Abstract
Background
Free-dose combination treatment with basal insulin and short-acting glucagon-like peptide-1 receptor agonists (GLP-1 RAs) reduces hyperglycemia via complementary targeting of fasting and postprandial blood glucose levels, however, in the real world, due to injection burden and clinical inertia, the full efficacy may not be able to translate into clinical and economic benefits.
Objective
The aim of the study was to evaluate treatment persistence and associated outcomes in patients with type 2 diabetes (T2D) treated with a GLP-1 RA in free-dose combination with basal insulin.
Methods
Claims data were extracted on US adults with T2D with ≥1 prescription claim for both a GLP-1 RA and a basal insulin from July 1, 2008 to June 30, 2013, and continuous health plan coverage for 6 months prior to (baseline) and 12 months after the index date (follow-up period). Outcomes analyzed for patients stratified by treatment persistence included glycemic control, hypoglycemia, and health care costs and resource utilization. Multivariate analyses were used to examine factors associated with persistence or hypoglycemia.
Results
The analysis included 7,320 patients, of whom 16.9% were persistent with free-dose combination treatment. The median time to treatment discontinuation was 133 days. Compared with nonpersistent patients, persistent patients had greater glycated hemoglobin A1c (A1C) reductions (−0.80% vs −0.42%; P=0.032), were more likely to achieve A1C <7.0% (39% vs 22%; P<0.001), and were less likely to experience hypoglycemia (9.5% vs 6.8%; P=0.002). Persistent patients also had significantly fewer hospitalizations and shorter hospital stays. While prescription costs were significantly higher (all-cause: $14,691 vs $10,791; P<0.001; diabetes-related: $8,142 vs $5,124; P<0.001), total medical charges were significantly lower (all-cause: $28,405 vs $40,292; P=0.001; diabetes-related: $11,114 vs $15,203; P=0.003) for persistent patients compared with nonpersistent patients.
Conclusion
This retrospective claims study of US patients with T2D showed that, although persistence with concurrent GLP-1 RA and basal insulin treatment is low, improved treatment persistence is associated with greater A1C reductions and lower total medical charges.
Keywords: basal insulin, GLP-1 receptor agonist, treatment persistence, type 2 diabetes
What is already known about this subject
Free-dose combination treatment for type 2 diabetes (T2D) with a glucagon-like peptide-1 receptor agonist (GLP-1 RA) plus basal insulin has been used for patients who were not able to achieve A1c target with basal insulin alone. Evidence indicates that due to the complementary effects of short-acting GLP-1 RAs on postprandial glucose (PPG) and basal insulins on fasting plasma glucose (FPG), combination therapy represents an attractive option for treatment of patients with T2D with inadequate glycemic control with standard of care oral anti-diabetic medications or basal insulin alone.
In order for antihyperglycemic therapies to be effective, patients must persist with therapy. Persistence with antihyperglycemic therapies, including basal insulin or GLP-1 RAs, has previously been reported to be associated with positive clinical outcomes and reduced health care utilization and costs. To date, real-world data on the duration of persistence with free-dose combination therapy of a GLP-1 RA plus basal insulin are limited.
What this study adds
The findings from this real-world study using health care claims data show that persistence with GLP-1 RA plus basal insulin free-dose combination therapy was low (17%) over 12 months in US patients with T2D. The greatest risk of discontinuing therapy was early on in the use of combination therapy. Approximately 20% of patients had discontinued within the first month and close to 40% within the first 3 months.
Patients with better medication persistence with GLP-1 RA plus basal insulin combination therapy had better clinical outcomes in terms of glycemic control and hypoglycemia episodes during the 12-month follow-up period. Medical resource utilization and costs were lower among treatment-persistent patients with T2D.
Introduction
Current treatment guidelines for patients with T2D recommend a patient-centered strategy based on tackling progressive worsening of glycemic control through treatment intensification. Treatment begins with lifestyle changes (eg, dietary changes, increased exercise), followed by the addition of single or multiple oral antidiabetes drugs (OADs) in order to achieve and maintain glycemic control.1,2 As T2D progresses, deterioration in pancreatic β-cell function necessitates the use of insulin therapy to maintain glycemic control in the majority of cases.2
Initiation of insulin treatment using a basal insulin is recommended, usually as an addition to OADs when glycated hemoglobin A1c (A1C) target is not achieved after ~3 months.2 Basal insulins provide effective reduction of FPG levels, but increases in PPG may be inadequately controlled, and there is a risk of hypoglycemia and weight gain compared with OAD therapy alone.3,4 Long-acting basal analog insulins such as insulin glargine 100 units/mL have proved to be safe and efficacious in long-term randomized trials and in high-quality meta-analyses,5,6 while newer basal analog insulins, such as insulin glargine 300 units/mL and ultra-long-acting insulin degludec, may have further advantages in terms of reducing weight gain and hypoglycemia.7,8
GLP-1 RAs are glucoregulatory agents that enhance β-cell function and reduce body weight in patients with T2D.9 GLP-1 RAs are a recommended option for treatment intensification in patients with T2D not achieving glycemic targets on single OAD treatment.1,2 Clinical evidence suggests that GLP-1 RAs are associated with weight loss during treatment and a lower risk of hypoglycemia than basal insulins.3,10–14 Both short- and long-acting GLP-1 RAs are available and have different therapeutic profiles. While long- (eg, albiglutide and exenatide LAR) and intermediate-acting (eg, liraglutide) GLP-1 RAs primarily target FPG, short-acting GLP-1 RAs (eg, exenatide and lixisenatide) primarily act through slowing gastric emptying and therefore target PPG, making them an appropriate partner of basal analog insulins that target FPG.3,10,15 Discontinuation of GLP-1 RAs is commonly due to gastrointestinal side effects such as nausea and vomiting.
