Abstract
Reactive oxygen species (ROS), a by-product of aerobic metabolism were initially studied in context to their damaging effect but recent decades witnessed significant advancements in understanding the role of ROS as signaling molecules. Contrary to earlier views, it is becoming evident that ROS production is not necessarily a symptom of cellular dysfunction but it might represent a necessary signal in adjusting the cellular machinery according to the altered conditions. Stomatal movement is controlled by multifaceted signaling network in response to endogenous and environmental signals. Furthermore, the stomatal aperture is regulated by a coordinated action of signaling proteins, ROS-generating enzymes, and downstream executors like transporters, ion pumps, plasma membrane channels, which control the turgor pressure of the guard cell. The earliest hallmarks of stomatal closure are ROS accumulation in the apoplast and chloroplasts and thereafter, there is a successive increase in cytoplasmic Ca2+ level which rules the multiple kinases activity that in turn regulates the activity of ROS-generating enzymes and various ion channels. In addition, ROS also regulate the action of multiple proteins directly by oxidative post translational modifications to adjust guard cell signaling. Notwithstanding, an active progress has been made with ROS signaling mechanism but the regulatory action for ROS signaling processes in stomatal movement is still fragmentary. Therefore, keeping in view the above facts, in this mini review the basic concepts and role of ROS signaling in the stomatal movement have been presented comprehensively along with recent highlights.
Keywords: Calcium, Guard Cell, Reactive oxygen species, Stomatal movement, Transcription factors
1. Introduction
About 2.7 billion years ago, life originated on the earth under a reducing environment. In due course of evolution, with the introduction of oxygen-evolving photosynthetic organisms, molecular oxygen (O2) evolved in the atmosphere that transformed the reducing atmosphere into oxidized one. Since that time, reactive oxygen species (ROS) became an inevitable companion of aerobic life [1]. ROS such as superoxide radical (O2•⁻), hydroxyl radical (•OH), and hydrogen peroxide (H2O2) are either produced by redox (oxidation–reduction) reactions or they are the active derivative of O2. These ROS are permanently generated in chloroplasts, mitochondria, peroxisomes, cytosol, and apoplast and are highly reactive and toxic that can oxidatively damage lipids, proteins, and nucleic acids [2], [3], [4], [5], [6], [7] (Table 1). However, recent studies instead of discussing their toxic nature, have concentrated towards their signaling role in several key physiological processes of plants [7], [8], [9], [10]. Their participation in signaling pathway can only be achieved when there is a tight balance between ROS production and their scavenging [10], [11]. The involvement of ROS in developmental processes as a signaling molecule suggests that during the course of evolution, plants might have evolved the capacity to achieve a high degree of tolerance over ROS toxicity [12], [13].
Table 1.
Various sites of ROS production and their impacts on plants.
| Types of ROS | Organelle | Main sources of ROS production | References |
|---|---|---|---|
| O2•⁻, H2O2,•OH | Chloroplast (electron transport chain; ETC) | -During stress conditions, NADP supply decreases thereby resulting into overload on ETC due to which electron leaks from ferrodoxin to O2 and reduces O2 into O2•− (Mehler reaction). | [69] |
| In PSI, electrons may leak from 2Fe-2S and 4Fe-4S clusters in the ETC of PSII | [70] | ||
| -In PSII, also electrons may leak from QA and QB (an acceptor side of ETC) to O2 thereby leading to O2•− production. | |||
| O2•⁻, H2O2 | Peroxisomes (photorespiratory glycolate oxidase reaction, the fatty acid β-oxidation) | -During photorespiration, the glycolate oxidase mediated oxidation of glycolate results into H2O2 production. | [71], [72] |
| -During β-oxidation of fatty acid C3 or β-carbon of acyl-CoA oxidises to produce -CoA and an acyl-CoA molecule lacking two carbon. The first step is catalyzed by acyl CoA oxidases where flavin adenine dinucleotide (FAD) is a cofactor of acyl CoA oxidases, and the electrons are passed to molecular oxygen to produce H2O2. | [73], [74] | ||
| O2•⁻, H2O2,•OH | Mitochondria (ETC or respiratory chain) | -When NAD+ linked substrates for NADH dehydrogenase (complex I) are limited, electron start to flow in reverse direction i.e, from succinate dehydrogenase segment (complex II) to complex I. Due to this reason, O2 reduces into O2•− in the flavoprotein region of complex I. | [75] |
