Abstract
Bacillus cereus bacteraemia can be severe, especially among patients with haematologic malignancy. We retrospectively reviewed first episodes of true B. cereus bacteraemia (more than one positive bottle plus signs of infection) at our institution between 1997 and 2013 with the aim to compare haematologic versus nonhaematologic patients and analyse episodes with complicated outcome. Among 56 episodes of positive-blood cultures for B. cereus, 21 were considered significant. Median age was 54 years (range 23–82 years). Ten patients (48%) had a haematologic malignancy; all were neutropenic at the time of B. cereus bacteraemia. Nonhaematologic patients were either intravenous drug users (n = 3, 14%), polytraumatized (n = 3, 14%) or had multiple chronic comorbidities (n = 5, 24%). Most episodes were hospital acquired (15, 71%). Sources of bacteraemia were intravascular catheter (n = 11, 52%), digestive tract (n = 6, 29%), drug injection (n = 3, 14%) and wound (n = 1, 5%). Adequate antibiotic therapy was provided to 18 patients (86%) during a median of 17 days (range 2–253 days). The intravascular catheter was removed in eight cases (42%). Three haematologic patients had a complicated course with neurologic complications (meningoencephalitis and cerebral abscesses). Complications appeared to be associated with catheter infection (100% of complicated cases vs. 29% of noncomplicated cases). In conclusion, B. cereus bacteraemia can have a complicated course in a subset of patients, mainly those with haematologic malignancy. Catheter infection may be associated with a worse outcome with frequent neurologic complications.
Keywords: Bacillus cereus, bacteraemia, central nervous system, intravascular catheter, neutropenia
Introduction
Bacillus cereus is a spore-building, Gram-positive rod that can survive in extreme environmental conditions and can persist in the hospital [1], [2]. While B. cereus is often considered a contaminant, it can also cause significant disease, which manifests in three distinct syndromes: food intoxication, localized infection and bacteraemia, with the latter sometimes being associated with haematogenous complications (e.g. endophtalmitis, cerebral abscesses) [3]. Certain clinical manifestations like necrotizing infections (e.g. endophtalmitis, fasciitis) might be caused by the release of to exotoxins such as proteases, phospholipases and haemolysins [2], [4], [5].
It is widely recognized that haematologic patients have a higher risk for invasive B. cereus infection [5], [6], [7], [8], but few data are available on other risk factors for complications of B. cereus bacteraemia (such as mortality and metastatic infections). The aims of the present study were to compare episodes between haematologic and nonhaematologic patients and to identify possible risk factors that may predispose patients to a complicated course of B. cereus bacteraemia.
Patients and Methods
Definition of cases
Cases with more than one positive blood culture bottle for B. cereus occurring at our institution (a 1500-bed university hospital) between January 1997 and April 2013 were identified through the microbiologic database. We included all patients presenting with a first episodes of true B. cereus bacteraemia, defined as more than one positive blood culture bottles and signs of sepsis [9] or localized infection.
Data collection
The following data were extracted from patient charts: demographic characteristics, site of hospitalization, underlying disease, presence of neutropenia (neutrophil count <0.5 × 109/L), previous chemotherapy, treatment with corticoids or other immunosuppressive drugs, source of bacteraemia, presence of a central venous catheter (CVC) and its management, symptoms and signs of sepsis and localized infection and antibiotic treatment. Outcome criteria were mortality at 30 days, presence of haematogenous complications (meningitis, cerebral abscesses, infective endocarditis or endophtalmitis) and relapse of B. cereus bacteraemia.
The study was approved by the local institutional review board of the University of Lausanne, Switzerland.
Identification and antibiotic susceptibility
B. cereus was identified from blood cultures through Gram stain appearance, colony morphology and haemolysis on blood agar, positive lecithinase on egg yolk agar or matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Antimicrobial susceptibility testing was performed using disk diffusion and was interpreted according to Clinical and Laboratory Standards Institute criteria used for staphylococci (due to the absence of criteria for Bacillus species other than B. anthracis) until 2010, and Etest interpreted with nonspecific European Committee for Antimicrobial Susceptibility Testing (EUCAST) criteria for Gram-positive bacteria was used thereafter.