Combination treatment with a GLP-1 RA plus basal insulin has been approved by the US Food and Drug Administration and is recommended in guidelines.2 Review of the clinical evidence indicates that owing to the complementary effects of short-acting GLP-1 RAs on PPG and basal insulins on FPG, the two types of agents represent an attractive option for intensifying the treatment of patients with T2D and inadequate glycemic control.16,17 Studies have shown that combination treatment with a GLP-1 RA and basal insulin increases glycemic control without weight gain or increased risk of hypoglycemia.18–21
In order for antihyperglycemic therapies to be effective, patients must persist with therapy. Persistence with antihyperglycemic therapies, including basal insulin or GLP-1 RAs, has previously been reported to be associated with positive clinical outcomes and reduced health care utilization and costs.22,23 To date, real-world data on persistence with a combination therapy of a GLP-1 RA plus basal insulin are limited.24,25
The aim of this study was to evaluate treatment persistence – and outcomes associated with persistence – with free-dose GLP-1 RA plus basal insulin combination therapy in a large population of patients with T2D identified from a medical claims database.
Methods
Study design and patients
This was a retrospective (January 1, 2008−June 30, 2014) database claims study using the Optum Clinformatics™ Data Mart (LabRx; Eden Prairie, MN, USA) database comprising 12−13 million annual covered lives. The database included health care claims data from the health plans of patients with United Health Group commercial Administrative Services Only (ASO) insurance and fully insured patients; both medical and pharmacy coverages were included.
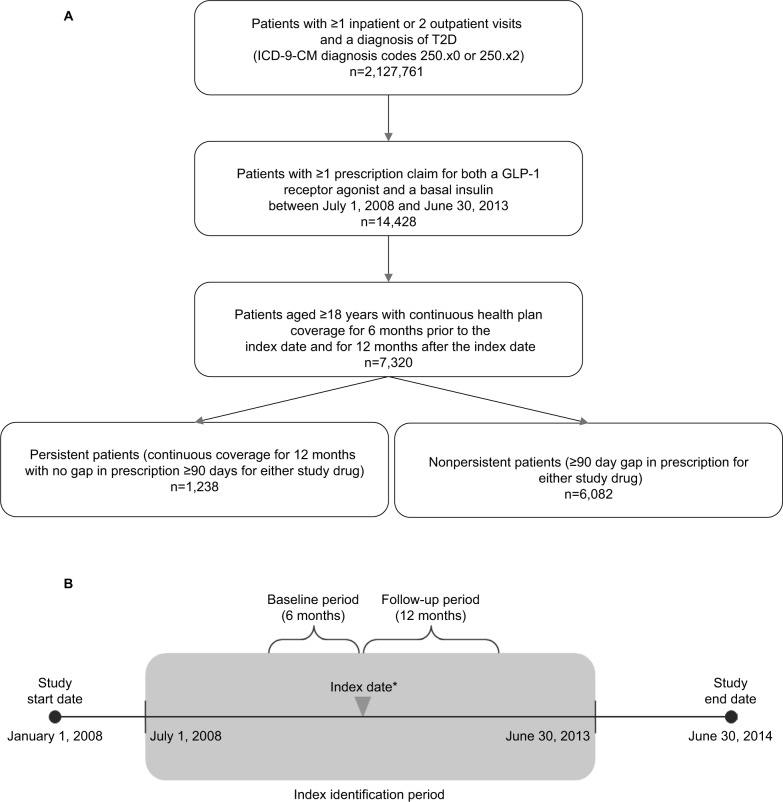
Included patients were aged ≥18 years with ≥1 inpatient or 2 outpatient visits (≥30 days apart) as recorded in the claim database, with a primary or secondary diagnosis of T2D (identified by ICD-9-CM diagnosis codes 250.x0 [Type II or unspecified type, not stated as uncontrolled] or 250.x2 [Type II or unspecified type, uncontrolled]);26 had ≥1 prescription claim for both a GLP-1 RA (either short-acting exenatide or intermediate-acting liraglutide) and a basal insulin (NPH insulin, insulin glargine, or insulin detemir) between July 1, 2008, and June 30, 2013, as recorded in the claim database; and continuous health plan coverage for 6 months prior to the index date (baseline period) and for 12 months after the index date (follow-up period; Figure 1A). The database collected data in anonymous way, retrospectively, and thus written consent was not obtained. The data used for this study also did not involve the interaction or interview with any patient and was fully de-identified. Therefore, this study is exempt from Institutional Review Board overview under the Common Rule (45 CFR ×46.101(b)(4)) and written consent under the US regulation.
Figure 1.
(A) Participant flow chart. (B) Schematic of study design.
Notes: *The initiation of the second drug in the combination therapy (eg, insulin plus GLP-1, or GLP-1 plus insulin) is defined as the index event, with the corresponding date as the index date. To ensure that patients received combination therapy after the index date, they were required to have ≥14 days of overlap for both therapies in the 90 days after the index date.
Abbreviations: T2D, type 2 diabetes; GLP-1, glucagon-like peptide-1.