| -Also ubiquinone-cytochrome region (complex III) of respiratory chain produces O2•− . A fully reduced ubiquinone provides an electron to cytochrome C1 thereby leaving an unstable highly reducing ubisemiquinone radical that is favourable for the leakage of electrons to O2 and, thus, to O2•− formation. | [76] | ||
| Enzymes of mitochondrial matrix | -Aconitase, directly produces ROS. | [77], [78] | |
| O2•⁻, H2O2 | Endoplasmic reticulum (Cyt P450) | -The NAD(P)H-dependent electron transport includes Cyt P450. Organic substrate (RH) first reacts with Cyt P450 and after that is reduced by a flavoprotein to produce an intermediate (Cyt P450R-). This intermediate and triplet oxygen both have one unpaired electron so can readily react with each other. This oxygenated complex (Cyt P450-ROO-) can be reduced by cytochrome b or rarely the complexes may decompose thereby releasing O2•−. | [79] |
| O2•⁻, H2O2 | Plasma membrane (NADPH oxidase; respiratory burst oxidase) | -Plasma membrane located NADPH oxidase especially during stress condition catalyzes the electron transport from cytoplasmic NADPH to O2 to produce O2•−, which may further dismutated to H2O2 by SOD activity. | [36], [80], [81] |
| H2O2 | Apoplast (Oxalate oxidase, Amine oxidases) | -Oxalate oxidase mainly releases H2O2 from oxalic acid and leads into apoplastic H2O2 accumulation. | [82], [83] |
| -Oxidative deamination of polyamines (i.e., spermine, putrescine, and spermidine) is mainly catalyzed by amine oxidases by using FAD as a cofactor. | [84] |
Stomata are present on underside part of leaf and surrounded by a pair of guard cells. The movement (opening and closing) of the stomatal aperture is controlled by turgor pressure generated in the guard cells. Apart from this, the stomatal movement is also regulated by soil and air water content, light intensity and quality, air pollutants, and CO2 like environmental factors [14], [15]. Furthermore, guard cells have anion channels that are located in the plasma membrane and are activated by several stimuli such as Abscisic acid (ABA) [16], NADPH metabolism [17], and anion fluxes across the plasma membrane via voltage-gated K+ transport channels [15]. ROS are key signals involved in the regulation of stomatal closure [15], [18]. During regulation of stomatal movement, ROS are firstly generated in apoplast of guard cell thereafter activate the anion channels via sensing and signaling. In this review, we have discussed studies from past and that are being performed in recent days with context to interaction of ROS and other components during stomatal movement.
2. ROS and stomatal opening/closing: a cascade of signaling network
Stomata are broadly known for mediating photosynthetic CO2 exchange and for the efficient use of water for generating the transpirational pull for the ascent of sap [18] (Fig. 1). Stomatal pore size is regulated by guard cells through a combination of environmental and endogenous signals that affect stomatal movement [19], [20]. Stomatal movement mediated by ROS have created tremendous interest in their signaling mechanisms as well as network. Each network has unique and distinct receptors and early signaling elements but they also have common components, for instances, plasma membrane anion channels and K+ channels through which solute fluxes drive water influx/efflux during actual stomatal movement. ROS are reported as vital participants in guard cell signaling; in particular, H2O2 plays a key role in ABA -induced stomatal closure [21], [22] (Fig. 1). Water stress is a common symptom of plants growing in dry soil, as water lost from leaves surpasses the amount taken up by the roots and leads to cellular dehydration, damage, and finally death. Cellular dehydration also occurs, when plants are exposed to other abiotic stresses that limit water supply, such as anaerobic conditions resulting from root flooding or cold and salt stress. Under water stress condition, plants close their stomata as a defence response in order to minimize the loss of water, and the stomatal movement during this stress is regulated by redistribution and synthesis of ABA. ABA alters the gene expression which controls other ameliorative responses such as the maintenance of root water uptake, synthesis of osmoprotective proteins, and various metabolic changes [23], [24], [25]. Oxidative stress is a common characteristic of various abiotic stresses which disturbs the redox balance of cell thereby increasing the ROS production that are controlled either by antioxidant enzymes or by reaction with antioxidant molecules.
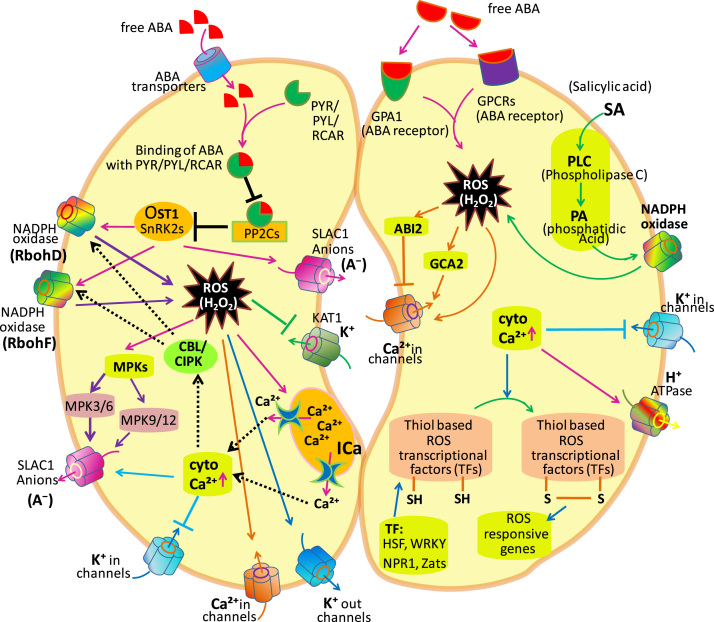
Fig. 1.