Definitions of source of bacteraemia, adequate antibiotic treatment and complicated bacteraemia
Bacteraemia was categorized as hospital onset if it occurred >48 hours after admission. The source of B. cereus bacteraemia was ascertained by using the following criteria: for catheter-related bloodstream infection (CRBSI), no apparent source for bloodstream infection except catheter or positive catheter tip culture for B. cereus (≥15 CFU); for digestive tract, significant abdominal disease confirmed by imaging (e.g. ileus, perforation) or neutropenic enterocolitis (clinical or radiologic signs); for drug injection, active intravenous drug use (IVDU) and temporal association between B. cereus bacteraemia and drug injection; and for open fracture/wound, sign of infection or wound culture positive for B. cereus.
Antibiotic treatment was considered adequate if the B. cereus isolate was susceptible to the antimicrobial prescribed (or if there was a high likelihood of susceptibility based on published data [10]) and dosed in accordance with current recommendations. Antibiotic treatment was categorized as empirical if administered within 24 hours of collection of the blood culture and before susceptibility was known. Targeted antibiotic treatment referred to the treatment continued or initiated on the day that antibiogram results were reported. Haematogenous complications were defined by using following criteria: for meningitis, at least one clinical sign (headache, neck stiffness, impaired level of consciousness) associated with pleocytosis (>4 white blood cells/mm3); for brain abscess, at least one clinical sign (headache, focal neurologic deficit, impaired level of consciousness) and radiologic evidence; for infective endocarditis, definite diagnosis according to the Duke criteria [11]; and endophtalmitis, clinical signs and positive ophthalmologic examination.
Statistical analysis
For comparison between groups, we used Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous variables. Calculations were performed with Stata 12.0 (StataCorp, College Station, TX, USA) and GraphPad Prism 6.00 (GraphPad Software, La Jolla, CA, USA).
Results
Clinical characteristics
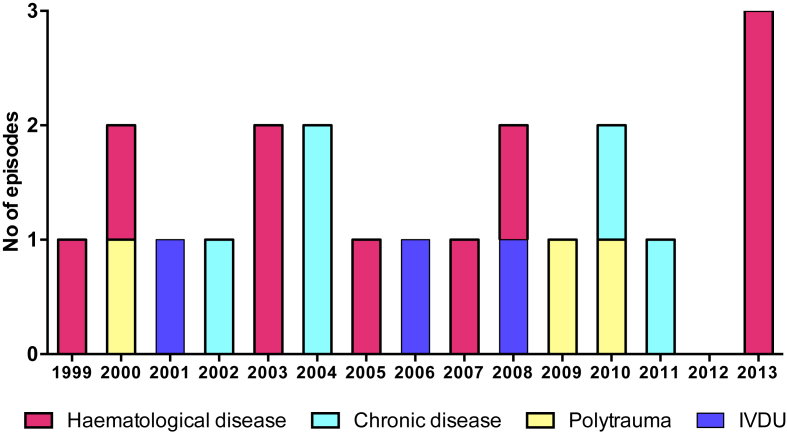
During the study period, we identified 56 episodes of positive blood cultures for B. cereus, among which 33 were excluded due to contamination (n = 31), relapse (n = 1) and missing clinical data (n = 1); 21 were retained as true bacteraemia. Among those, median patient age was 54 years (range 23–82 years) and the majority were men (n = 14, 67%). Underlying haematologic malignancy was observed in 10 patients (48%), chronic disease associated with some form of immunosuppression (autoimmune hepatitis, solid tumour, end-stage renal disease, diabetes mellitus) in five (24%), IVDU in three (14%) and polytrauma in three (14%). Six patients (29%) experienced breakthrough bacteraemia while receiving antibiotic treatment with cephalosporins or penicillins. The annual incidence of B. cereus bacteraemia stratified according to underlying condition is shown in Fig. 1. Of note, three cases among haematologic patients clustered in the first months of 2013. However, no common source of infection was identified.
Fig. 1.
Timing of infection and underlying diseases. Haematologic disease (n = 10): acute myeloid leukemia (n = 5), myelodysplastic syndrome (n = 3), acute lymphatic leukemia (n = 2). Chronic disease (n = 4): end-stage renal disease (n = 2), autoimmune hepatitis (n = 1), diabetes mellitus (n = 1), pseudomyxoma peritonei (n = 1). Polytrauma (n = 3). Intravenous drug use (IVDU, n = 3).
Most episodes were hospital onset (71%). Community-onset episodes were either associated with IVDU or end-stage renal disease. Fever was present in 18 patients (86%), abdominal symptoms (i.e. abdominal pain, vomiting, diarrhea) in six (29%), neurologic symptoms (including confusion, lethargy, generalized headache, blurred vision, photopsia, dysarthria, dysdiadochokinesia, hemiparesis and hemineglect) in four (19%).
