Abstract
Chronic mucocutaneous candidiasis (CMC) is characterized by recurrent or persistent infections affecting the nails, skin and oral and genital mucosae caused by Candida spp., mainly Candida albicans. CMC is an infectious phenotype in patients with inherited or acquired T-cell deficiency. Patients with autosomal-dominant (AD) hyper IgE syndrome (HIES), AD signal transducer and activator of transcription 1 (STAT1) gain-of-function, autosomal-recessive (AR) deficiencies in interleukin (IL)-12 receptor β1 (IL-12Rβ1), IL-12p40, caspase recruitment domain-containing protein 9 (CARD9) or retinoic acid-related orphan receptor γT (RORγT) or AR autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) develop CMC as a major infectious phenotype that is categorized as Syndromic CMC. In contrast, CMC disease (CMCD) is typically defined as CMC in patients in the absence of any other prominent clinical signs. This definition is not strict; thus, CMCD is currently used to refer to patients presenting with CMC as the main clinical phenotype. The etiology of CMCD is not related to genes that cause severe combined immunodeficiency or combined immunodeficiency, nor to genes responsible for Syndromic CMC. Four genetic etiologies, AR IL-17 receptor A, IL-17 receptor C and ACT1 deficiencies, and AD IL-17F deficiency, are reported to underlie CMCD. Each of these gene defects directly has an impact on IL-17 signaling, suggesting their nonredundant role in host mucosal immunity to Candida. Here, we review current knowledge focusing on IL-17 signaling and the genetic etiologies responsible for, and associated with, CMC.
Introduction
Candida albicans is a ubiquitous fungus and commensal yeast in humans. It can occasionally be pathogenic causing oral thrush, intertrigo and genital candidiasis in healthy populations. However, in immunocompromised hosts, Candida can cause chronic mucocutaneous or invasive infections. Chronic mucocutaneous candidiasis (CMC) is characterized by recurrent or persistent infections affecting the nails, skin and oral and genital mucosae caused by Candida spp., often C. albicans.1, 2 CMC is one of the infectious phenotypes in patients with inherited or acquired T-cell deficiencies.3, 4 These clinical observations demonstrate the pivotal role of T-cell immunity in host defense against superficial Candida infections. Recent studies have revealed that Th17 cells, together with other cells expressing retinoic acid-related orphan receptor γT (RORγT), such as γδT cells and group 3 innate lymphoid cells, produce interleukin (IL)-17 and have an essential role in host defense against mucocutaneous Candida infections in mice and humans.2, 3, 5, 6 In contrast, invasive fungal infections are also observed in patients with quantitative and/or qualitative disorders of neutrophils, such as chronic granulomatous disease (CGD), autosomal-recessive (AR) caspase recruitment domain-containing protein 9 (CARD9) deficiency and neutropenic conditions.7, 8
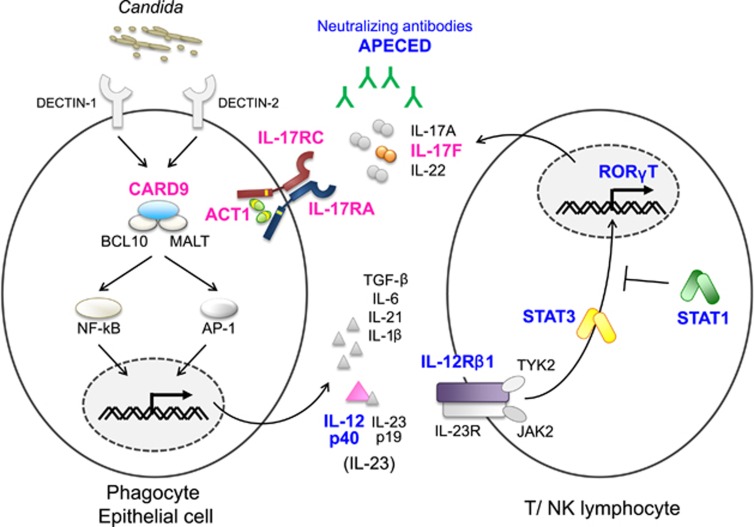
Patients with autosomal-dominant (AD) hyper IgE syndrome (HIES), AD signal transducer and activator of transcription 1 (STAT1) gain-of-function (GOF), AR autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED), or AR CARD9, IL-12 receptor β1 (IL-12Rβ1), IL-12p40 or RORγT deficiencies, develop CMC as one of the major infectious phenotypes associated with the other clinical and infectious manifestations.2, 3, 4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 These specific conditions are designated as Syndromic CMC (Table 1) and occur in association with impaired IL-17 immunity (Figure 1). Patients with AD HIES develop CMC and staphylococcal infections associated with other clinical manifestations, such as elevated serum IgE, characteristic facial features, pneumatocele and retained primary teeth. These patients have severely decreased frequencies of circulating IL-17A- and IL-22-producing T