Targets of reactive oxygen species (ROS) in guard cells. The ROS with sensors play a key role in stomatal movement, which not only supervise ROS concentration in and out of the cell, but also respond to ROS signals. Here the abbreviations are; different channels: NADPH oxidase channels (RbohD and RbohF); S-type anion channel; SLAC1; calcium channels (Ca+in), potassium channels KAT1 (K+in channel), etc. Receptors; G protein-coupled receptor (GPCR); ABA receptor (PYL/PYL/RCAR); type 2C protein phosphatase (PP2C); open stomata 1 (Ost1); Sucrose nonfermenting-related protein kinase 2 (SnRK2s); Arabidopsis α-subunit of the trimeric G protein (GPA1). Calcium dependent protein kinase; MPK, mitogen-activated protein kinase (MPK3/6, MPK9/12); Growth Controlled by Abscisic Acid 2 (GCA2); ABA insensitive 2 (ABI2); Ca2+-permeable (ICa); Calcineurin-B Like Proteins (CBLs) proteins; CBL Interacting Protein Kinases (CIPKs), transcriptional factors; HSFs, heat shock transcription factors; Zats, zinc finger proteins; WRKYs, WRKY transcription factors; NPR1, nonexpressor of pathogenesis-related genes 1 (modified after Song et al. [18]).
The phytohormone ABA is synthesized in shoots, roots, and particularly in seeds, veins, and guard cells [26] and plays an important role in various physiological processes, such as development and the regulation of stomatal function in response to abiotic stresses. In case of high salinity and water stress, ABA starts to accumulate in the plant cell and its accumulation directs the changes in gene expression and stomatal closure, with subsequent decrease in transpiration and water loss [27]. Gaseous exchange decreases as an outcome of stomatal closure thereby resulting into decrease in photosynthetic activity [18], [26]. Under stress conditions, ABA concentration increases due to release from its conjugated forms or enhanced biosynthesis and decreased degradation. These steps taking place within the affected cell or in neighbouring cells results in uptake of ABA by non-stressed cells; ABA in cells is sensed by the ABA receptors. The regulatory network of ABA involves three major components of ABA receptor; the PYRABACTIN (PYR1)/PYR1-LIKE (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR; i.e., PYR/PYL/RCAR [28], [29], a type-2C protein phosphatase (PP2C; a negative regulator), and a SNF1-related protein kinase 2 (SnRK2; a positive regulator). These component shows a double negative regulatory system (PYR/PYL/RCAR—|PP2Cs; PP2Cs —|SnRK2) [30], [31]. In guard cells, ABA is sensed by PYL/PYR/RCAR (PYLs), which binds to ABA. PYLs change their conformation and then interact and inhibit PP2Cs. PP2Cs interact with Sucrose-Non-Fermenting Kinase 1 (SNF1)-related SnRK2s protein kinase open stomata 1 (OST1), leading in dephosphorylation of Ser/Thr residues present at the activation loop of the SnRK2s, resulting in its inactivation [32]. Therefore, it is concluded that ABA interacts with PYLs complex, inactivates the inhibitory function of PP2Cs, and activate SnRK2 protein kinase OST1 [30], [31]. Activated OST1 directly binds with and phosphorylates SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1), the anion channel that mediates the release of anions from guard cells, promoting stomatal closure [33], [34]. OST1 also interacts and phosphorylates with plasma membrane-bound N terminus of respiratory burst oxidase homolog D (RBOHD) and F (RBOHF) protein and in the guard cells of ost1 knockout mutant, the ABA-induced ROS generation is eliminated thereby suggesting that OST1 catalyzes ROS production (H2O2) mediated by NADPH oxidase [33], [34]. Recently, Shi et al. [35] reported that OST1 compromised the CO2-induced H2O2 and NO accumulation, upregulation of SLAC1 expression, and reduced stomatal aperture.
Kwak et al. [36] have reported that H2O2 application in guard cells activates ABA-mediated activation of the hyperpolarization-regulated Ca2+-permeable (ICa) channels and produces concurrent cytosolic Ca2+ increase, and this activation was found to be damaged in the ABA-insensitive gca2 mutant. The plasma membrane-bound anion channels that are activated by elevated cytosolic Ca2+ concentrations cause a membrane depolarization resulting to the hang-over of inward K+ KAT1 channels [37]. Upon Ca2+ binding, CALCINEURIN-B LIKE PROTEINS (CBLs) interact and regulate the CBLINTERACTING PROTEIN KINASES (CIPKs) activity [38]. CBL1/CBL9-CIPK26 complex interact and phosphorylates RBOHF, which is located at the plasma membrane thereby suggesting that CIPK26-mediated RBOHF regulation occurs at the plasma membrane and not by the CBL-CIPK dependent translocation regulatory mechanism [38], [39]. Further, the Ca2+-CBL-activated kinase i.e. CIPK26 mediated phosphorylation of RBOHF resulted in enhanced ROS production [39] (Fig. 1).