Source of B. cereus bacteraemia
The source of bacteraemia was CRBSI in 11 cases (52%): short-term CVC (n = 5), port (n = 3), tunnelled haemodialysis catheter (n = 2) and arterial catheter (n = 1). In six cases (29%) bacteraemia originated from the digestive tract: all patients with an abdominal source had either abdominal symptoms (n = 4) or pathologic findings on computed tomography (n = 4; bowel wall thickening, ileus). Finally, source of infection was drug use in three (14%) and wound infection (confirmed by multiple positive wound swab cultures) in one (5%).
Microbiology
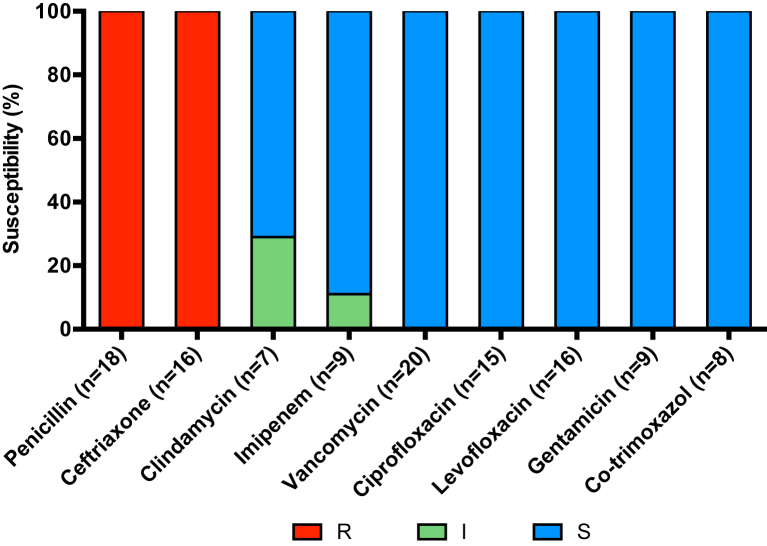
Concomitant pathogens were identified in seven episodes of B. cereus bacteraemia. The frequency of polymicrobial infections was similar between haematologic and nonhaematologic patients. Cerebrospinal fluid was collected in two cases (IVDU-associated bacteraemia and meningoencephalitis) and was sterile. Results of susceptibility testing are shown in Fig. 2. All tested isolates were susceptible to vancomycin (20/20), fluoroquinolones (16/16), gentamicin (9/9) and cotrimoxazole (8/8), while they were resistant to penicillin (18/18) and ceftriaxone (16/16). Imipenem susceptibility was assessed in nine cases. One isolate had a minimal inhibitory concentration (MIC) of 3 mg/L. Only three of seven tested isolates were susceptible to clindamycin.
Fig. 2.
Antibiotic susceptibility of 21 Bacillus cereus strains. I, intermediate; R, resistant; S, susceptible.
Treatment characteristics
Empirical treatment (including vancomycin or carbapenems) was adequate in six cases (26%), while adequate targeted treatment was administered in 18 cases (86%). Two immunocompetent patients (one IVDU and one polytraumatized) were treated with amoxicillin/clavulanic acid, and a haematologic patient was treated with imipenem despite an MIC of 3 mg/L. Of note, no complication was observed among these patients treated with inadequate antibiotics.
Median duration of adequate antibiotic treatment was 16.5 days (range 2–253 days). Nineteen patients (91%) had a CVC at time of B. cereus bacteraemia. The CVC was removed in eight cases (42%), including three cases of CRBSI. Median delay to device removal was 1.5 days (range 0–16 days).
Haematologic patients
All but one patient with haematologic diseases had nosocomial bacteremia and were neutropenic at time of bacteraemia. Median duration of neutropenia was 11 days (range 5–180 days). Eight patients had either induction or consolidation chemotherapy for acute leukaemia (cytarabine was administered to seven patients). Sources of bacteraemia were the intestinal tract (n = 5, 50%) and the CVC (n = 5, 50%).
In this group, median delay to adequate antibiotic treatment was 1 day (range 0–7 days). The CVC was removed in 42% of cases after a median delay of 4.5 days. No significant difference in clinical and treatment characteristics was found between haematologic and nonhaematologic patients (Table 1).
Table 1.