Definition and measurement of combination therapy persistence
The index date for initiation of combination therapy was the date of initiation of the second therapy; ≥14 days of overlap for both therapies during the 90 days following initiation was required for inclusion. Treatment-persistent patients were defined as those patients without a prescription gap of ≥90 days in either the GLP-1 RA or the basal insulin treatment during the 12-month follow-up period. Discontinuation of either drug in the combination therapy was considered as nonpersistence. Patients were grouped into persistent and nonpersistent study cohorts (Figure 1B).
Study outcomes
A number of indices of glycemic control were compared between the study cohorts including: mean baseline A1C value, defined as the last A1C value during the baseline period or <15 days after the index date (if multiple values were available, the measurement closest to the index date was used); follow-up A1C value, defined as the last A1C value within a 3-month window at the end of the 12-month follow-up period (if multiple A1C values were recorded, the measurement closest to the end of the follow-up period was used); change in A1C, defined as the change in A1C values between baseline and follow-up; and the proportion of patients who achieved a target A1C <7.0% during the baseline and follow-up periods.
The frequency of hypoglycemia was also assessed. Hypoglycemic events were defined as any health care encounter (outpatient, inpatient, or emergency department [ED] visit) with a primary or secondary ICD-9-CM diagnosis code for hypoglycemia (ICD-9-CM codes: 250.8 [diabetes with other specified manifestations]; 251.0 [hypoglycemic coma]; 251.1 [other specified hypoglycemia]; or 251.2 [hypoglycemia, unspecified]).26
Health care resource utilization and health care costs, including all claims and associated costs incurred for inpatient, outpatient, and pharmacy services, were assessed as recorded in the claim database and compared between groups.
Statistical analysis
Descriptive statistics were used to compare the cohorts that were persistent and nonpersistent with basal insulin and GLP-1 RA combination therapy. P-values for unadjusted comparisons were calculated by χ2 test or analysis of variance where appropriate. A P-value <0.05 was used to determine the level of statistical significance.
A multivariate regression analysis was used to control for key patient characteristics and to examine the factors associated with combination treatment persistence: the dependent variable was drug persistence (yes or no) and independent variables were patient demographics information (age; gender; region; Charlson Comorbidity Index [CCI] score; baseline diabetes-related medication usage; baseline A1C; presence of baseline hypoglycemia, hypertension, and hyperlipidemia; and baseline total health care charges).
Generalized linear regression models with appropriate data transformation and data distribution were used to evaluate the impact of persistence vs nonpersistence with basal insulin and GLP-1 RA combination therapy on A1C outcomes, all-cause medical and total health care charges, and diabetes-related medical and total health care charges. The risk of hypoglycemia was analyzed by logistic regression. For both these analyses, the dependent variables were change in A1C, risk of hypoglycemia, all-cause medical and total health care charges, and diabetes-related medical and total health care charges. The independent variables were patient drug persistence status, patient demographics information including age, gender, region, CCI score, baseline diabetes-related medication usage, baseline A1C, presence of baseline hypoglycemia, and baseline total health care charge.
Results
Baseline patient characteristics
A total of 7,320 patients met the inclusion criteria and were included in the analysis. The baseline characteristics of the study population are shown in Table 1. During the 12-month follow-up period, 1,238 patients (16.9%) were persistent and 6,082 patients (83.1%) were nonpersistent with combination treatment.
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | Total (n=7,320) | Persistent (n=1,238) | Nonpersistent (n=6,082) | P-valuea |
|---|---|---|---|---|
| Age in years, mean (SD) | 56.9 (10.4) | 57.8 (9.4) | 56.7 (10.5) | <0.001 |
| Female, % (n) | 50.2 (3,674) | 43.9 (543) | 51.5 (3,131) | <0.001 |
| CCI score, mean (SD) | 2.01 (1.71) | 1.86 (1.70) | 2.04 (1.72) | <0.001 |
| Comorbidity, % (n) | ||||
| Hypertension | 67.1 (4,909) | 62.9 (779) | 67.9 (4,130) | <0.001 |
| Cardiovascular disease | 71.5 (5,231) | 67.0 (830) | 72.4 (4,401) | <0.001 |
| Renal disease | 8.8 (644) | 8.7 (108) | 8.8 (536) | 0.920 |
| A1Cb, %, mean (SD) | 8.89 (1.83) | 8.59 (1.67) | 8.94 (1.85) | 0.006 |
| Hypoglycemia during the baseline period, % (n) | 5.2 (380) | 3.6 (45) | 5.5 (335) | 0.007 |
