The proteasome, which mediates the ubiquitin-dependent degradation of intracellular proteins, is well recognized as an important anticancer target (Figure 1). So far, three inhibitors of this multiprotease complex have received FDA approval for treating multiple myeloma: the peptide boronic acids bortezomib and ixazomib, and the peptide epoxyketone carfilzomib.[1] Several other proteasome inhibitors have entered clinical trials, including the peptide boronic acid delanzomib and the peptide epoxyketone oprozomib.[2]
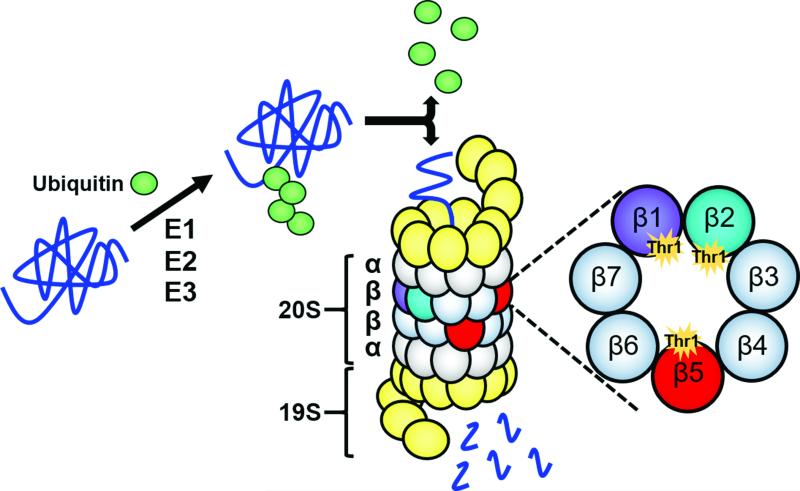
Figure 1.
The ubiquitin-proteasome pathway for protein degradation. A series of three enzymes – E1s, E2s, and E3s – assemble a polyubiquitin chain on a substrate protein to mark it for degradation by the proteasome. The polyubiquitin chain is recognized by the proteasome's 19S regulatory cap, which removes the chain and feeds the protein into the 20S proteasome core. Each of the two outer rings of the 20S core contains seven α subunits (α1-α7), while each of the two inner rings contains seven β subunits (β1-β7). Only β1, β2, and β5 are catalytically active; they contain active sites harboring catalytic N-terminal threonine (Thr1) residues. These three subunits work together to degrade the incoming protein to short peptides.
Although the FDA-approved proteasome inhibitors have achieved major breakthroughs in treating multiple myeloma, they have shown limited efficacy in treating most other types of cancer. This and other limitations, including severe side effects and inevitable drug resistance, continue to fuel the quest for new proteasome inhibitors that exhibit improved safety and efficacy profiles in and beyond multiple myeloma. Gaining a detailed understanding of the structural features of proteasomes and of the molecular interactions of existing inhibitors with the proteasome's active sites is a crucial step towards designing inhibitors that meet these criteria.
Crystallographic studies have provided a wealth of information on proteasome structure and function. Since these studies began in the 1990s, researchers have solved the crystal structures of proteasome core complexes derived from yeast, bovine, murine, and, most recently, human cells.[3] The 20S core complexes of these disparate species have strikingly similar structures. Their cylindrical shapes are built of four axially-stacked heptameric rings. Each of the two inner rings contains three catalytically-active subunits – β1, β2, and β5 – whose active sites are sequestered within the complex's interior.[3a] The proteasome's catalytic subunits are members of the N-terminal nucleophile (Ntn) hydrolase family – distinguishing them from most other proteases in mammalian cells (Figure 1).[4]
The crystal structures of 20S proteasomes in complex with peptide boronic acid or peptide epoxyketone inhibitors have also helped explain how these inhibitors interact with the proteasome's active sites.[3c, 3d, 5] The structure of the bortezomib-bound yeast proteasome revealed that, as expected, the boron atom of bortezomib's boronic acid pharmacophore reacts with the Oγ atom of the proteasome's catalytic threonine residue to form a stable yet reversible tetrahedral adduct.[5a] Conversely, based on the structure of the yeast proteasome complexed with the natural product peptide epoxyketone inhibitor epoxomicin, Groll et al concluded that the reaction of this inhibitor with the catalytic threonine results in the formation of a six-membered morpholino ring.[5b] They proposed that the formation of this six-membered ring occurs in two steps: an initial step in which the catalytic threonine's Oγ atom attacks the epoxyketone pharmacophore's carbonyl group to form a hemiketal, and a second step in which the catalytic threonine's N-terminal amino group attacks the epoxide α carbon to form the irreversible morpholino adduct (Figure 2). The requirement for the N-terminal amino group – in addition to the side chain nucleophile – of the catalytic threonine residue for forming this adduct appeared to explain the exquisite specificity of peptide epoxyketones for proteasomes over non-proteasomal proteases.[5b]
