org/10.1136/bmj.326.7404.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol. Psychiatry. 2004;9(4):386–392. doi: 10.1038/sj.mp.4001401. http://dx.doi.org/10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Billard T, Le Bars D, Zimmer L. PET radiotracers for molecular imaging of serotonin 5-HT1A receptors. Curr. Med. Chem. 2014;21(1):70–81. doi: 10.2174/09298673113209990215. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Johansson IM, Wang MD, Seckl JR, Backstrom T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology. 2001;74(2):135–142. doi: 10.1159/000054679. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J. Psychiatr. Res. 2008;42(6):433–442. doi: 10.1016/j.jpsychires.2007.05.004. http://dx.doi.org/10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DM, Najlerahim A, Procter AW, Francis PT, Murphy E. Circumscribed changes of the cerebral cortex in neuropsychiatric disorders of later life. Proc. Natl. Acad. Sci. USA. 1989;86(23):9504–9508. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C, Hedberg E, Andersch MA, Sundblad B. The serotonin reuptake inhibitor paroxetine is superior to the noradrenaline reuptake inhibitor maproltiline in the treatment of premenstrual syndrome. Neuropsychopharmacology. 1995;12:167–176. doi: 10.1016/0893-133X(94)00076-C. [DOI] [PubMed] [Google Scholar]
- Castagne V, Porsolt RD, Moser P. Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur. J. Pharmacol. 2009;616(1–3):128–133. doi: 10.1016/j.ejphar.2009.06.018. http://dx.doi.org/10.1016/j.ejphar.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT1 binding sites in depressed suicides. Psychopharma-col. (Berl.) 1990;102(4):544–548. doi: 10.1007/BF02247138. [DOI] [PubMed] [Google Scholar]
- Claustre Y, Benavides J, Scatton B. Potential mechanisms involved in the negative coupling between serotonin 5-HT1A receptors and carbachol-stimulated phosphoinositide turnover in the rat hippocampus. J. Neurochem. 1991;56(4):1276–1285. doi: 10.1111/j.1471-4159.1991.tb11422.x. [DOI] [PubMed] [Google Scholar]
- Czesak M, Le Francois B, Millar AM, Deria M, Daigle M, Visvader JE, Albert PR. Increased serotonin-1 A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. J. Biol. Chem. 2012;287(9):6615–6627. doi: 10.1074/jbc.M111.293027. http://dx.doi.org/10.1074/jbc.M111.293027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montigny C, Blier P. Effects of antidepressant treatments on 5-HT neurotransmission: electrophysiological and clinical studies. Adv. Biochem. Psychopharmacol. 1984;39:223–239. [PubMed] [Google Scholar]
- Dencker SJ, Malm U, Roos B, Werdinius B. Acid monoamine metabolites of cerebrospinal fluid in mental depression and mania. J. Neurochem. 1966;13:1545–1548. doi: 10.1111/j.1471-4159.1966.tb04320.x. [DOI] [PubMed] [Google Scholar]
- Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J. Neurochem. 2001;78(6):1185–1200. doi: 10.1046/j.1471-4159.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1 A receptor imaging in depression. Nuclear Med. Biol. 2000;27(5):499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol. Psychiatry. 1999;46(10):1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Flugge G, Pfender D, Rudolph S, Jarry H, Fuchs E. 5HT1A–receptor binding in the brain of cyclic and ovariecto-mized female rats. J. Neuroendocr. 1999;11(4):243–249. doi: 10.1046/j.1365-2826.1999.00317.x. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, McKittrick CR, Mendelson SD, McEwen BS. Effect of 5,7-dihydroxytryptamine, ovariectomy and gonadal steroids on serotonin receptor binding in rat brain. Neuroendocrinology. 1994;59(3):245–250. doi: 10.1159/000126665. [DOI] [PubMed] [Google Scholar]
- Frey BN, Skelin I, Sakai Y, Nishikawa M, Diksic M. Gender differences in alpha-[(11)C]MTrp brain trapping, an index of serotonin synthesis, in medication-free individuals with major depressive disorder: a positron emission tomography study. Psychiatry Res. 2010;183(2):157–166. doi: 10.1016/j.pscychresns.2010.05.005. http://dx.doi.org/10.1016/j.pscychresns.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Wong DT. Serotonin uptake and serotonin uptake inhibition. Ann. N.Y. Acad. Sci. 1990;600:68–78. doi: 10.1111/j.1749-6632.1990.tb16873.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: Their projections in the guina-pig small intestine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- Gitlin M, Pasnau R. Psychiatric syndromes linked to reproductive function in women: a review of current knowledge. Am. J. Psychiatry. 1989;146:1413–1422. doi: 10.1176/ajp.146.11.1413. [DOI] [PubMed] [Google Scholar]
- Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang YY, Mann JJ, Parsey RV. Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol. Psychiatry. 2013;74(1):26–31. doi: 10.1016/j.biopsych.2012.11.012. http://dx.doi.org/10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006):930–933. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, Lammertsma AA. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage. 1998;8(4):426–440. doi: 10.1006/nimg.1998.0379. http://dx.doi.org/10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- Halbreich H, Tworek U. Altered serotonergic activity in women with dysphoric premenstrual syndromes. Int. J. Psychiatry Med. 1993;23:1–27. doi: 10.2190/J2W0-RTGD-NYKK-FF77. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. http://dx.doi.org/10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997;745(1–2):96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9(3):155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgrave N, Parsey RV. Imaging the serotonin 1 A receptor using [11C]WAY100635 in healthy controls and major depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368(1615):20120004. doi: 10.1098/rstb.2012.0004. http://dx.doi.org/10.1098/rstb.2012.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland RH, Wilson MG, Kornstein SG, Clayton AH, Trivedi MH, Wohlreich MM, Fava M. Factors predicting reduced antidepressant response: experience with the SNRI duloxetine in patients with major depression. Ann. Clin. Psychiatry. 2008;20(4):209–218. doi: 10.1080/10401230802437639. http://dx.doi.org/10.1080/10401230802437639. [DOI] [PubMed] [Google Scholar]
- Hull P, D'Arcy C. Isoretinoin use and subsequent suicide. Am. J. Clin. Dermatol. 2003;4:493–505. doi: 10.2165/00128071-200304070-00005. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. http://dx.doi.org/10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jonnakuty C, Gragnoli C. What do we know about serotonin? J. Cell. Physiol. 2008;217:301–306. doi: 10.1002/jcp.21533. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Nordstrom AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39(3):1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. http://dx.doi.org/10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Marttunen M, Karlsson H, Kaprio J, Hillevi A. Minor change in the diagnostic threshold leads into major alteration in the prevalence estimate of depression. J. Affect. Disord. 2010;122(1–2):96–101. doi: 10.1016/j.jad.2009.06.025. http://dx.doi.org/10.1016/j.jad.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Sullivan GM, Yang J, Ogden RT, Miller JM, Oquendo MA, DeLorenzo C. Quantification of the Serotonin 1A receptor using PET: identification of a potential biomarker of major depression in males. Neuropsychopharmacology. 2015;40(7):1692–1699. doi: 10.1038/npp.2015.15. http://dx.doi.org/10.1038/npp.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR National Comorbidity Survey, Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. http://dx.doi.org/10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsunoka T, Ikeda M, Kawashima K, Okochi T, Kitajima T, Iwata N. Serotonin 1A receptor gene and major depressive disorder: an association study and meta-analysis. J. Hum. Genet. 2009;54(11):629–633. doi: 10.1038/jhg.2009.84. http://dx.doi.org/10.1038/jhg.2009.84. [DOI] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Fukuo Y, Okochi T, Matsunaga S, Umene-Nakano W, Iwata N. The serotonin 1A receptor gene confer susceptibility to mood disorders: results from an extended meta-analysis of patients with major depression and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(2):105–118. doi: 10.1007/s00406-012-0337-4. http://dx.doi.org/10.1007/s00406-012-0337-4. [DOI] [PubMed] [Google Scholar]
- Kumar JS, Mann JJ. PET tracers for serotonin receptors and their applications. Cent. Nerv. Syst. Agents Med. Chem. 2014;14(2):96–112. doi: 10.2174/1871524914666141030124316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B, Khare SK, Ram VJ, Goel A, Olivier B, Soudijn W, Wang QM. Progress in Drug Research: Birkhäuser Basel. 2012 [Google Scholar]