cells, probably associated with impaired STAT3-dependent signaling downstream of IL-6, IL-21 and/or IL-23.15, 17, 19, 20 The presence of CMC is also identified in one patient with AR HIES with TYK2 mutation.21 However, a follow-up study reported that the core clinical phenotype of TYK2 deficiency is mycobacterial and/or viral infections, with an association of CMC.22 Patients with APECED present with CMC in addition to polyendocrinopathy and ectodermal dysplasia.23, 24 These patients produce neutralizing autoantibodies against IL-17A, IL-17F and/or IL-22, leading to development of CMC.9, 13, 25 Neutralizing antibodies against these Th17-produced cytokines are also identified in patients with thymoma who develop CMC.9 Patients with AR CARD9 deficiency develop CMC, deep dermatophytosis and invasive fungal infections.7, 8, 26 They present with decreased frequency of circulating IL-17-producing T cells and impaired neutrophil-killing of C. albicans. Patients with AR IL-12p40 or IL-12Rβ1 deficiency develop Mendelian susceptibility to mycobacterial disease (MSMD), a primary immunodeficiency with selective host susceptibility to intracellular bacteria such as Mycobacterium bovis BCG, environmental mycobacteria and Salmonella that is associated with impaired IL-12-induced interferon gamma (IFN-γ) signaling.27, 28, 29 These patients occasionally develop mild CMC and show decreased frequencies of circulating IL-17A- and IL-22-producing T cells as a result of impaired IL-23 responses.10, 16, 17
Table 1. Syndromic CMC and CMCD: clinical and immunological phenotype and molecular defects/genetic etiologies.
| Disease | Frequency of CMC | Other infections | Associated symptoms | Immunological phenotype | Gene | Inheritance | Refs |
|---|---|---|---|---|---|---|---|
| Syndromic CMC | |||||||
| HIES | 85% | Staphylococcus, Aspergillus | Eczema, scoliosis, pneumatocoele, hyperextensibility, dysmorphic facial features, retention of primary teeth | Increased serum IgE, eosinophilia, decreased IL-17-producing T cells | STAT3 | AD | 14, 17, 19, 20, 78 |
| APECED | 70–98% | Ectodermal dysplasia, autoimmune dysfunciton of parathyroid and adrenal glands, alopecia | Neutralizing antibodies against IL-17A, IL-17F and/or IL-22 | AIRE | AR | 9, 23, 24, 25 | |
| CARD9 deficiency | 35–86% | Dermatophytes, Candida, brain abscess | Decreased IL-17-producing T cells, impairment of C. albicans-killing by neutrophils | CARD9 | AR | 7, 8, 18, 26 | |
| IL-12Rβ1 and IL-12p40 deficiency | 6–25% | Mycobacterium, Salmonella | Decreased IL-17-producing T cells, impaired IL-12 signaling | IL12RB1 IL12B | AR | 10, 11, 16, 27, 28, 29 | |
| STAT1 gain-of-function | 98% | Bacteria, viruses, fungi, mycobacteria | Aneurysm, autoimmune diseases, endocrine diseases | Decreased IL-17-producing T cells, decreased switched memory B cells | STAT1 | AD | 30, 31, 32, 33, 34, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 |
| RORγT deficiency | 6/7 (86%) | Mycobacterium | Lack of peripheral lymph node, thymic hypoplasia | Defect of MAIT, type 1 NKT, IL-17-producing T cells, impaired antigen-specific IFN-γ production | RORC | AR | 12 |
| CMCD | |||||||
| IL-17RA deficiency | 3/3 (100%) | Staphylococcus | No response to IL-17A, IL-17E and IL-17F | IL17RA | AR | 38, 72 | |
| IL-17RC deficiency | 3/3 (100%) | No response to IL-17A and IL-17F | IL-17RC | AR | 40 | ||
| IL-17F deficiency | 5/7 (70%) | Impaired IL-17F and IL-17A/F function | IL17F | AD | 38, 71 | ||
| ACT1 deficiency | 2/2 (100%) | Staphylococcus | No response to IL-17A, IL-17E, and IL-17F | TRAF3IP2 | AR | 39 | |
Abbreviations: AD, autosomal-dominant; APECED, autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy; AR, autosomal-recessive; CARD9, caspase recruitment domain-containing protein 9; CMC, chronic mucocutaneous candidiasis; CMCD, CMC disease; HIES, hyper IgE syndrome; IFN-γ, interferon gamma; IL, interleukin; RORγT, retinoic acid-related orphan receptor γT.