Patient characteristics
| Characteristic | Haematologic (n = 10) | Nonhaematologic (n = 11) |
|---|---|---|
| Age (years) | 55.5 (27–78) | 44 (23–82) |
| Men | 6 (60) | 8 (73) |
| Nosocomial acquisition | 9 (90) | 6 (55) |
| Source of infection | ||
| CRBSI | 5 (50) | 6 (55) |
| Digestive tract | 5 (50) | 1 (9) |
| IVDU | 0 | 3 (27) |
| Open fracture/wound | 0 (0) | 1 (9) |
| Polymicrobial infection | 4 (40) | 3 (27) |
| Neutropenia duration before bacteraemia (days) | 11 (5–180) | NR |
| Mucositis | 5 (50) | NR |
| Fever | 10 (100) | 8 (73) |
| Adequate antibiotic treatment | 9 (90) | 9 (82) |
| Vancomycin | 2 (22) | 4 (44) |
| Imipenem | 5 (56) | 4 (44) |
| Other | 2 (22) | 1 (12) |
| Duration of adequate treatment (days) | 18 (13–253) | 14 (2–84) |
| Presence of catheter at time of bacteraemia | 10 (100) | 9 (82) |
| Catheter removal | 4 (40) | 4 (44) |
| Delay until catheter removal (days) | 4.5 (1–16) | 1 (0–2) |
| Hospitalization in ICU | 4 (40) | 4 (36) |
| Relapse | 1 (10) | 0 |
| Death at 30 days | 1 (10) | 1 (9) |
Values are median (range) or n (%). No significant difference was found between haematologic and nonhaematologic patients.
CRBSI, catheter-related bloodstream infection; ICU, intensive care unit; IVDU, intravenous drug use; NR, not reported.
Complications of B. cereus bacteraemia
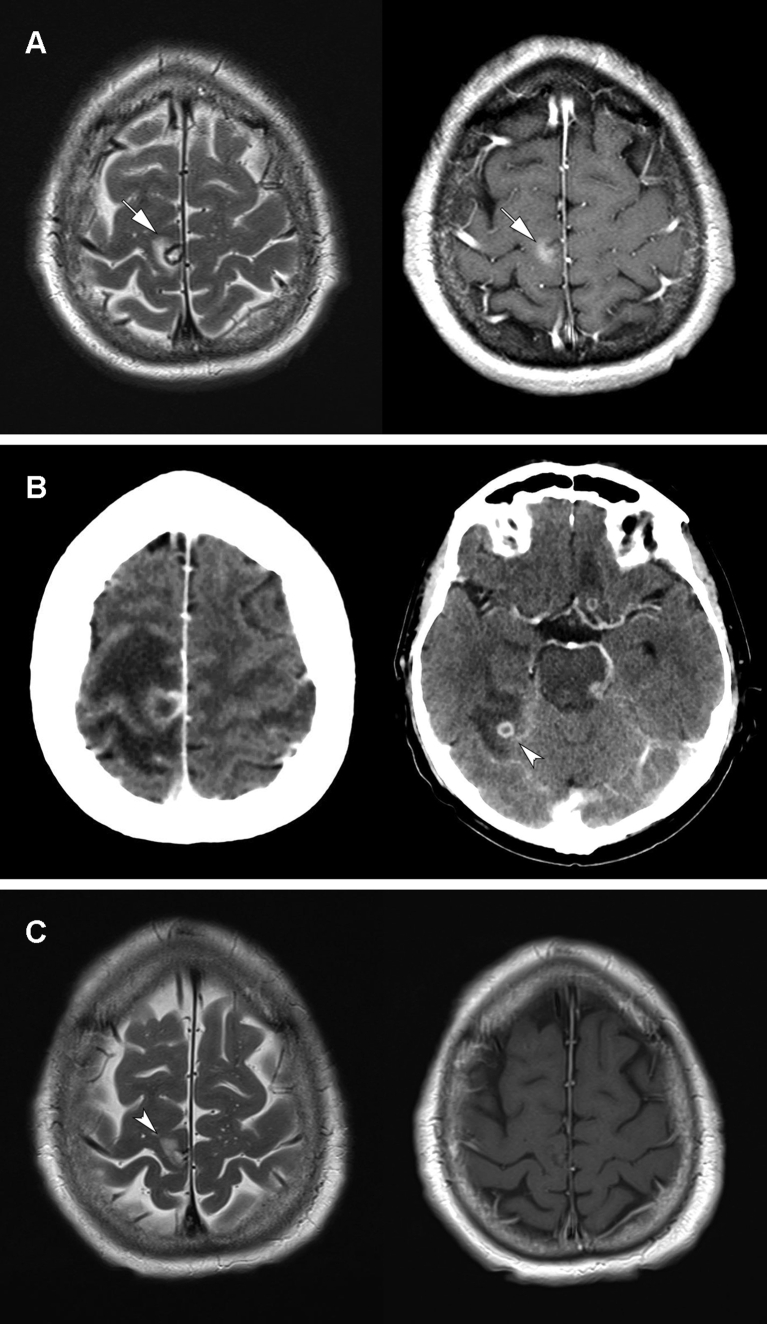
Three patients (14%) had central nervous system (CNS) complications: one had meningoencephalitis (impaired mental status, cerebrospinal fluid pleocytosis) and two had cerebral abscesses. An illustrative case of CNS abscess is depicted in Fig. 3. In the other case, B. cereus bacteraemia and cerebral abscesses appeared 1 month after stopping antibiotic treatment for the first episode, which was managed with port retention and vancomycin lock. All three patients with neurologic complications had a haematologic malignancy. No other hematogenous complications, such as infective endocarditis (echocardiography was performed in nine patients, two of whom underwent transoesophageal echocardiography) or endophtalmitis, were identified.