- Parry LB. Psychobiology of premenstrual dysphoric disorder. Semin. Reprod. Endocrinol. 1997;15:55–68. doi: 10.1055/s-2008-1067968. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J. Neurosci. 2003;23(25):8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. An exploratory factor analysis of the Tail Suspension Test in 12 inbred strains of mice and an F2 intercross. Brain Res. Bull. 2003;60(3):223–231. doi: 10.1016/s0361-9230(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Lovenberg W, Jequier E, Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science. 1967;155(3759):217–219. doi: 10.1126/science.155.3759.217. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21(2 Suppl):99S–105S. doi: 10.1016/S0893-133X(99)00040-8. http://dx.doi.org/10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Mann JJ, et al. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch. Gen. Psychiatry. 1992;49:442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- Manocha M, Khan WI. Serotonin and GI disorders: an update on clinical and experimental studies. Clin. Transl. Gastroenterol. 2012;3:e13. doi: 10.1038/ctg.2012.8. http://dx.doi.org/10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz M, Verge D, Gozlan H, Pichat L, Hamon M. Autoradiographic evidence for the heterogeneity of 5-HT1 sites in the rat brain. Brain Res. 1984;291(1):159–163. doi: 10.1016/0006-8993(84)90664-4. [DOI] [PubMed] [Google Scholar]
- Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry. 2013;70(10):1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. http://dx.doi.org/10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- Maswood S, Stewart G, Uphouse L. Gender and oestrus cycle effects of the 5-HT1A agonist 8-OH-DPAT on hypothalamic serotonin. Pharmacol. Biochem. Behav. 1995;51:807–813. doi: 10.1016/0091-3057(95)00038-x. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. Plos. Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. http://dx.doi.org/10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendels J, Frazer A, Fitzgerald R, Ramsey T, Stokes J. Biogenic amine metabolites in cerebrospinal fluid of depressed and manic patients. Science. 1972;175:1380–1382. doi: 10.1126/science.175.4028.1380. [DOI] [PubMed] [Google Scholar]
- Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34(10):2275–2284. doi: 10.1038/npp.2009.54. http://dx.doi.org/10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Zanderigo F, Oquendo MA, Mann JJ, Parsey RV. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol. Psychiatry. 2013;74(10):760–767. doi: 10.1016/j.biopsych.2013.03.021. http://dx.doi.org/10.1016/j.biopsych.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff PB, Axelrod J. Biochemistry and catecholamines. Ann. Rev. Biochem. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Campbell IC, Costa JL. The brain serotonergic system in the affective disorders. Prog. Neuropsychopharmacol. 1978;2(1):5–31. doi: 10.1016/0364-7722(78)90019-x. [DOI] [PubMed] [Google Scholar]
- Naudon L, El Yacoubi M, Vaugeois JM, Leroux-Nicollet I, Costentin J. A chronic treatment with fluoxetine decreases 5-HT(1 A) receptors labeling in mice selected as a genetic model of helplessness. Brain Res. 2002;936(1–2):68–75. doi: 10.1016/s0006-8993(02)02548-9. [DOI] [PubMed] [Google Scholar]
- O'Reilly KC, Trent S, Bailey SJ, Lane MA. 13-cis-Retinoic acid alters intracellular serotonin, increases 5-HT1A receptor, and serotonin reuptake transporter levels in vitro. Exp. Biol. Med. 2007;232(9):1195–1203. doi: 10.3181/0703-RM-83. http://dx.doi.org/10.3181/0703-RM-83. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekstrom JC, Svenningsson P, Stiedl O. The role of 5-HT(1 A) receptors in learning and memory. Behav. Brain Res. 2008;195(1):54–77. doi: 10.1016/j.bbr.2008.02.023. http://dx.doi.org/10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Parker G, Brotchie H. Gender differences in depression. Int. Rev. Psychiatry. 2010;22(5):429–436. doi: 10.3109/09540261.2010.492391. http://dx.doi.org/10.3109/09540261.2010.492391. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mann JJ. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol. Psychiatry. 2010;68(2):170–178. doi: 10.1016/j.biopsych.2010.03.023. http://dx.doi.org/10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol. Psychiatry. 2006;59(2):106–113. doi: 10.1016/j.biopsych.2005.06.016. http://dx.doi.org/10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89(1):267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Weissman MM. A brain-based endophenotype for major depressive disorder. Annu. Rev. Med. 2011;62:461–474. doi: 10.1146/annurev-med-010510-095632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, Weinberger DR. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol. Psychiatry. 2008;13(7):709–716. doi: 10.1038/mp.2008.32. http://dx.doi.org/10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am. J. Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. http://dx.doi.org/10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol. Rev. 1999;51(3):533–591. [PubMed] [Google Scholar]