Figure 1.
Inborn errors of IL-17 immunity. Phagocytes recognize C. albicans via pattern recognition receptors and produce proinflammatory cytokines, such as IL-6 and IL-23. These proinflammatory cytokines activate T cells via STAT3 and upregulate RORγT expression, leading to production of IL-17A, IL-17F and IL-22. Impairment in IL-23-induced STAT3-mediated signaling in AD HIES and AR IL-12Rβ1 and IL-12p40 deficiencies cause Syndromic CMC. Neutralizing autoantibodies against IL-17A, IL-17F and IL-22 in patients with APECED impair IL-17 signaling, underlying Syndromic CMC. Patients with AR RORγT deficiency show developmental defects of Th17 cells, resulting in Syndromic CMC. They also develop MSMD, probably caused by impairment of IFN-γ production associated with mycobacterial infections. AD STAT1 gain-of-function was originally identified as a genetic etiology of CMCD. However, it can be categorized as Syndromic CMC based on its broad clinical manifestations. The majority of patients with GOF-STAT1 display a decreased frequency of IL-17-producing cells. Defects in four genes (encoding IL-17F, IL-17RA, IL-17RC and ACT1) that are directly involved in IL-17 signaling have been identified in patients with CMCD. Blue: Syndromic CMC-related molecules and neutralizing antibodies (APECED). Magenta: CMCD-related molecules.
In 2011, AD STAT1-GOF was found to be responsible for CMC disease (CMCD), typically defined as CMC in patients without any other prominent clinical signs.30, 31 Subsequent studies revealed that AD STAT1-GOF is the major genetic etiology of CMCD, explaining more than half of all CMCD cases.32, 33, 34 In the classification of primary immunodeficiency compiled by the Primary Immunodeficiency Expert Committee of the International Union of Immunological Societies, AD STAT1-GOF, together with four genetic etiologies directly related to defective IL-17 signaling, is categorized as CMC, which is often referred to as CMCD.35, 36 However, recent studies revealed that patients with GOF mutations in STAT1 present with broad clinical manifestations, including bacterial, viral, mycobacterial and invasive fungal infections, autoimmune diseases, aneurysms and tumors.33, 34 Therefore, AD STAT1-GOF is categorized as Syndromic CMC in this review.
Recently, a new primary immunodeficiency due to biallelic mutations in RORC, encoding RORγ and RORγT, was identified (designated as AR RORγT deficiency).12 RORγT is a master transcription factor of Th17 cells; thus, these patients showed a markedly decreased frequency of circulating IL-17A- and IL-22-producing T cells, which probably underlies the CMC seen in these patients. Surprisingly, all RORγT-deficient patients also developed MSMD, probably because of the impairment of IFN-γ production associated with mycobacterial infections.12
The definition of CMCD is typically CMC in patients without any other prominent clinical signs.1, 37 However, this definition is not strict, and some CMCD patients have other infectious diseases, such as infection with Staphylococcus aureus.38, 39 Therefore, the term CMCD is now used to refer to patients presenting with CMC as the major clinical phenotype, and its etiology is neither related to genes known to cause severe combined immunodeficiency or combined immunodeficiency, nor genes responsible for Syndromic CMC. Four of the five genes implicated in CMCD (IL17F, IL17RA, IL-17RC and TRAF3IP2/ACT1) are directly involved in IL-17 signaling (Table 1).38, 39, 40 These genetic disorders clearly reveal the pure and nonredundant role of IL-17 in mucocutaneous immunity to Candida in humans (Figure 1). Here, we review current knowledge of IL-17-signaling defects and the genetic etiologies of Syndromic CMC and CMCD.