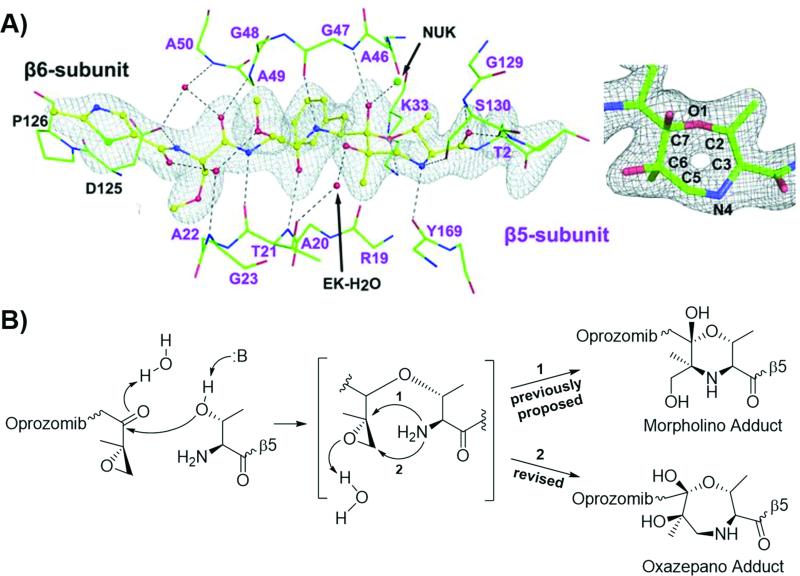
Figure 2.
New structural data revises our understanding of the reaction between the epoxyketone pharmacophore of an inhibitor and the catalytic threonine residue of the proteasome. A) Illustration of oprozomib complexed with the β5 active site (left), with a close-up view of the seven-membered ring adduct (right). From J. Schrader, F. Henneberg, R. A. Mata, K. Tittmann, T. R. Schneider, H. Stark, G. Bourenkov, A. Chari, Science 2016, 353, 594-598. Reprinted with permission from AAAS. B) Previously-proposed and newly-revised reaction mechanisms.
In a recent Science article, Schrader et al enhance our knowledge of the proteasome's active sites, and of how these sites interact with peptide boronic acid and peptide epoxyketone inhibitors. Using an optimized protocol, they solved the structures of 20S proteasomes from human HeLa cervical carcinoma cells at an unprecedented 1.8 Å resolution – a considerable improvement over the 2.6-Å structure of the human erythrocyte 20S proteasome reported previously.[3d, 6] They also acquired high-resolution cocrystal structures of human 20S proteasomes with six different inhibitors, including the clinically-relevant inhibitors bortezomib, ixazomib, delanzomib, and oprozomib.[6]
The most important observations derived from Schrader et al's study require us to revise our long-held (>15 years) conception of the mechanism by which peptide epoxyketone proteasome inhibitors react with the proteasome's catalytic threonine residues. Specifically, the cocrystal structures of the human 20S proteasome with three different peptide epoxyketone proteasome inhibitors – oprozomib (solved at 1.9 Å resolution), epoxomicin (solved at 2.4 Å resolution), and dihydroeponemycin (solved at 2.0 Å resolution) – reflected the formation of a seven-membered, 1,4-oxazepano adduct between the inhibitor and the catalytic threonine residue within the β5 active site.[6] This finding diverges from the previously-reported formation of the 1,4-morpholino adduct and indicates that, in the second step of the inhibitory reaction, the N-terminal amino group of the proteasome's catalytic threonine attacks the β carbon, rather than the α carbon, of the inhibitor's epoxide (Figure 2).[3c, 3d, 5b, 6] Schrader et al also indicated that the peptide ketoaldehyde inhibitor Z-LLY-ketoaldehyde forms a 1,4-morpholino adduct with β5's catalytic threonine residue, contrasting the 5,6-dihydro-2H-1,4-oxazino ring product proposed by Gräwert et al.[6-7]
Through cluster quantum chemical calculations and kinetic assays, Schrader et al further evaluated the differences between the inhibitory reactions that form six-membered versus seven-membered rings.[6] Based on the calculated pathways of these reactions, they identified the cyclization step as the bottleneck of both reactions. Their results also indicated that, although the six-membered ring product is more thermodynamically stable than the seven-membered ring product, the greater strain of the transition state of the former pathway causes the latter pathway to be favored from a kinetic standpoint. The results of kinetic assays also support that seven-membered ring formation is kinetically favored over six-membered ring formation.