| GLP-1 RA % (n) | ||||
| Exenatide | 23.7 (1,734) | 25.2 (312) | 23.4 (1,422) | 0.169 |
| Liraglutide | 8.0 (586) | 7.6 (94) | 8.1 (492) | 0.557 |
| Basal insulin, % (n) | ||||
| Insulin glargine | 39.4 (2,881) | 39.8 (493) | 39.3 (2,388) | 0.714 |
| Insulin detemir | 12.5 (917) | 12.6 (156) | 12.5 (761) | 0.932 |
| NPH insulin | 3.1 (226) | 2.5 (31) | 3.2 (195) | 0.193 |
| Concomitant antihyperglycemic medicine, % (n) | ||||
| Metformin | 57.3 (4,194) | 56.9 (704) | 57.4 (3,490) | 0.738 |
| Sulfonylurea | 36.7 (2,686) | 37.8 (468) | 36.5 (2,218) | 0.375 |
| DPP-4 inhibitor | 16.5 (1,208) | 16.8 (208) | 16.4 (1,000) | 0.756 |
| Thiazolidinedione | 20.4 (1,497) | 22.0 (273) | 20.1 (1,224) | 0.126 |
| Meglitinides | 2.4 (177) | 3.2 (39) | 2.3 (138) | 0.066 |
| α-Glucosidase inhibitor | 0.68 (50) | 0.65 (8) | 0.69 (42) | 0.863 |
| All-cause health care resource utilization, mean (SD) | ||||
| Number of admissions | 0.09 (0.37) | 0.08 (0.36) | 0.09 (0.38) | 0.250 |
| Number of outpatient claims | 9.53 (11.06) | 8.84 (12.04) | 9.66 (10.85) | 0.018 |
| Number of ED claims | 0.27 (1.30) | 0.18 (0.94) | 0.29 (1.36) | 0.010 |
| Diabetes-related health care resource utilization, mean (SD) | ||||
| Number of admissions | 0.05 (0.25) | 0.04 (0.19) | 0.06 (0.26) | 0.017 |
| Number of outpatient claims | 4.30 (6.54) | 4.07 (7.29) | 4.34 (6.37) | 0.185 |
| Number of ED claims | 0.06 (0.38) | 0.04 (0.30) | 0.06 (0.40) | 0.174 |
| All-cause health care resource costs in $, mean (SD) | ||||
| Inpatient and outpatient costs | 13,529 (46,717) | 10,972 (34,691) | 14,049 (48,789) | 0.035 |
| ED costs | 263 (2,019) | 204 (2,070) | 275 (2,008) | 0.263 |
| Prescription costs | 3,499 (3,533) | 3,793 (3,509) | 3,439 (3,535) | 0.001 |
| Diabetes-related health care resource costs in $, mean (SD) | ||||
| Inpatient and outpatient costs | 5,815 (28,023) | 4,357 (22,326) | 6,112 (29,039) | 0.045 |
| ED costs | 114 (1,091) | 63 (539) | 124 (1,171) | 0.072 |
| Prescription costs | 1,414 (1,331) | 1,624 (1,493) | 1,372 (1,291) | <0.001 |
Notes:
Persistent vs nonpersistent;
defined as the last A1C value during the baseline period or <15 days after the index date; if multiple A1C values were available, the measurement closest to the index date was used in the analysis.
Abbreviations: A1C, glycated hemoglobin A1c; CCI, Charlson Comorbidity Index; DPP-4, dipeptidyl peptidase; ED, emergency department; GLP-1 RA, glucagon-like peptide-1 receptor agonist; SD, standard deviation.
Compared with the persistent cohort, nonpersistent patients were significantly younger (57.8 years vs 56.7 years, respectively; P<0.001), significantly more likely to be female (43.9% vs 51.5%, respectively; P<0.001), and had a significantly higher CCI score (1.86 vs 2.04, respectively; P<0.001). Compared with the persistent cohort, nonpersistent patients were significantly more likely to have hypertension (62.9% vs 67.9%, respectively; P<0.001). The percentage of patients with renal disease did not differ between persistent and non-persistent cohorts. Nonpersistent patients also had a higher baseline mean A1C than persistent patients (persistent: 8.59% vs nonpersistent: 8.49%; P=0.006), and more of them had experienced hypoglycemia during the baseline period than persistent patients (3.6% vs 5.5%, respectively; P=0.007; Table 1).
In comparison with persistent patients, nonpersistent patients had significantly more all-cause outpatient (8.84 vs 9.66, respectively; P=0.018) and ED claims at baseline (0.18 vs 0.29, respectively; P=0.010) and more baseline diabetes-related admissions (0.04 vs 0.06, respectively; P=0.017). Compared with persistent patients, patients who were nonpersistent with combination therapy had higher baseline all-cause costs ($10,972 vs $14,049, respectively; P=0.035) and baseline diabetes-related outpatient and inpatient costs ($4,357 vs $6,112, respectively; P=0.045), but had lower baseline prescription costs ($1,624 vs $1,372, respectively; P<0.001; Table 1).
Treatment persistence and clinical outcomes
The median time to treatment discontinuation for the overall study population was 133 days (Figure 2); 22.4% of patients who discontinued did so in the first month and 39.5% discontinued within the first 3 months of treatment. Over the 12-month follow-up period, the mean reduction in A1C was significantly greater in combination-treatment-persistent patients compared with nonpersistent patients (−0.80% vs −0.42%, respectively; P=0.032). A significantly greater proportion of persistent patients achieved endpoint A1C <7.0% (39% vs 22%; P<0.001). Significantly fewer patients in the persistent cohort experienced hypoglycemia during the follow-up period (6.8% vs 9.5%; P=0.002).
Figure 2.
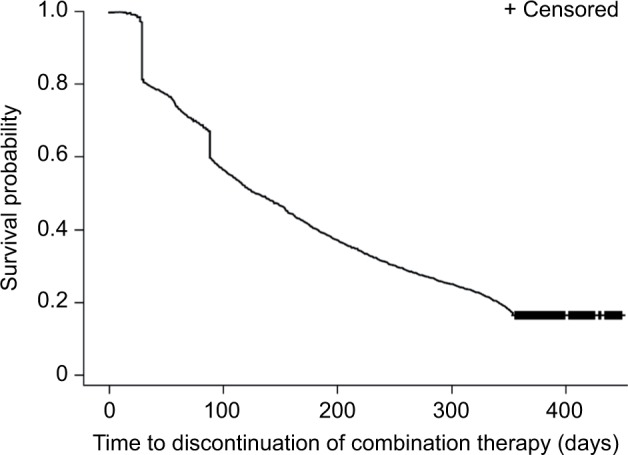
Kaplan–Meier curve of median time to discontinuation of combination therapy.