IL-17 cytokines, receptors and signaling
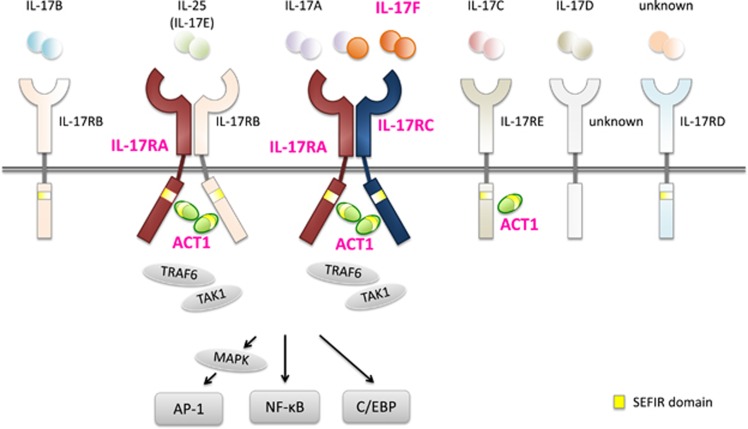
The IL-17 cytokine family consists of six members (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F), whereas the IL-17 receptor family consists of five members (IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE; Figure 2).41 IL-17 cytokines form disulfide-linked homodimers, and IL-17A and IL-17F can also form heterodimers (IL-17A/F). IL-17A and IL-17F share the strongest sequence homology and are produced by a distinct subset of T helper cells, Th17. Receptors for all IL-17 cytokines also form homodimers or heterodimers, and each combination recognizes distinct IL-17 cytokines, with IL-17RA as the common subunit for each complex. For example, the receptor complex formed by IL-17RA/C recognizes IL-17A and IL-17F, whereas the complex formed by IL-17RA/B recognizes IL-17E (CD25). In each case, signaling triggers recruitment of ACT1 as an adaptor molecule for downstream signaling (Figure 2). IL-17A and IL-17RA are the original members of the IL-17 cytokine and receptor families, respectively, and, as such, are commonly referred to as IL-17 and IL-17R. Mutations in four genes, IL17F, IL17RA, IL-17RC and TRAF3IP2 (which encode ACT1) that directly relate to IL-17A/F-induced, IL-17RA/C-mediated signaling, have been identified in patients with CMCD.38, 39, 40 Furthermore, mutations in IL17RA and TRAF3IP2 also affect IL-17E-induced, IL-17RA/B-mediated signaling (Figure 2). These clinical and experimental observations strongly suggest that defects in IL-17 signaling have a pivotal role in host mucocutaneous immunity against Candida in humans.
Figure 2.
IL-17 and IL-17 receptor family. The IL-17 cytokine family consists of six members (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F), whereas the IL-17R family consists of five members (IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE). IL-17 cytokines form disulfide-linked homodimers, whereas IL-17A and IL-17F can form heterodimers. Functional receptors for IL-17 family cytokines are thought to consist of homodimers or heterodimers. Upon stimulation, ACT1 is recruited to IL-17RA, IL-17RB and/or IL-17RC (and probably IL-17RE) by homotypic dimerization of two SEFIR domains, and activates the nuclear factor-κB (NFκB), mitogen-activated protein (MAP) kinase and CCAAT enhancer-binding protein (C/EBP) signaling pathways. In this pathway, mutations in four genes (IL17F, IL17RA, IL-17RC and TRAF3IP2/ACT1) have been identified in patients with CMCD. These mutations are directly related to IL-17A/F-induced, IL-17RA/RC-mediated signaling, and mutations in IL17RA and TRAF3IP2 also affect IL-17E-induced, IL-17RA/IL-17RB-mediated signaling. Thus, effective host mucocutaneous immunity against Candida in humans is critically dependent on functional and effective IL-17A/F-induced, IL-17RA/C-mediated signaling. Magenta: CMCD-related molecules.