The contributions of Schrader et al provide important insight for proteasome inhibitor design. Currently, the clinical development of proteasome inhibitors remains limited to inhibitors falling within the peptide boronic acid or peptide epoxyketone classes, as they are regarded as having acceptably low activity against non-proteasomal proteases. But these new findings suggest the possibility that the so-far-unparalleled specificity of the epoxyketone pharmacophore for the proteasome's catalytic threonine residues can be extended to other classes of proteasome inhibitors yet to be developed. Importantly, they indicate that the second electrophile of a dual-electrophilic pharmacophore can be placed not one carbon, but two carbons, away from the first (i.e., in the β position) to promote formation of the kinetically-favored seven-membered ring.[6] One might envision, for example, generating inhibitors analogous to the peptide halomethyl ketone cysteine/serine protease inhibitors but in which the leaving group is attached to the β carbon instead of to the α carbon. Exploration of these possibilities may yield inhibitors with improved proteasome selectivity relative to peptide boronic acids and improved pharmacokinetic profiles over those of peptide epoxyketones. It is hoped that such improvements would in turn lead to enhanced anticancer efficacy and reduced toxicity, thereby benefiting patients with multiple myeloma as well as those with other types of cancer.
Acknowledgements
We would lke to thank the National Institutes of Health (grant R01 CA188354 to K.B.K.) and Basic Science Research Program, National Research Foundation of Korea, Ministry of Science, ICT and Future Planning (NRF-2014R1A1A3050645 to W.L.) for financially supporting this work.
References
- 1.a Steele JM. J. Oncol. Pharm. Pract. 2013;19:348–354. doi: 10.1177/1078155212470388. [DOI] [PubMed] [Google Scholar]; b Muz B, Ghazarian RN, Ou M, Luderer MJ, Kusdono HD, Azab AK. Drug Des. Devel. Ther. 2016;10:217–226. doi: 10.2147/DDDT.S93602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Gallerani E, Zucchetti M, Brunelli D, Marangon E, Noberasco C, Hess D, Delmonte A, Martinelli G, Böhm S, Driessen C, De Braud F, Marsoni S, Cereda R, Sala F, D'Incalci M, Sessa C. Eur. J. Cancer. 2013;49:290–296. doi: 10.1016/j.ejca.2012.09.009. [DOI] [PubMed] [Google Scholar]; b U.S. National Library of Medicine, U.S. National Institutes of Health ClinicalTrials.gov [Google Scholar]
- 3.a Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]; b Unno M, Mizushima T, Morimoto Y, Tomisugi Y, Tanaka K, Yasuoka N, Tsukihara T. Structure. 2002;10:609–618. doi: 10.1016/s0969-2126(02)00748-7. [DOI] [PubMed] [Google Scholar]; c Huber EM, Basler M, Schwab R, Heinemeyer W, Kirk CJ, Groettrup M, Groll M. Cell. 2012;148:727–738. doi: 10.1016/j.cell.2011.12.030. [DOI] [PubMed] [Google Scholar]; d Harshbarger W, Miller C, Diedrich C, Sacchettini J. Structure. 2015;23:418–424. doi: 10.1016/j.str.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Brannigan JA, Dodson G, Duggleby HJ, Moody PCE, Smith JL, Tomchick DR, Murzin AG. Nature. 1995;378:416–419. doi: 10.1038/378416a0. [DOI] [PubMed] [Google Scholar]
- 5.a Groll M, Berkers CR, Ploegh HL, Ovaa H. Structure. 2006;14:451–456. doi: 10.1016/j.str.2005.11.019. [DOI] [PubMed] [Google Scholar]; b Groll M, Kim KB, Kairies N, Huber R, Crews CM. J. Am. Chem. Soc. 2000;122:1237–1238. [Google Scholar]
- 6.Schrader J, Henneberg F, Mata RA, Tittmann K, Schneider TR, Stark H, Bourenkov G, Chari A. Science. 2016;353:594–598. doi: 10.1126/science.aaf8993. [DOI] [PubMed] [Google Scholar]
- 7.Gräwert MA, Gallastegui N, Stein M, Schmidt B, Kloetzel P-M, Huber R, Groll M. Angew. Chem. Int. Ed. 2011;50:542–544. doi: 10.1002/anie.201005488. [DOI] [PubMed] [Google Scholar]