Fig. 3.
Brain imaging of haematogenous cerebral abscesses complicating Bacillus cereus bacteraemia in a 63-year-old man with relapsing acute myeloid leukemia. At day 13 after induction chemotherapy, the patient presented with neutropenic fever, headache, photopsia and left leg paresis. Magnetic resonance imaging (MRI) at first neurologic symptoms revealed a focal lesion in the paracentral lobule with focal oedema and contrast enhancement on T2- and T1-weighted images (arrows) (A). B. cereus was grown from blood cultures on the same day. Contrast-enhanced computed tomography performed to investigate neurologic deterioration (progression of paresis, lethargy and hemineglect) showed a significant increase in perilesional edema and appearance of new abscesses (arrowhead) (B). Antibiotic therapy (imipenem switched to oral levofloxacin at discharge), initially associated with high-dose corticoids, was carried on for a total of 8.5 months. Neurologic deficits regressed completely. Last follow-up MRI T2- and T1-weighted images showed an inactive lesion with minimal gliosis and no contrast enhancement (arrowhead) (C).
Two patients died during the 30 days after B. cereus bacteraemia (10%). One death in a patient with autoimmune hepatitis was possibly related to B. cereus bacteraemia, while the other patient died of invasive aspergillosis.
Because features of the group of patients with nonhaematologic diseases were heterogeneous, we limited the analysis of complications to haematologic patients (Table 2). While demographics and underlying disease did not differ between the two groups, CRBSI was the source of bacteraemia in all complicated cases as compared to 29% of uncomplicated cases. Overall, catheter management was similar between the two groups. Of note, the only recurrence was related to catheter retention.
Table 2.
Risk factors for complicated outcome in haematologic patients
| Characteristic | Complicated (n = 3) | Not complicated (n = 7) |
|---|---|---|
| Age (years) | 57 (46–64) | 54 (27–78) |
| Male | 2 (33) | 4 (57) |
| Active haematologic disease | ||
| Myelodysplastic syndrome | 1 (33) | 2 (29) |
| AML | 2 (66) | 3 (43) |
| ALL | 0 (0) | 2 (29) |
| Cytarabine chemotherapy | 2 (66) | 5 (71) |
| Source of infection | ||
| Catheter-related bloodstream infection | 3 (100) | 2 (29) |
| Digestive tract | 0 (0) | 5 (71) |
| Intestinal mucositis | 0 (0) | 4 (57) |
| Skin rash | 1 (33) | 3 (43) |
| Polymicrobial infection | 1 (33) | 3 (43) |
| Adequate antibiotic treatment (days) | 3 (100) | 6 (86) |
| Duration | 21 (15–253) | 17.5 (13–20) |
| Delay until adequate antibiotic | 1 (1) | 4 (0–7) |
| Presence of catheter at time of bacteraemia | 3 (100) | 7 (100) |
| No catheter removal | 1 (33) | 5 (71) |
| Delay until removal (days) | 3 (1–5) | 10 (4–16) |
| Hospitalization in ICU | 2 (66) | 2 (29) |
| ICU days of hospitalization | 8.5 (4–13) | 10.5 (4–17) |
| Death at 30 days | 0 (0) | 1 (14) |
Values are median (range) or n (%).
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CRBSI, catheter-related bloodstream infection; ICU, intensive care unit.