Classification of Syndromic CMC
AD Hyper IgE Syndrome
HIES is a primary immunodeficiency disease, which is characterized by elevated serum IgE levels, recurrent staphylococcal skin abscesses, eczema and pulmonary infections. It was first described in 1966 and was originally named Job's syndrome.42 HIES has either a dominant or recessive pattern of autosominal inheritance, with the rare AR HIES largely shown to be caused by mutations in DOCK8 (OMIM ID: 243700); in addition, AD HIES has been shown to be mainly caused by germline heterozygous STAT3 mutations (OMIM ID: 147060).15 STAT3 has a central role in signal transduction downstream of multiple cytokines, including IL-6, IL-10, IL-17, IL-22, IL-23 and IL-27. The STAT3 mutations identified in AD HIES patients are loss-of-function (LOF) and exert a dominant-negative effect on wild-type STAT3-mediated signaling.15 In addition to their infectious phenotype, patients with STAT3 mutations present with multiple clinical manifestations, including characteristic facial features, high-arched palate, retained primary teeth, scoliosis, osteoporosis and hyperextensibility of joints. STAT3-deficient patients also frequently develop CMC associated with other infectious and clinical manifestations. A large cohort study, collecting 60 patients with germline STAT3 mutations, revealed that 85% of the STAT3-mutated patients develop CMC, including oral (63%), genital (18%), cutaneous (16%) and esophageal (8%) candidiasis and chronic onychomycosis (57%).43 C. albicans was the major pathogen isolated in 88% of collected samples obtained from infected sites.43 These patients have severely decreased frequency of circulating IL-17A- and IL-22-producing T cells. Furthermore, naive CD4+ T cells isolated from patients demonstrated significantly impaired differentiation into Th17 cells, probably associated with impaired STAT3-dependent signaling downstream of IL-6, IL-21 and IL-23.15, 17, 19, 20 Together, AD HIES patients who develop CMC as one of the many clinical manifestations associated with impaired Th17 differentiation are thus categorized as Syndromic CMC.
AR APECED
APECED, also called APS-1 syndrome, is an AR inherited disorder caused by biallelic mutations in AIRE (OMIM ID:240300). Affected patients suffer from autoimmune polyendocrinopathy, such as Addison's disease, hypoparathyroidism and hypogonadism. They also develop alopecia areata, vitiligo and ectodermal dystrophy, such as nail dystrophy, or dental enamel dysplasia. CMC is one of the major infectious phenotypes of APECED, observed in up to 98% of patients (Table 1).25 Patients with APECED develop neutralizing autoantibodies against cytokines IL-17A (41%), IL-17F (75%) and/or IL-22 (91%).9 However, no other anticytokine autoantibodies have been detected in these patients (including against IL-6, IL-10, IL-12, IL-18, IL-21, IL-23 or IFN-γ).13 Peripheral blood mononuclear cells from APECED patients with CMC show decreased IL-17F and IL-22 secretion in vitro, following stimulation with heat-killed C. albicans hyphae,9 and this cellular phenotype is correlated with the presence of neutralizing antibodies against IL-17A, IL-17F and/or IL-22 in patient serum.9 These results clearly reveal that autoimmunity in APECED targets not only endocrine organs, but also IL-17 immunity via production of high titer of neutralizing antibodies, resulting in a specific impairment of host mucosal immunity to C. albicans.9
AR CARD9 deficiency