Several works have suggested that apoplastic ROS accumulation actively participates in the initiation of stomatal closure [40], [41]. According to Okuma et al. [42] reduced glutathione (GSH) concentrations decreases by increasing ABA levels in guard cells and in GSH-knockout mutants enhanced ABA-induced stomatal closure was observed. In cad2-1 mutant of A. thaliana lacking gamma-glutamylcysteine synthase (catalyzes the first step in GSH biosynthesis), an increase in H2O2 level by the hyperpolarized-activated Ca2+ channel in plasma membrane of the guard cell was observed along with an increase in H2O2-induced stomatal closure [43]. As the cytosolic GSH in the guard cell was induced by ABA and not by H2O2, it had been suggested that apoplastic ROS signal might alter the responsiveness of the guard cells to ABA by stimuli other than ABA itself [42], [43], but this view has not been experimentally evidenced so far. ROS are suggested to elevate the free ABA levels either by enhancing ABA biosynthesis or by inhibiting ABA degradation [18], [26], [44]. Therefore, increased ROS levels might result into increased ABA accumulation while increased ABA might results into increased ROS generation thereby forming a positive feedback loop in mediating stomatal closure.
It is commonly known that ROS (such as O2•− and H2O2) and NO are produced in response to similar stimuli and with similar kinetics. In the leaves of Phaseolus aureus, exogenous H2O2 triggered NO generation in the guard cells [45]. These findings were supported by Neill et al. [46] who reported that nitric oxide synthase (NOS) as well as nitrate reductase (NR) both are necessary for ABA-induced NO generation in the guard cell of Arabidopsis. NO induces MAPK activity, cGMP, and Ca2+ production that are vital for ABA-induced stomatal closure under stress conditions [46]. ABA and H2O2 can directly participate in stomatal closure via NO-independent signaling. NO increases the activity of antioxidant protective enzymes and proteins via MAPK or by other signaling pathways. For instance, in order to struggle with increased ROS, superoxide dismutase (SOD) activity might increase along with the ascorbate peroxidase (APX) and catalase (CAT), and dehydrins like proteins can be produced in order to improve the effects of cell dehydration [46] (Fig. 2).
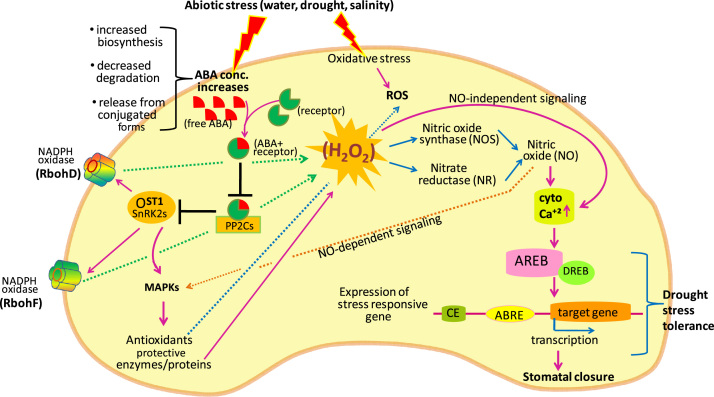
Fig. 2.
During stress condition, ABA accumulates in the guard cells via different ways. It enters into guard cells via ABA transporters, synthesized in response to signals like increased ROS, or accumulates as a result of decreased degradation or release of ABA from conjugated sources. The accumulated ABA interacts with the PYR/PYL/RCAR receptor, and inhibits the PP2Cs, which will result into activation of OST1 and phosphorylation and activation of NADPH oxidase (Rboh). NADPH oxidase facilitates H2O2 generation via signaling pathway·H2O2 induces NO generation by nitric oxide synthase (NOS)-like enzyme(s) and nitrate reductase (NR) that result in the opening of ROS-regulated Ca2+ channels. NO enhances antioxidant gene and enzyme activity via MAPK signaling pathways. H2O2 directly induces ROS-regulated Ca2+ channels (NO-independent signaling) thereby increasing Ca2+(Cyt). Elevated Ca2+(Cyt) induces the expression of abscisic acid–responsive element binding (AREB) protein that binds to the ABA–responsive element (ABRE) motif in the promoter region of ABA-inducible genes. The expression of ABA-responsive genes requires a combination of an ABRE and a coupling element (CE) for a functional promoter. AREB also interacts physically with dehydration responsive element binding (DREB) proteins for the expression of stress responsive genes, leading to stomatal closure under drought conditions.
Under drought condition, H2O2 increases cytosolic Ca2+(cyt) either directly by activating Ca2+ in channels or indirectly by inducing nitric oxide (NO) synthesis.. Increased Ca2+(Cyt) induces the expression of abscisic acid–responsive element binding (AREB) protein. The AREB, a basic domain/leucine zipper transcription factor, binds to the ABA–responsive element (ABRE) motif in the promoter region of ABA-inducible genes. According to Fujita et al. [47] and Nakashima and Yamaguchi-Shinozaki [48], the expression of ABA-responsive genes requires more than one ABRE or a combination of an ABRE and a coupling element (CE) for a functional promoter. The ABRE mainly mediates downstream gene expression in the ABA-signaling pathway. According to Narusaka et al. [49], the dehydration responsive element/C-repeat (DRE/CRT) motif in the promoters of drought-responsive genes, is a binding region for an ABA-independent dehydration responsive element binding (DREB) transcription factor and functions as a CE for ABRE in ABA-dependent gene expression [49]. According to Lee et al. [50], DREB proteins interact physically with AREB/ABF proteins for the expression of stress responsive gene (Fig. 2).