Discussion
In this series of 21 patients with B. cereus bacteraemia, we identified four distinct groups of patients according to their underlying condition: haematologic malignancies, chronic diseases (e.g. solid tumour, end-stage renal disease), typically associated with a lower degree of immunosuppression as compared to haematologic malignancies, polytrauma and IVDU.
Haematologic patients are a well-known risk group for B. cereus bacteraemia due to neutropenia and a break in the mucosal barrier after high-dose chemotherapy [12] and the skin barrier due to CVCs. B. cereus colonizes not only the environment and skin but also the intestinal tract, as shown in stool studies of healthy subjects and hospitalized patients [13]. Autopsies of patients with fatal B. cereus bacteraemia have revealed necrotizing intestinal lesions, highlighting the association between mucositis/neutropenic enterocolitis and B. cereus bacteraemia [14], [15]. Our series highlights the important role of neutropenia (90% of patients), cytarabine chemotherapy (70%) and enterocolitis (50%) in the pathogenesis of invasive B. cereus disease. The median delay of 11 days between onset of neutropenia and B. cereus bacteraemia suggests that B. cereus infection is a late event in the course of neutropenia, unlike other bacterial infections (e.g. Gram-negative or streptococcal bacteraemia). This is most likely explained by resistance to the β-lactams commonly used for the initial treatment of febrile neutropenia. Intravascular catheters are an important risk factor for B. cereus bacteraemia in this population of patients. B. cereus is able to produce biofilms and adhere to foreign bodies [2], [17], thus preventing eradication of infection. Guidelines for the management of CRBSI recommend removing the CVC within 72 hours of onset of B. cereus bacteraemia [18], as studies indicate that the risk of recurrence is higher if the catheter is retained [19], [20], [21]. Our own case of recurrence confirms this risk.
A striking feature of B. cereus among haematologic patients is the ability to invade the CNS and produce meningitis, abscesses or haemorrhage [14], [22], [23], [24], [25]. The pathogenesis of CNS invasion is unknown. Intrathecal chemotherapy has been discussed by some authors [7], but it is unlikely to explain most of the cases because the association with bacteraemia points to a haematogenous route of infection. In our series, neurologic complications were found in three of ten haematologic patients. The outcome was favourable with long-term antibiotic treatment, and no drainage of the cerebral abscesses was necessary.
We tried to identify risk factors for CNS involvement in haematologic patients by comparing episodes complicated by CNS disease with uncomplicated episodes. The only possible predictor was catheter-related infection: all three patients with CNS complications had CRBSI, while among patients with uncomplicated course the digestive tract was the most common source. Although the small sample size prevents us from drawing a definitive conclusion, we believe that this is an interesting observation. Two previous studies have addressed risk factors for complications of B. cereus bacteraemia in haematologic patients. In a review of 46 published episodes, possible predictors of poor outcome were neutropenia, presence of a CVC and neurologic symptoms [5]. A paediatric study of 12 patients identified corticosteroid and cephalosporin exposure, neutropenia and intrathecal therapy as possible risk factors for CNS complications [7]. Some authors have described an association with intestinal mucositis [7], [8]. None of our patients with CNS complications experienced abdominal symptoms.
Previous studies have highlighted the presence of a CVC as a risk factor for poor outcome [5], [22] and recurrence [6], [19], [20]. To our knowledge, this is the first description of a possible association between catheter infection and complicated B. cereus bacteraemia.
Invasive B. cereus infections among nonhaematologic patients are usually localized infections. In contrast, B. cereus bacteraemia is rarely reported, with the notable exception of IVDU, where infections are related to heroin contamination [26], [27]. Bacteraemia is often transient; however, complications such as endocarditis and endophtalmitis may occur [3]. In our series, B. cereus bacteraemia among IVDU had a benign course, even without adequate antibiotic treatment, thus calling into question the role of antibiotic treatment of B. cereus bacteraemia with spontaneous resolution.
B. cereus is usually resistant to all β-lactams except carbapenems via a broad-spectrum β-lactamase [10]. As shown in our series, resistance to carbapenems may occur, as already described in studies from Japan [22], [28], [29], [30]. Resistance to clindamycin and fluoroquinolones has also been reported [22], [31]. Antibiotic treatment should be tailored according to susceptibility testing; no data are available to recommend specific regimens.