CARD9 is an intracellular adaptor molecule that, together with its binding partners BCL10, Malt1 and NEMO, mediates signals from C-type lectin-like receptors, Dectin-1 and Dectin-2, to induce transcription and production of proinflammatory cytokines via nuclear factor-κB (NFκB) signaling. In 2009, a primary immunodeficiency, which associates with a genetic defect of CARD9, was identified in the patients who suffer from CMC and invasive fungal infections (OMIM ID:212050).7 A homozygous mutation, Q295X, in CARD9 was identified in those patients.7 Subsequent studies revealed that patients with AR CARD9 deficiency also suffer from deep dermatophytosis, invasive Exophiala dermatitidis, subcutaneous Phaeohyphomycosis and Candida-species meningoencephalitis and/or colitis, and are thus considered Syndromic CMC.18, 26, 44, 45 There are several reports describing a decreased frequency of circulating IL-17-producing cells in CARD9-deficient patients, probably explaining the clinical phenotype of CMC.7, 18, 45 On the other hand, several studies also report that the frequency of circulating IL-17-producing cells in CARD9-deficient patients is equivalent to healthy controls.44, 46 Therefore, there is some controversy regarding the frequency of circulating IL-17-producing cells in CARD9-deficient patients. Neutrophils kill both serum-opsonized and unopsonized C. albicans via distinct mechanisms; reactive oxygen species production by the NADPH oxidase system has an important role for neutrophil-killing of serum-opsonized Candida, whereas neutrophil-killing of unopsonized Candida requires complement receptor type 3 (CR3) and CARD9.47 Neutrophils from CARD9-deficient patients show a selective C. albicans-killing defect that is CR3- and CARD9-dependent, but NADPH oxidase-independent.8 Furthermore, patients with AR CARD9 deficiency are particularly predisposed to meningoencephalitis caused by Candida species.18 This might be explained by the enhanced requirement of CR3- and CARD9-dependent neutrophil-killing in the limited access of plasma proteins that is required for opsonization in cerebrospinal fluids,8 as well as the finding that neutrophils from CARD9-deficient patients normally inhibit germination of Aspergillus fumigatus, consistent with the clinical observation that no CARD9-deficient patients were reported to have Aspergillus species infection.8, 18
AR IL-12Rβ1 deficiency and AR IL-12p40 deficiency
IL-12, IL-23, IL-27 and IL-35 belong to the IL-12 cytokine family. Functional IL-12 (also called IL-12p70) consists of a heterodimer of IL-12p35 and IL-12p40 subunits, each of which has distinct effector functions. IL-12p40 is a common component of both IL-12 and IL-23; IL-12 drives T helper 1 (Th1) differentiation, whereas IL-23 is critical for Th17 survival and expansion. IL-12Rβ1 combines with IL-12Rβ2 or IL-23R to form high-affinity receptors for IL-12 or IL-23, respectively. IL-12 binds to the IL-12R complex (IL-12Rβ1 and IL-12Rβ2), on T lymphocytes and NK cells, and induces IFN-γ production. IL-23 binds to its receptor complex (IL-12Rβ1 and IL-23R) on Th17 cells and has an important role in maintenance of Th17 cells and induction of IL-17 and IL-22.
AR-complete IL-12Rβ1 deficiency (OMIM ID: 614891) is the most common genetic cause of MSMD, explaining 44% of MSMD patients with a known genetic etiology.48 The first cases of AR-complete IL-12Rβ1 deficiency were reported in 1998.27, 28 From the first identification, a total of 180 patients from 136 kindreds have since been reported.48 A large cohort study, collecting 141 patients from 102 kindreds with AR-complete IL-12Rβ1 deficiency, revealed its heterogeneous clinical manifestations. Mycobacterial disease (83%), Salmonellosis (43%) and CMC (23%) were the three major infectious phenotypes reported in symptomatic patients.11 Moreover, 78% of BCG-vaccinated patients developed BCG disease. In contrast, 8 of the 29 genetically affected siblings were asymptomatic (27%), suggesting incomplete penetrance of this disorder.