Unlike ABA, salicylic acid (SA) mediates ROS production in guard cells via peroxidase-catalyzed reaction not via NADPH oxidases [51]. Indeed, in the SA-accumulating mutant siz1, the reduced stomatal apertures were inhibited by the application of peroxidase inhibitors such as azide and salicylhydroxamic acid (SHAM; inhibitor of SA-dependent ROS production) but not by the NADPH oxidase inhibitor i.e. diphenyliodonium chloride (DPI) (inhibitor of ABA-dependent ROS production) [52]. Pre-treatment with CAT and SOD, inhibited the SA-induced stomatal closure thereby suggesting that extracellular ROS are involve in stomatal movement [41]. Furthermore, SHAM (a peroxidase inhibitor) completely eliminates SA-induced stomatal closure while neither atrbohD and atrbohF mutation nor DPI (an inhibitor of NADPH oxidase) impairs SA-induced stomatal closure [41]. ROS accumulation in guard cell was considerably increased by SA, but those ROS were holdback by exogenous SHAM, SOD, and CAT. According to Khokon et al. [41], SA was failed to stimulate Ca2+(cyt) oscillations while K+in channel activity was suppressed by SA, in guard cells. These findings point out that SA induces stomatal closure along with extracellular ROS generation mediated by SHAM-sensitive peroxidase, intracellular ROS accumulation and inactivation of K+in channels [41]. In contrary to this, Kalachova et al. [53], reported that SA-induced stomatal closure is impaired by DPI [an NADPH oxidases (NOX) inhibitor that inhibits ROS production] treatment and NOX deficient plants showed inhibited stomatal reaction even after exposure to exogenous SA [53]. Thus, it can be concluded that NOX plays a critical role in stomatal closure in response to SA also. ROS are also generated by G protein-coupled receptor (GPCR), Arabidopsis α-subunit of the trimeric G protein (GPA1), and salicylic acid signaling network complexes i.e. phospholipase C (PLC) and phosphatidic acid (PA) either directly or by activating NOX, which leads to increased cytosolic Ca2+(cyt). Further, based on the observation, a fundamental link between ABA and SA signaling has been suggested, as in ABA-deficient aba2-1 mutant no longer stomata were closed in response to exogenously applied SA; while SA-deficient nahG and sid2 mutant responded normally to ABA in guard cells [54], [55]. These findings, therefore, imply that SA signaling acts in upstreaming of ABA signaling and signifies that interaction between SA and ROS can differ under different concentrations and conditions.
Another phytohormone methyl jasmonate (MeJA) has also been known to elicit the ROS generation in guard cells [56]. MeJA-induced stomatal closure was suppressed by exogenous application of DPI. In the same study, the NADPH oxidase double mutant atrbohD/F, MeJA could not induced stomatal closing. These observations suggest that major sources of ROS in MeJA induced signaling in guard cell is NADPH oxidases AtrbohD/F [57]. In the rcn1 mutant (mutation in gene encoding a regulatory subunit of protein phosphatase type 2A (PP2A)) of A. thaliana, MeJA failed to elicit the ROS and NO generation [58]. These findings suggest that in MeJA induced signaling of guard cell, RCN1-regulates PP2As function by upstreaming of ROS and NO generation.
When addressing oxidative stress signaling, the role played by transcription factors cannot be neglected. Any stimulus which increases ROS and/or decreases antioxidant activity of the cell, disturbs the redox balance and thus induces oxidative stress. Several redox-controlled transcription factors have been identified. Thiol groups are probably important in redox signal transduction, including ROS sensing by receptor kinases that mediates stomatal closure in response to H2O2 [59]. For instances, a bacterial H2O2 sensor i.e. OxyR was firstly identified transcription factor in Salmonella species and Escherichia coli [60], [61]. OxyR, activated by H2O2, is a homodimer formed by an intermolecular disulphide bridge which brings out some significant alterations in the structure of protein [60], while deactivated by enzymatic reduction with glutaredoxin 1 (Grx1); the gene encoding Grx1 regulated by OxyR.
Among the transcriptional factors, heat shock transcription factors (HSFs) are potential ROS sensors. HSFs are necessary not only for defence/protection against high-temperature stress, but also involved in the modulation of different abiotic stress responses [62], [63]. HSFs play a central role in the early sensing of H2O2 and participate in signaling crosstalk with several key components of H2O2 signaling [64]. According to Miller and Mittler [65] HSFs play the role of molecular peroxide sensor and cause the conformational changes and multimer formation thereby altering the H2O2 concentrations during stress which concomitantly leads to transcriptional activation of their target genes. The member of other transcription factor families i.e. GRAS, Myb, RAV, WRKY, and Zat are also activated by ROS [66]. During abiotic stresses and in response to wound-induced signaling, the expression of a zinc finger protein Zat12 (Zat12 is a vital part of the oxidative stress response signal transduction network of Arabidopsis) is activated at the transcriptional level as determined by fusion between the reporter gene luciferase and the Zat12 promoter [67]. The nonexpressor of pathogenesis-related genes 1(NPR1) transcription factor, is responsible for the modulation in the alterations of gene expression during systemic acquired resistance in plants. NPR1 exists as an oligomer in non-activation state, which is maintained by intermolecular disulphide bonds; while on activation, NPR1 get reduced to a monomeric form which after that accumulates in the nucleus and alters the gene expression [68].