Our study has several limitations. First, because of the small sample size, it was underpowered to compare clinical characteristics of patients according to underlying conditions or to identify further risk factors for complicated B. cereus bacteraemia. Second, when dealing with B. cereus, contamination is always an issue. We made every effort to exclude blood culture contamination by selecting only cases with at least two positive blood culture bottles and associated compatible symptoms. Third, in the absence of positive cultures of the affected site, both the source attribution of B. cereus bacteraemia and the definition of CNS involvement may be arbitrary. Definition of CRBSI is particularly difficult, because the CVC was removed in less than half of cases. Therefore, like others [19], we relied on the indirect criteria of absence of an alternative source. Finally, management of patients has changed during the 16-year-long study period. This may have affected our ability to compare the episodes.
In conclusion, while B. cereus bacteraemia is mostly benign, its clinical course can be severe in a subset of patients. Haematologic disease and catheter-related infection appear to be risk factors for a complicated course. Optimal management in this patient group includes adequate antibiotic therapy and catheter removal unless an alternative source is apparent (e.g. intestinal mucositis). Future studies should further characterize predictors of a complicated course among both haematologic and nonhaematologic patients.
Acknowledgement
We thank H. Alwan for English-language revision.
Conflict of Interest
None declared.
References
- 1.Dubouix A., Bonnet E., Alvarez M., Bensafi H., Archambaud M., Chaminade B. Bacillus cereus infections in traumatology–orthopaedics department: retrospective investigation and improvement of healthcare practices. J Infect. 2005;50:22–30. doi: 10.1016/j.jinf.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Kotiranta A., Lounatmaa K., Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189–198. doi: 10.1016/s1286-4579(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 3.Tuazon C.U., Murray H.W., Levy C., Solny M.N., Curtin J.A., Sheagren J.N. Serious infections from Bacillus sp. JAMA. 1979;241:1137–1140. [PubMed] [Google Scholar]
- 4.Bottone E.J. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue D., Nagai Y., Mori M., Nagano S., Takiuchi Y., Arima H. Fulminant sepsis caused by Bacillus cereus in patients with hematologic malignancies: analysis of its prognosis and risk factors. Leuk Lymphoma. 2010;51:860–869. doi: 10.3109/10428191003713976. [DOI] [PubMed] [Google Scholar]
- 6.Cotton D.J., Gill V.J., Marshall D.J., Gress J., Thaler M., Pizzo P.A. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol. 1987;25:672–674. doi: 10.1128/jcm.25.4.672-674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaur A.H., Patrick C.C., McCullers J.A., Flynn P.M., Pearson T.A., Razzouk B.I. Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin Infect Dis. 2001;32:1456–1462. doi: 10.1086/320154. [DOI] [PubMed] [Google Scholar]
- 8.Rhee C., Klompas M., Tamburini F.B., Fremin B.J., Chea N., Epstein L. Epidemiologic investigation of a cluster of neuroinvasive Bacillus cereus infections in 5 patients with acute myelogenous leukemia. Open Forum Infect Dis. 2015;2:ofv096. doi: 10.1093/ofid/ofv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Chest Physicians; Society of Critical Care Medicine American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 10.Turnbull P.C., Sirianni N.M., LeBron C.I., Samaan M.N., Sutton F.N., Reyes A.E. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J Clin Microbiol. 2004;42:3626–3634. doi: 10.1128/JCM.42.8.3626-3634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J.S., Sexton D.J., Mick N., Nettles R., Fowler V.G., Jr., Ryan T. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 12.Hogan W.J., Letendre L., Litzow M.R., Tefferi A., Hoagland H.C., Pruthi R.K. Neutropenic colitis after treatment of acute myelogenous leukemia with idarubicin and cytosine arabinoside. Mayo Clin Proc. 2002;77:760–762. doi: 10.4065/77.8.760. [DOI] [PubMed] [Google Scholar]