The first case of AR-complete IL-12p40 deficiency (OMIM ID: 614890) was identified in 1998 in a patient born to consanguineous parents who developed disseminated infection with BCG and S. enteritidis.29 A follow-up study, collecting 49 patients from 30 kindreds, revealed that patients with AR-complete IL-12p40 deficiency develop recurrent infections due to Salmonella (36.4%) and mycobacteria (25%).10 Strikingly, BCG disease was observed in 40 of the 41 patients (97.5%) who were vaccinated with BCG. Moreover, CMC was also reported in three patients (6%). The clinical penetrance of IL-12p40 deficiency is incomplete, with 33.3% of genetically affected relatives of index cases showing no symptoms. Therefore, AR-complete IL-12p40 and IL-12Rβ1 deficiencies are clinical phenocopies that show increased susceptibility to intracellular pathogens and develop CMC.10, 11, 48
Genetic defects in IL12B or IL12RB1, which encode IL-12p40 or IL-12Rβ1, respectively, affect both IL-12- and IL-23-induced signaling. Leukocytes from patients with AR-complete IL-12p40 deficiency show a complete absence of IL-12p40, IL-12 and IL-23 proteins.10, 17, 29 T-cell blasts from IL-12Rβ1-deficient patients have undetectable cell surface protein expression of IL-12Rβ1, and thus a complete lack of cellular responses to IL-12 and IL-23.11 In both cases, the lack of IL-12 protein itself or cellular response to IL-12 results in poor production of IFN-γ by T and NK cells, and is the pathogenic mechanism responsible for susceptibility to intracellular pathogens, such as mycobacteria and Salmonella. In contrast, the absence of IL-23 protein or defective cellular responses to IL-23 forms the likely molecular cause of CMC in these patients.10, 16, 17, 49, 50 Indeed, patients with AR-complete IL-12p40 or IL-12Rβ1 deficiencies show decreased frequencies of circulating IL-17-producing cells, albeit a less severe reduction than observed in patients with AD HIES. This difference may explain the disparity in the frequency and severity of CMC between AD HIES and AR IL-12p40/IL-12Rβ1 deficiencies.10, 11, 17, 43
AD STAT1-GOF
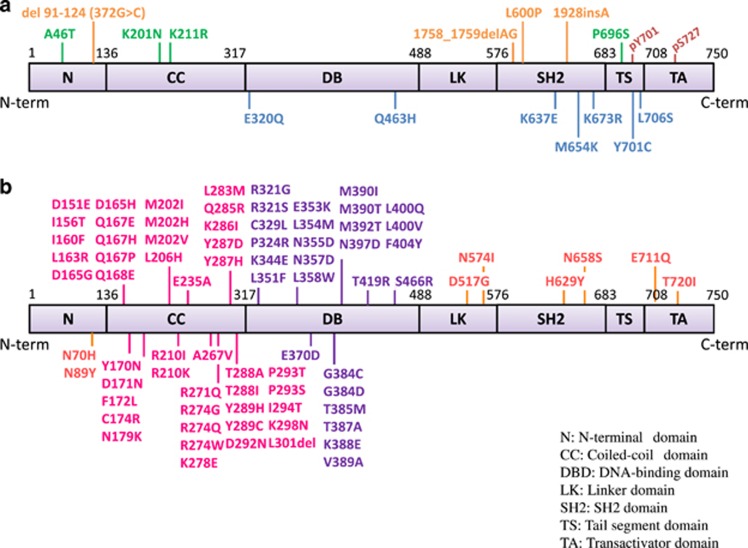
Germline mutations in STAT1 cause diverse range of primary immunodeficiencies (Figure 3).51 Patients with biallelic hypomorphic or LOF-STAT1 mutations (AR STAT1 deficiency; OMIM ID: 613796), which partially or completely impair STAT1 protein expression, show susceptibility to viruses and intracellular bacteria.48 The infectious phenotype observed in patients with AR-partial STAT1 deficiency is milder than in those with AR-complete STAT1 deficiency who require hematopoietic stem cell transplantation to avoid life-threatening infections. Germline monoallelic hypomorphic or LOF-STAT1 mutations are responsible for AD MSMD (AD STAT1 deficiency; OMIM ID: 614892). These STAT1 mutations do not disturb STAT1 protein expression, but exert a dominant-negative effect on IFN-γ-induced STAT1-mediated signaling.48 In 2011, monoallelic GOF-STAT1 (OMIM ID: 614162) mutations were shown to cause the AD form of CMCD (Table 1).30, 31 These mutations impair dephosphorylation of STAT1, leading to hyperphosphorylation of STAT1 Tyr701 in response to IFN-γ, IFN-α/β and IL-27 stimulation. This finding has enabled the development of a simple flow cytometry-based STAT1 functional test to facilitate the diagnosis of CMCD patients with GOF-STAT1 mutations.32 GOF-STAT1 mutations are preferentially identified in the coiled-coil domain and DNA-binding domain of STAT1, whereas there are no obvious hot spots for LOF-STAT1 mutations (Figure 3).30, 31, 33, 48, 51, 52 Moreover, GOF-STAT1 mutations are a major mechanism of molecular pathogenesis of CMCD, and are reported to explain more than half of the cases of this disorder.30, 31, 32, 33, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65
Figure 3.