3. Conclusion and future directives
Undoubtedly, in recent years an extraordinary development regarding the role of ROS in stomatal movement has been made. In this mini review, we have discussed the mechanistic action of ROS signaling for regulating stomatal movement. We have also speculated the possible signaling pathways with special emphasis on involvement of hormones, several transcription factors, and messengers. This review is a comprehensive work in which mechanistic model of cell system is also provided. However, many more facts remain to be unravelled, which will provide the answer to the questions like: How ROS or redox homeostasis is sensed by guard cells? What is the link between ROS-modulated gene networks and other stomatal signaling networks? Does any redox sensor based on thiols, exist in guard cells? If so, do they interact with guard cell signaling networks? Answering these questions will explicate the fragmentary picture of stomatal signaling and signaling networks which will fill the loopholes of the studied mechanisms till date.
Acknowledgements
The University Grants Commission, New Delhi, is acknowledged for providing financial support to Rachana Singh, Parul Parihar, and Samiksha Singh to carry out this work. Dr. Vijay Pratap Singh is thankful to Department of Biotechnology, New Delhi for financial support (file no. BT/PR12980/BPA/118/80/2015) to carry out this work.
Contributor Information
Vijay Pratap Singh, Email: vijaypratap.au@gmail.com.
Sheo Mohan Prasad, Email: profsmprasad@gmail.com.
References
- 1.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. The reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki N., Koussevitzky S., Mittler R., Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35:259–270. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandalio L.M., Rodríguez-Serrano M., Romero-Puertas M.C., del Rio L.A. Role of peroxisomes as a source of reactive oxygen species (ROS) signalling molecules. Subcell. Biochem. 2013;69:231–255. doi: 10.1007/978-94-007-6889-5_13. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S.M., Kumar S., Parihar P., Singh A., Singh R. Evaluating the combined effects of pretilachlor and UV-B on two Azolla species. Pest. Biochem. Physiol. 2015;128:45–56. doi: 10.1016/j.pestbp.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor D., Sharma R., Handa N., Kaur H., Rattan A., Yadav P. Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Plant Sci. 2015;3:13. [Google Scholar]
- 6.Karuppanapandian T., Moon J.C., Kim C., Manoharan K., Kim W. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011;5:709–725. [Google Scholar]
- 7.Singh R., Singh S., Parihar P., Mishra R.K., Tripathi D.K., Singh V.P., Chauhan D.K., Prasad S.M. Reactive Oxygen Species (ROS): beneficial Companions of plants' developmental processes. Front. Plant Sci. 2016;7:1299. doi: 10.3389/fpls.2016.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Breusegem F.V. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Wrzaczek M., Brosche M., Kangasjarvi J. ROS signaling loops: production, perception, regulation. Curr. Opin. Plant Biol. 2013;16:575–582. doi: 10.1016/j.pbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Baxter A., Mittler R., Suzuki N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage and anti-oxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;2012:1–26. [Google Scholar]
- 12.Bhattacharjee S. Membrane lipid peroxidation and its conflict of interest: the two faces of oxidative stress. Curr. Sci. 2014;107:1811–1823. [Google Scholar]
- 13.Mattila H., Khorobrykh S., Havurinne V., Tyystjärvi E. Reactive oxygen species: reactions and detection from photosynthetic tissues. J. Photochem. Photobiol. B. 2015;152:176–214. doi: 10.1016/j.jphotobiol.2015.10.001. (Pt. B) [DOI] [PubMed] [Google Scholar]
- 14.Sun Z., Jin X., Albert R., Assmann S.M. Multi-level modeling of light induced stomatal opening offers new insights into its regulation by drought. PLOS Comput. Biol. 2014;10:e1003930. doi: 10.1371/journal.pcbi.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata Y., Mori I.C., Munemasa S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015;66:369–392. doi: 10.1146/annurev-arplant-043014-114707. [DOI] [PubMed] [Google Scholar]
- 16.Roelfsema M.R., Hedrich R., Geiger D. Anion channels: master switches of stress responses. Trends Plant Sci. 2012;17:221–229. doi: 10.1016/j.tplants.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Leterrier M., Barroso J.B., Valderrama R., Begara-Morales J.C., Sánchez-Calvo B., Chaki M., Luque F., Viñegla B., Palma J.M., Corpas F.J. Peroxisomal NADP-isocitrate dehydrogenase is required for Arabidopsis stomatal movement. Protoplasma. 2016;253:403–415. doi: 10.1007/s00709-015-0819-0. [DOI] [PubMed] [Google Scholar]
- 18.Song Y., Miao Y., Song C.P. Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol. 2014;201:1121–1140. doi: 10.1111/nph.12565. [DOI] [PubMed] [Google Scholar]