- 13.Thuler L.C., Velasco E., de Souza Martins C.A., de Faria L.M., da Fonseca N.P., Dias L.M. An outbreak of Bacillus species in a cancer hospital. Infect Control Hosp Epidemiol. 1998;19:856–858. doi: 10.1086/647746. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama N., Mitani K., Tanaka Y., Hanazono Y., Motoi N., Zarkovic M. Fulminant septicemic syndrome of Bacillus cereus in a leukemic patient. Intern Med. 1997;36:221–226. doi: 10.2169/internalmedicine.36.221. [DOI] [PubMed] [Google Scholar]
- 15.Le Scanff J., Mohammedi I., Thiebaut A., Martin O., Argaud L., Robert D. Necrotizing gastritis due to Bacillus cereus in an immunocompromised patient. Infection. 2006;34:98–99. doi: 10.1007/s15010-006-5019-6. [DOI] [PubMed] [Google Scholar]
- 17.Auger S., Ramarao N., Faille C., Fouet A., Aymerich S., Gohar M. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl Environ Microbiol. 2009;75:6616–6618. doi: 10.1128/AEM.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mermel L.A., Allon M., Bouza E., Craven D.E., Flynn P., O'Grady N.P. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassar R., Hachem R., Jiang Y., Chaftari A.M., Raad I. Management of Bacillus bacteremia: the need for catheter removal. Medicine (Baltimore) 2009;88:279–283. doi: 10.1097/MD.0b013e3181b7c64a. [DOI] [PubMed] [Google Scholar]
- 20.Park H.G., Choi S.H. Long-term central venous catheter salvage in patients with Bacillus bacteremia. Medicine (Baltimore) 2010;89:346. doi: 10.1097/MD.0b013e3181f25680. [DOI] [PubMed] [Google Scholar]
- 21.Srivaths P.R., Rozans M.K., Kelly E., Jr., Venkateswaran L. Bacillus cereus central line infection in an immunocompetent child with hemophilia. J Pediatr Hematol Oncol. 2004;26:194–196. doi: 10.1097/00043426-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Uchino Y., Iriyama N., Matsumoto K., Hirabayashi Y., Miura K., Kurita D. A case series of Bacillus cereus septicemia in patients with hematological disease. Intern Med. 2012;51:2733–2738. doi: 10.2169/internalmedicine.51.7258. [DOI] [PubMed] [Google Scholar]
- 23.Hansford J.R., Phillips M., Cole C., Francis J., Blyth C.C., Gottardo N.G. Bacillus cereus bacteremia and multiple brain abscesses during acute lymphoblastic leukemia induction therapy. J Pediatr Hematol Oncol. 2014;36:e197–e201. doi: 10.1097/MPH.0b013e31828e5455. [DOI] [PubMed] [Google Scholar]
- 24.Psiachou-Leonard E., Sidi V., Tsivitanidou M., Gompakis N., Koliouskas D., Roilides E. Brain abscesses resulting from Bacillus cereus and an Aspergillus-like mold. J Pediatr Hematol Oncol. 2002;24:569–571. doi: 10.1097/00043426-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Vodopivec I., Rinehart E.M., Griffin G.K., Johncilla M.E., Pecora N., Yokoe D.S. A cluster of CNS infections due to B. cereus in the setting of acute myeloid leukemia: neuropathology in 5 patients. J Neuropathol Exp Neurol. 2015;74:1000–1011. doi: 10.1097/NEN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 26.McLauchlin J., Mithani V., Bolton F.J., Nichols G.L., Bellis M.A., Syed Q. An investigation into the microflora of heroin. J Med Microbiol. 2002;51:1001–1008. doi: 10.1099/0022-1317-51-11-1001. [DOI] [PubMed] [Google Scholar]
- 27.Tuazon C.U., Hill R., Sheagren J.N. Microbiologic study of street heroin and injection paraphernalia. J Infect Dis. 1974;129:327–329. doi: 10.1093/infdis/129.3.327. [DOI] [PubMed] [Google Scholar]
- 28.Katsuya H., Takata T., Ishikawa T., Sasaki H., Ishitsuka K., Takamatsu Y. A patient with acute myeloid leukemia who developed fatal pneumonia caused by carbapenem-resistant Bacillus cereus. J Infect Chemother. 2009;15:39–41. doi: 10.1007/s10156-008-0654-8. [DOI] [PubMed] [Google Scholar]
- 29.Kiyomizu K., Yagi T., Yoshida H., Minami R., Tanimura A., Karasuno T. Fulminant septicemia of Bacillus cereus resistant to carbapenem in a patient with biphenotypic acute leukemia. J Infect Chemother. 2008;14:361–367. doi: 10.1007/s10156-008-0627-y. [DOI] [PubMed] [Google Scholar]
- 30.Sakai C., Iuchi T., Ishii A., Kumagai K., Takagi T. Bacillus cereus brain abscesses occurring in a severely neutropenic patient: successful treatment with antimicrobial agents, granulocyte colony-stimulating factor and surgical drainage. Intern Med. 2001;40:654–657. doi: 10.2169/internalmedicine.40.654. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi K., Kami M., Ikeda M., Kishi Y., Murashige N., Tanosaki R. Fulminant septicemia caused by Bacillus cereus following reduced-intensity umbilical cord blood transplantation. Haematologica. 2005;90:ECR06. [PubMed] [Google Scholar]