Health care utilization and costs
Persistent patients had a significantly lower number of all-cause hospitalizations (0.18 vs 0.25; P=0.003) and diabetes-related hospitalizations (0.10 vs 0.14; P=0.006) than nonpersistent patients, respectively. Persistent patients also had shorter hospital stays than nonpersistent patients (all-cause length of stay: 0.86 vs 1.39 days, respectively; P=0.011; diabetes-related length of stay: 0.35 vs 0.57 days, respectively; P=0.014). Other total and diabetes-related health care utilizations were generally comparable between the cohorts (Table 2).
Table 2.
Health care utilization in the 12-month follow-up period
| Characteristics | Total (n=7,320) | Persistent (n=1,238) | Nonpersistent (n=6,082) | P-valuea |
|---|---|---|---|---|
| All-cause, mean (SD) | ||||
| Number of admissions | 0.24 (0.72) | 0.18 (0.61) | 0.25 (0.74) | 0.003 |
| Number of outpatient claims | 22.69 (21.95) | 23.01 (24.66) | 22.62 (21.36) | 0.571 |
| Number of ED claims | 0.67 (2.91) | 0.54 (2.37) | 0.70 (3.01) | 0.074 |
| Length of in-hospital stay (days) | 1.30 (6.62) | 0.86 (5.17) | 1.39 (6.88) | 0.011 |
| Diabetes-related, mean (SD) | ||||
| Number of admissions | 0.13 (0.43) | 0.10 (0.37) | 0.14 (0.44) | 0.006 |
| Number of outpatient claims | 9.35 (11.80) | 9.44 (15.04) | 9.33 (11.03) | 0.760 |
| Number of ED claims | 0.12 (0.65) | 0.10 (0.64) | 0.12 (0.66) | 0.266 |
| Length of in-hospital stay (days) | 0.53 (2.76) | 0.35 (1.69) | 0.57 (2.93) | 0.014 |
Note:
Persistent vs nonpersistent.
Abbreviations: ED, emergency department; SD, standard deviation.
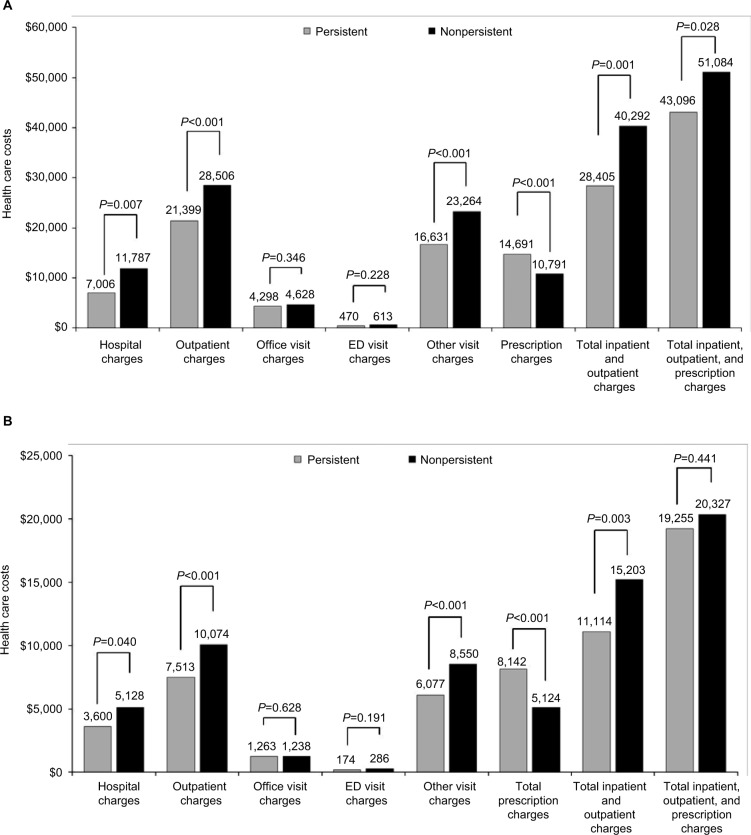
All-cause and diabetes-related total inpatient and outpatient charges were significantly lower for the persistent cohort than the nonpersistent cohort ($28,405 vs $40,292, respectively; P=0.001), as were total diabetes-related medical charges ($11,114 vs $15,203, respectively; P=0.003). Prescription costs were significantly higher in the persistent cohort than in the nonpersistent cohort (total costs: $14,691 vs $10,791, respectively; P<0.001; diabetes-related costs: $8,142 vs $5,124; P<0.001). Office visit and ED costs were comparable between the two cohorts (Figure 3).
Figure 3.
All-cause (A) and diabetes-related (B) health care costs over the 12 months of follow-up.
Abbreviation: ED, emergency department.