- 19.Kim T.H., Bohmer M., Hu H., Nishimura N., Schroeder J.I. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signalling. Annu. Rev. Immunol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra B.B., Acharya B.R., Granot D., Assmann S.M., Chen S.M. The guard cell metabolome: functions in stomatal movement and global food security. Front. Plant Sci. 2015;6:334. doi: 10.3389/fpls.2015.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki N., Miller G., Salazar C., Mondal H.A., Shulaev E., Cortes D.F. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell. 2013;25:3553–3569. doi: 10.1105/tpc.113.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittler R., Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell. 2015;27:64–70. doi: 10.1105/tpc.114.133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaves M.M., Marococ J.P., Pereira J.S. Understanding plant responses to drought—from genes to the whole plant. Funct. Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- 25.Seki M., Umezawa T., Urano K., Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Boursiac Y., Léran S., Corratgé-Faillie C., Gojon A., Krouk G., Lacombe B. ABA transport and transporters. Trends Plant Sci. 2013;18:325–333. doi: 10.1016/j.tplants.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Shinozaki K., Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 29.Joshi-Saha A., Valon C., Leung J. A brand new START: abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011;4 doi: 10.1126/scisignal.2002164. [DOI] [PubMed] [Google Scholar]
- 30.Klingler J.P., Batelli G., Zhu J.K. ABA receptors: the START of a new paradigm in phytohormone signaling. J. Exp. Bot. 2010;61:3199–3210. doi: 10.1093/jxb/erq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umezawa T., Nakashima K., Miyakawa T., Kuromori T., Tanokura M., Shinozaki K. Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol. 2010;51:1821–1839. doi: 10.1093/pcp/pcq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X.L., Jiang L., Xin Q., Liu Y., Tan J.X., Chen Z.Z. Structural basis and functions of abscisic acid receptors PYLs. Front. Plant Sci. 2015;6:88. doi: 10.3389/fpls.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., Romeis T., Hedrich R. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjärvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA. 2012;109:10593–10598. doi: 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi K., Li X., Zhang H., Zhang G., Liu Y., Zhou Y., Xia X., Chen Z., Yu J. Guard cell hydrogen peroxide and nitric oxide mediate elevated CO2-induced stomatal movement in tomato. New Phytol. 2015;208:342–353. doi: 10.1111/nph.13621. [DOI] [PubMed] [Google Scholar]
- 36.Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014;202:35–49. doi: 10.1111/nph.12613. [DOI] [PubMed] [Google Scholar]
- 38.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Drerup M.M., Schlücking K., Hashimoto K., Manishankar P., Steinhorst L., Kuchitsu K., Kudla J. The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant. 2013;6:559–569. doi: 10.1093/mp/sst009. [DOI] [PubMed] [Google Scholar]
- 40.An Z., Jing W., Liu Y., Zhang W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008;59:815–825. doi: 10.1093/jxb/erm370. [DOI] [PubMed] [Google Scholar]
- 41.Khokon A.R., Okuma E., Hossain M.A., Munemasa S., Uraji M., Nakamura Y., Mori I.C., Murata Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011;34:434–443. doi: 10.1111/j.1365-3040.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 42.Okuma E., Jahan M.S., Munemasa S., Hossain M.A., Muroyama D., Islam M.M., Ogawa K., Watanabe-Sugimoto M., Nakamura Y., Shimoishi Y., Mori I.C., Murata Y. Negative regulation of abscisic acid-induced stomatal closure by glutathione in Arabidopsis. J. Plant Physiol. 2008;168:2048–2055. doi: 10.1016/j.jplph.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Munemasa S., Muroyama D., Nagahashi H., Nakamura Y., Mori I.C., Murata Y. Regulation of reactive oxygen species mediated abscisic acid signaling in guard cells and drought tolerance by glutathione. Front Plant Sci. 2013;4:472. doi: 10.3389/fpls.2013.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daszkowska-Golec A., Szarejko I. Open or close the gate - stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci. 2013;4:138. doi: 10.3389/fpls.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lum H.K., Butt Y.K.C., Lo S.C.L. Hydrogen peroxide induces a rapid production of nitric oxide in mung bean (Phaseolus aureus) Nitric Oxide: Biol. Chem. 2002;6:205–213. doi: 10.1006/niox.2001.0395. [DOI] [PubMed] [Google Scholar]
- 46.Neill S., Barros R., Bright J., Desikan R., Hancock J., Harrison J., Morris P., Ribeiro D., Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 47.Fujita M., Fujita Y., Maruyama K., Seki M., Hiratsu K., Ohme-Takagi M., Tran L.P., Yamaguchi-Shinozaki K., Shinozaki K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004;39:863–876. doi: 10.1111/j.1365-313X.2004.02171.x. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima K., Yamaguchi-Shinozaki K. ABA signaling in stress response and seed development. Plant Cell Rep. 2013;32:959–970. doi: 10.1007/s00299-013-1418-1. [DOI] [PubMed] [Google Scholar]
- 49.Narusaka Y., Nakashima K., Shinwari Z.K., Sakuma Y., Furihata T., Abe H., Narusaka M., Shinozaki K., Yamaguchi-Shinozaki K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003;34:137–148. doi: 10.1046/j.1365-313x.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 50.Lee S.J., Kang J.Y., Park H.J., Kim M.D., Bae M.S., Choi H.I., Kim S.Y. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol. 2010;153:716–727. doi: 10.1104/pp.110.154617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori I.C., Schroeder J.I. Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetical mechanotransduction. Plant Physiol. 2001;135:702–708. doi: 10.1104/pp.104.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miura K., Okamoto H., Okuma E., Shiba H., Kamada H., Hasegawa P.M., Murata Y. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 2012;73:91–104. doi: 10.1111/tpj.12014. [DOI] [PubMed] [Google Scholar]