- Placidi GP, et al. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol. Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, Dinkel JJ, Mohs RC. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebocontrolled trial. Am. J. Psychiatry. 2007;164(6):900–909. doi: 10.1176/ajp.2007.164.6.900. http://dx.doi.org/10.1176/appi.ajp.164.6.900. [DOI] [PubMed] [Google Scholar]
- Rasmuson S, Olsson T, Henriksson BG, Kelly PA, Holmes MC, Seckl JR, Mohammed AH. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression binding in the rat hippocampus. Brain Res. Mol. Brain Res. 1998;53(1–2):285–290. doi: 10.1016/s0169-328x(97)00317-3. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001;92(2–3):179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40–52. doi: 10.1016/j.neuron.2009.12.003. http://dx.doi.org/10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley WT, Treiber FA, Woods MG. Anger and hostility in depression. J. Nerv. Ment. Dis. 1989;177(11):668–674. doi: 10.1097/00005053-198911000-00002. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Nishikawa M, Leyton M, Benkelfat C, Young SN, Diksic M. Cortical trapping of alpha-[(11)C]methyl-l-tryptophan, an index of serotonin synthesis, is lower in females than males. Neuroimage. 2006;33(3):815–824. doi: 10.1016/j.neuroimage.2006.08.004. http://dx.doi.org/10.1016/j.neuroimage.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C] WAY-100635: effects of depression and antidepressant treatment. Arch. Gen. Psychiatry. 2000;57(2):174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med. Hypotheses. 2007;69(6):1305–1308. doi: 10.1016/j.mehy.2007.03.021. http://dx.doi.org/10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1 A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. http://dx.doi.org/10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry. 1965;122(5):509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Rubinow DR. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714–726. doi: 10.1001/jamapsychiatry.2015.0111. http://dx.doi.org/10.1001/jamapsychiatry.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat hippocampal slices in vitro. J. Neurosci. 1995;15(11):7217–7225. doi: 10.1523/JNEUROSCI.15-11-07217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Hirvonen J, Hines CS, Henter ID, Svenningsson P, Pike VW, Innis RB. Serotonin-1A receptors in major depression quantified using PET: controversies, confounds, and recommendations. Neuroimage. 2012;59(4):3243–3251. doi: 10.1016/j.neuroimage.2011.11.029. http://dx.doi.org/10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Rose N. Biomarkers in psychiatry. Nature. 2009;460(7252):202–207. doi: 10.1038/460202a. http://dx.doi.org/10.1038/460202a. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res. 2003;37(5):357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J. Neurosci. 1998;18(18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Schmidt PJ, Danaceau M, Murphy DL, Rubinow DR. Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist m-chlorophenylpiperazine in women with premenstrual syndrome and controls. J. Clin. Endocrinol. Metab. 1997;82:1220–1228. doi: 10.1210/jcem.82.4.3905. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Parsey RV. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol. Psychiatry. 2009;66(3):223–230. doi: 10.1016/j.biopsych.2009.01.028. http://dx.doi.org/10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Milak M, Miller JM, Burke A, Ogden RT, Mann JJ. Positron emission tomography quantification of serotonin(1A) receptor binding in suicide attempters with major depressive disorder. JAMA Psychiatry. 2015;72(2):169–178. doi: 10.1001/jamapsychiatry.2014.2406. http://dx.doi.org/10.1001/jamapsychiatry.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Austin MC. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int. J. Neuropsychopharmacol. 2009;12(2):155–168. doi: 10.1017/S1461145708009012. http://dx.doi.org/10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traskman L, Asberg M, Bertilsson L, Sjostrand L. Monoamine metabolites in CSF and suicidal behavior. Arch. Gen. Psychiatry. 1981;38:631–638. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. doi: 10.1126/science.1078197. http://dx.doi.org/10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Winkler D, Pjrek E, Kasper S. Anger attacks in depression-evidence for a male depressive syndrome. Psychother. Psychosom. 2005;74(5):303–307. doi: 10.1159/000086321. http://dx.doi.org/10.1159/000086321. [DOI] [PubMed] [Google Scholar]
- World Health, O. Global Burden of Disease 2004 Update. 2004 from 〈〈 http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html〉〉.
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94(1):251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]