Multivariable regression analyses
Multivariate regression analysis showed that baseline basal insulin use was associated with a higher likelihood of persistence with combination therapy (odds ratio [OR]: 2.00 [1.148, 3.484]; P=0.014), whereas a higher baseline A1C was associated with a lower likelihood of persistence with combination therapy (OR: 0.89 [0.817, 0.967]; P=0.006). Furthermore, multivariate regression analysis showed that persistence with combination therapy was associated with a greater reduction in A1C between baseline and 12-month follow-up (estimate [95% confidence interval]): (−0.57 [−0.866, −0.272]; P=0.002) and that higher baseline A1C measurements were also associated with greater reductions in A1C (−0.53 [−0.594, −0.464]; P<0.001) during the follow-up period (Table 3). Persistence with combination therapy was also a predictor of lower all-cause inpatient and outpatient medical costs during the follow-up period (estimate [95% confidence interval]: −0.24 [−0.43, −0.05]; P=0.013) and a predictor of lower diabetes-related inpatient and outpatient medical charges (estimate [95% confidence interval]: −0.45 [−0.66, −0.24]; P<0.001) during the follow-up period. Conversely, older age and baseline hypertension were predictors of higher all-cause inpatient and outpatient costs during the follow-up period (estimate [95% confidence interval]: 0.02 [−0.43, −0.05]; P<0.001 and 0.26 [0.10, 0.43]; P=0.002, respectively). Moreover, presence of hypertension at baseline was also associated with higher diabetes-related costs (estimate [95% confidence interval]: −0.50 [0.31, 0.69]; P<0.001).
Table 3.
Predictors of A1C change
| Predictor | Estimate (%) | SE | 95% CI | P-value |
|---|---|---|---|---|
| Persistent (vs nonpersistent) | −0.5689 | 0.1516 | −0.8661, −0.2717 | 0.002 |
| Age (per year) | −0.0028 | 0.0068 | −0.0161, 0.0105 | 0.678 |
| Female (vs male) | −0.0835 | 0.1166 | −0.3119, 0.1450 | 0.474 |
| US region (vs South) | ||||
| Midwest | 0.2129 | 0.2206 | −0.2195, 0.6453 | 0.335 |
| Northeast | 0.1120 | 0.2262 | −0.3312, 0.5553 | 0.620 |
| Unknown | 0.3471 | 0.5706 | −0.7712, 1.4653 | 0.543 |
| West | 0.1384 | 0.1570 | −0.1693, 0.4461 | 0.378 |
| Health plan type (vs point of service) | ||||
| Health Maintenance Organization | 0.0040 | 0.1573 | −0.3044, 0.3124 | 0.980 |
| Exclusive Provider Organization | 0.0174 | 0.1717 | −0.3190, 0.3539 | 0.919 |
| Indemnity | −0.0851 | 1.0411 | −2.1257, 1.9555 | 0.935 |
| Preferred Provider Organization | 0.5761 | 0.3446 | −0.0994, 1.2515 | 0.095 |
| Other | 0.0316 | 0.2707 | −0.4990, 0.5621 | 0.907 |
| CCI score (vs 0) | ||||
| 1–2 | −0.3561 | 0.3495 | −1.0411, 0.3288 | 0.308 |
| 3–4 | −0.4984 | 0.3634 | −1.2107, 0.2139 | 0.170 |
| ≥5 | −0.2046 | 0.3892 | −0.9674, 0.5581 | 0.599 |
| Baseline hypoglycemia (yes vs no) | 0.1649 | 0.3050 | −0.4330, 0.7628 | 0.589 |
| Baseline hypertension (yes vs no) | 0.0315 | 0.1467 | −0.2560, 0.3190 | 0.830 |
| Baseline lipid disease (yes vs no) | −0.0569 | 0.1667 | −0.3837, 0.2699 | 0.733 |
| Baseline A1C (per %) | −0.5293 | 0.0331 | −0.5943, −0.4643 | <0.001 |
| Baseline usage of any OAD (yes vs no) | −0.1685 | 0.1435 | −0.4497, 0.1126 | 0.240 |
| Baseline usage of basal insulin (yes vs no) | −0.1724 | 0.2060 | −0.5761, 0.2313 | 0.403 |
| Baseline usage of any GLP-1 RA (yes vs no) | −0.3094 | 0.2186 | −0.7379, 0.1191 | 0.157 |
| Baseline all-cause total health care encounter charges (per $) | <0.0001 | <0.0001 | <0.0001, <0.0001 | 0.497 |
Abbreviations: A1C, glycated hemoglobin A1c; CCI, Charlson Comorbidity Index; CI, confidence interval; GLP-1 RA, glucagon-like peptide-1 receptor agonist; OAD, oral antidiabetes drug; SE, standard error.
After controlling for key patient characteristics, the risk for hypoglycemia did not significantly differ among study cohorts (OR [95% confidence interval]: 0.552 [0.283, 1.078]; P=0.082). Only health plan type “other” (2.731 [1.335, 5.588]; P=0.006) and previous history of hypoglycemia (13.549 [8.007, 22.726]; P<0.001) were predictive of hypoglycemia risk.