- 53.Kalachova T., Iakovenko O., Kretinin S., Kravets V. Involvement of phospholipase D and NADPH-oxidase in salicylic acid signalling cascade. Plant Physiol. Biochem. 2013;66:127–133. doi: 10.1016/j.plaphy.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Zeng W., He S.Y. A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 2010;153:1188–1198. doi: 10.1104/pp.110.157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montillet J.L., Hirt H. New checkpoints in stomatal defense. Trends Plant Sci. 2013;18:295–297. doi: 10.1016/j.tplants.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Leshem Y., Levine A. Zooming into sub-organellar localization of reactive oxygen species in guard cell chloroplasts during abscisic acid and methyl jasmonate treatments. Plant Signal. Behav. 2013;8:e25689. doi: 10.4161/psb.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suhita D., Raghavendra A.S., Kwak J.M., Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito N., Munemasa S., Nakamura Y., Shimoishi Y., Mori I.C., Murata Y. Roles of RCN1, regulatory a subunit of protein phosphatase 2A, in methyl jasmonate signalling and signal crosstalk between methyl jasmonate and abscisic acid. Plant Cell Physiol. 2008;49:1396–1401. doi: 10.1093/pcp/pcn106. [DOI] [PubMed] [Google Scholar]
- 59.Desikan R., Hancock J.T., Bright J., Harrison J., Weir I., Hooley R., Neill S.J. A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol. 2005;137:831–834. doi: 10.1104/pp.104.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stone J.R. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch. Biochem. Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 61.D’Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 62.Akerfelt M., Trouillet D., Mezger V., Sistonen L. Heat shock factors at a crossroad between stress and development. Ann. N. Y. Acad. Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- 63.Anckar J., Sistonen L. Regulation of HSF1function in the heat stress response: implications in aging and disease. Annu. Rev. Plant Biol. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 64.Pucciariello C., Parlanti S., Banti V., Novi G., Perata P. Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol. 2012;159:184–196. doi: 10.1104/pp.111.191122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller G., Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tripathy B.C., Oelmuller R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davletova S., Rizhsky L., Liang H., Shengqiang Z., Oliver D.J., Coutu J J., Shulaev V., Schlauch K., Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mou Z., Fan W., Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 69.Elstner E.F. Mechanisms of oxygen activation in different compartments of plant cells. In: Pell E.J., Steffen K.L., editors. Active Oxygen/Oxidative Stress and Plant Metabolism. American Society of Plant Physiologists; Rockville, Md, USA: 1991. pp. 13–25. [Google Scholar]
- 70.Cleland R.E., Grace S.C. Voltammetric detection of superoxide production by photosystem II. FEBS Lett. 1999;457:348–352. doi: 10.1016/s0014-5793(99)01067-4. [DOI] [PubMed] [Google Scholar]
- 71.Baker A., Graham A.I. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. Plant Peroxisomes: Biochemistry, Cell Biology and Biotechnological Applications. [Google Scholar]
- 72.Noctor G., Veljovic-Jovanovic S., Driscoll S., Novitskaya L., Foyer C.H. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann. Bot. 2002;89:841–850. doi: 10.1093/aob/mcf096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Graham I.A. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2007;59:115–142. doi: 10.1146/annurev.arplant.59.032607.092938. [DOI] [PubMed] [Google Scholar]
- 74.Khan B.R., Adham A.R., Zolman B.K. Peroxisomal Acyl-CoA oxidase 4 activity differs between Arabidopsis accessions. Plant Mol. Biol. 2012;78:45–58. doi: 10.1007/s11103-011-9843-4. [DOI] [PubMed] [Google Scholar]
- 75.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andreyev A.Y., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochem (Mosc.) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 78.Rasmusson A.G., Geisler D.A., Møller I.M. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion. 2008;8:47–60. doi: 10.1016/j.mito.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 80.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 81.Torres M.A., Dangl J.L., Jones J.D.G. Arabidopsis gp91phox homologues Atrbohd and Atrbohf are required foraccumulation of reactive oxygen intermediates in the plant defense response. Proc. Nat. Acad. Sci. USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem. J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lane B.G. Oxalate, germins, and higher-plant pathogens. IUBMB Life. 2002;53:67–75. doi: 10.1080/15216540211474. [DOI] [PubMed] [Google Scholar]
- 84.Cona A., Rea G., Angelini R., Federico R., Tavladoraki P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;11:80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]