Discussion
The findings from this real-world study using health care claims data show that persistence with GLP-1 RA plus basal insulin free-dose combination therapy was low in US patients with T2D; only 17% of patients persisted with concurrent treatment for a period of 12 months. The low treatment persistence might have been due to the burden of injections, clinical inertia, as well as gastrointestinal-related adverse events.27 In the clinical setting, the dosage of GLP-1 RAs is sometimes reduced due to adverse events during treatment. However, information about dose reductions due to adverse events is not captured by claims data, and so it was not possible to determine whether this occurred in the population studied in this analysis. Kaplan–Meier analysis indicated that the greatest risk of discontinuing therapy was early on in the use of combination therapy. Approximately 20% of patients had discontinued treatment within the first month and close to 40% within the first 3 months. However, patients who were persistent with GLP-1 RA plus basal insulin combination therapy had significantly improved glycemic control, and significantly fewer treatment-persistent patients reported hypoglycemia episodes during the 12-month follow-up period, which might be due to the effect of basal insulin or a reduction in food intake accompanying the appetite loss associated with GLP-1 RA administration. Total hospitalization rates and both all-cause and diabetes-related total medical costs were lower among treatment-persistent patients. Multivariate regression analyses supported these observations, showing that persistence with combination therapy is predictive for a greater reduction in A1C, and lower total and diabetes-related medical costs.
These findings add to and support previously published data from randomized clinical studies showing that the addition of a GLP-1 RA to basal insulin therapy leads to improvements in glycemic control.16,18–21,28–30 Evidence from both a systematic literature review of observational and clinical practice studies (N=5,000) and a meta-analysis of clinical trials indicates that combination therapy with basal insulin and GLP-1 RAs improves glycemic control without weight gain and with no increased risk of hypoglycemia.16,31
Importantly, our observations extend the limited current knowledge and understanding of the clinical and economic impact of treatment persistence with concurrent use of GLP-1 RA plus basal insulin combination therapy in insured US patients with T2D in clinical practice. Real-world clinical outcomes associated with combination treatment have been reported in two previous retrospective studies that used national US health claims data.24,25 Both studies reported that A1C levels significantly reduced from baseline during combination therapy with exenatide and glargine with no increase in hypoglycemia, and without weight gain, irrespective of the order in which the two agents were prescribed.24,25
One of these studies, using the Integrated Health Care Information Services Impact database, reported that persistence with combined treatment for 1 year was observed in one-third of patients; this rate is higher than that observed in our study (16.9%), which may be explained in part by the difference in methodology used to determine treatment persistence. A greater proportion of patients remained on insulin glargine therapy but discontinued exenatide, possibly due to the relative inconvenience of twice-daily injections of exenatide or due to adverse gastrointestinal effects associated with exenatide.24 Similarly, in our study, baseline basal insulin use was a predictor of combination treatment persistence, indicating that patients adding GLP-1 RA therapy were more likely to discontinue during follow-up, although data on the type of discontinued medicine were not available.
As previously noted, rates of discontinuation were higher during early combination therapy with the addition of a GLP1 RA. Adverse gastrointestinal events are more common in the early stages of treatment, especially during the first 8 weeks of treatment, with GLP-1 RAs32,33 and hypoglycemia is likely to be more common during the titration phase early in insulin treatment.34 However, there was no association between baseline hypoglycemia, which predicted follow-up hypoglycemia, and discontinuation rate. Neither was there any difference in the proportion of patients using OADs, such as sulfonylureas, which are likely to cause hypoglycemia in the persistent and nonpersistent cohorts. It may be that increased treatment complexity and additional injections are driving discontinuation, as suggested in the previous study by Levin et al,24 and new-generation combined insulin/GLP-1 RA pens may offer a solution to this putative driver of discontinuation.35 Additionally, if gastrointestinal adverse events are also driving discontinuation of combination therapy, these could be mitigated by the use of fixed-ratio titratable combinations due to their slow titration of the GLP-1 RA, thereby increasing persistence.
Previously, persistence with liraglutide has been shown to be associated with significantly lower medical costs compared with those who discontinued treatment, as well as with improved A1C outcomes.22 Similarly, patients who were persistent with basal insulin treatment over a 1-year period showed greater reductions in A1C and lower health care utilization than those who were not persistent.23 However, to our knowledge, the present study is the first to demonstrate that persistence with GLP-1 RA plus basal insulin combination treatment is associated with improved glycemic control, lower health care resource use, and lower medical costs.
As discussed above, data from clinical studies support the therapeutic potential of combining basal insulin and GLP-1 RAs in one single injection. Novel therapies that combine basal insulin and GLP-1 RAs in one single fixed-ratio injection, recently approved by the US Food and Drug Administration, will potentially benefit patients with clinical efficacy and improved medication persistence and patient experience.
In pivotal clinical trials, fixed-ratio combination of Insulin Glargine and Lixisenatide (iGlarLixi), has demonstrated superior A1c reduction compared with insulin glargine alone with no increased risk of hypoglycemia or weight gain. During the continued determination of the safety and efficacy of such combinations, the effects of the comorbidities of cardiovascular disease and chronic kidney disease (CKD) should be borne in mind.36
Limitations
The study had several limitations. As this is a retrospective database analysis, no causal relationship between persistence and outcomes can be established, and the data may not be representative of all patients with T2D. The reasons for treatment discontinuation in this study are unknown. This study used prescription data to determine discontinuation rates, but prescription orders do not mean that the medication was taken by patients as directed. Also, the methodology did not include patients who restarted medication after a prescription gap of ≥90 days. Claims data also do not provide detailed information about treatment, such as the order of administration of drugs, or dose reductions due to adverse events. Furthermore, not all episodes of hypoglycemia may have been reported in the database, resulting in underreporting bias. Finally, claim database records may be subject to coding error, but they are generally considered as high-quality data sources and have been used in many research studies.
Conclusion
In conclusion, the real-world outcomes data from this retrospective database study indicate that important health economic as well as clinical benefits are associated with persistence to combined GLP-1 RA and basal insulin treatment in patients with T2D. Further large-scale observational studies are required to explore the reasons for the low persistence rates observed with this novel combination treatment.
Acknowledgments
Writing/editorial support in the preparation of this manuscript, which was funded by Sanofi US, Inc., was provided by Rosalie Gadiot, PhD, of Excerpta Medica, who wrote the initial draft of the manuscript with direction from the authors.
Footnotes
Author contributions
Study concept and design were developed by Fan and Lin. Lin and Lingohr-Smith collected the data and performed the analyses. All authors contributed toward the data analysis, interpretation of the data, drafting and critically revising the manuscript.
All authors had full access to all the data in the study and agree to be accountable for all aspects of the work. Lin is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
This study was funded by Sanofi US, Inc. Melissa Lingohr-Smith and Jay Lin are employees of Novosys Health, under contract with Sanofi US, Inc. Tao Fan is an employee of Sanofi US, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm. Endocr Pract. 2015;21(4):438–447. doi: 10.4158/EP15693.CS. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JM, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus basal insulin in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 5.Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;7:CD006383. doi: 10.1002/14651858.CD006383.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ORIGIN Trial Investigators. Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 7.Russell-Jones D, Gall MA, Niemeyer M, Diamant M, Del Prato S. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: a meta-analysis of seven clinical trials. Nutr Metab Cardiovasc. 2015;25(10):898–905. doi: 10.1016/j.numecd.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Goldman J, White JR. New insulin glargine 300 U/mL for the treatment of type 1 and type 2 diabetes mellitus. Ann Pharmacother. 2015;49(10):1153–1161. doi: 10.1177/1060028015597915. [DOI] [PubMed] [Google Scholar]
- 9.Bunck MC, Diamant M, Corner A, et al. One-year treatment with exenatide improves beta-cell function, compared with basal insulin, in metformin treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated basal insulin in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunck MC, Corner A, Eliasson B, et al. One-year treatment with exenatide versus basal insulin: effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis. 2010;212(1):223–229. doi: 10.1016/j.atherosclerosis.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu C, Ostenson CG, Matthaei S, et al. Using exenatide twice daily or insulin in clinical practice: results from CHOICE. Diabetes Ther. 2013;4(2):285–308. doi: 10.1007/s13300-013-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464–473. doi: 10.1016/S2213-8587(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 15.Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther. 2015;6(3):239–256. doi: 10.1007/s13300-015-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- 17.Meier JJ, Rosenstock J, Hincelin-Méy A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care. 2015;38(7):1263–1273. doi: 10.2337/dc14-1984. [DOI] [PubMed] [Google Scholar]
- 18.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 19.Diamant M, Nauck MA, Shaginian R, et al. 4B Study Group Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763–2773. doi: 10.2337/dc14-0876. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu C, Rodbard HW, Cariou B, et al. BEGIN: VICTOZA ADD-ON (NN1250-3948) study group A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON) Diabetes Obes Metab. 2014;16(7):636–644. doi: 10.1111/dom.12262. [DOI] [PubMed] [Google Scholar]
- 21.Ahmann A, Rodbard HW, Rosenstock J, et al. NN2211-3917 Study Group Efficacy and safety of liraglutide vs. placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056–1064. doi: 10.1111/dom.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–355. doi: 10.1007/s12325-015-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes: measures, predictors and outcomes. Endocr Pract. 2014;20(1):52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 24.Levin P, Wei W, Wang L, Pan C, Douglas D, Baser O. Combination therapy with basal insulin and exenatide: real-world outcomes in patients with type 2 diabetes. Curr Med Res Opin. 2012;28(3):1–8. doi: 10.1185/03007995.2012.654850. [DOI] [PubMed] [Google Scholar]
- 25.Levin P, Wei W, Wang L, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17–25. doi: 10.4158/EP11097.OR. [DOI] [PubMed] [Google Scholar]
- 26. CDC.gov . Centers for Disease Control and Prevention International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Atlanta, GA: 2015. [Accessed June 14, 2016]. Available from: www.cdc.gov/nchs/icd/icd9cm.htm. [Google Scholar]
- 27.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with basal insulin and metformin: a proof-of-concept study. Diabetes Care. 2010;33(7):1509–1515. doi: 10.2337/dc09-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36(9):2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wit HM, Vervoort GM, Jansen HJ, de Grauw WJ, de Galan BE, Tack CJ. Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT) Diabetologia. 2014;57(9):1812–1819. doi: 10.1007/s00125-014-3302-0. [DOI] [PubMed] [Google Scholar]
- 31.Balena R, Hensley IE, Miller A, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15(6):485–502. doi: 10.1111/dom.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381(9861):117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 33.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger J. Uncovering undetected hypoglycemic events. Diabetes Metab Syndr Obes. 2012;5:57–74. doi: 10.2147/DMSO.S29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson SL, Trujillo JM. Lixisenatide in type 2 diabetes: latest evidence and clinical usefulness. Ther Adv Chronic Dis. 2016;7(1):4–17. doi: 10.1177/2040622315609312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moghissi ES. Treating patients with diabetes of long duration: GLP-1 receptor agonists and insulin in combination. J Am Osteopath Assoc. 2014;114(5 Suppl 2):S22–S29. doi: 10.7556/jaoa.2014.086. [DOI] [PubMed] [Google Scholar]