Germline STAT1 mutations identified in patients with primary immune deficiency. (a) Loss-of-function or hypomorphic mutations are shown above human STAT1α isoform. Germline STAT1 mutations identified in patients with an AR form of complete (orange) or partial (green) STAT1 deficiency. Germline STAT1 mutations identified in patients with AD MSMD are shown in blue. (b) Germline GOF-STAT1 mutations are shown above human STAT1α isoform. Gain-of-function mutations are preferentially identified in coiled-coil domain (magenta) and DNA-binding domain (purple) of STAT1. CC, coiled-coil domain; DB, DNA-binding domain; LK, linker domain; N, N-terminal domain; SH2, SH2 domain; TA, transcriptional activation domain; TS, tail segment.
Although CMC is the major infectious manifestation among the patients with GOF-STAT1 mutations, some patients develop fungal infections other than candidiasis, or bacterial and viral infections, mycobacterial infections, autoimmune disorders, including IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome) and/or even fatal combined immunodeficiency.53, 54, 56, 57, 58, 59, 60, 65, 66 Recently, a large cohort study investigating 274 patients from 167 kindreds reported in detail the clinical manifestations of patients with GOF-STAT1 mutations.34 In this large cohort, the majority of patients with STAT1-GOF mutations developed CMC (98%), with a median age at onset of 1 year. Many patients also suffered from bacterial infections (74%), mainly due to S. aureus (36%), and viral infections (37%) typically caused by Herpes viruses (88%), whereas others/some experienced invasive fungal infections (10%) and mycobacterial diseases (6%). In addition to the infectious phenotypes reported in these patients, over one-third also presented with autoimmune manifestations (37%), such as hypothyroidism (23%), type 1 diabetes (4%) and blood cytopenias (4%), highlighting the broad and devastating clinical symptoms that can be associated with CMC in many patients with GOF-STAT1 mutations. Therefore, based on these broad clinical manifestations, AD STAT1-GOF can be categorized as Syndromic CMC, rather than the original categorization of CMCD. Immunological test also detects B-cell defects in these patients, with reduced CD19+CD27+ memory B cells (49%) and low IgG2 (38%) or IgG4 (50%).34 Although impaired development of IL-17-producing T cells ex vivo is consistently observed in patients with GOF-STAT1 mutations,30 the molecular mechanism underlying such developmental defects of IL-17-producing T cells is unknown.30 In mice and humans, IFN-γ, IFN-α/β and IL-27, which predominantly signal via STAT1, inhibit IL-17 T-cell development.67 In contrast, IL-6, IL-21 and IL-23, which mainly signal via STAT3, promote IL-17 T-cell development.30, 51 Probably, IFN-γ-, IFN-α/β- and/or IL-27-induced enhanced STAT1 activity might inhibit IL-17 T-cell development in patients with GOF-STAT1 mutations. It is also possible that GOF-STAT1 mutations affect IL-6-, IL-21- and/or IL-23-induced STAT3 activity.2, 3, 6, 64 Further studies are required to fully understand the molecular and pathogenic mechanisms of GOF-STAT1 mutations underlying CMC.
AR RORγT deficiency
RORγT is a lineage-determining transcription factor of Th17 cells and has crucial role for Th17 development, Th17 effector cytokine production and expression of the Th17 chemokine receptor, CCR6.68 In 2015, germline homozygous mutations in RORC, which encodes RORγ and RORγT, were identified in seven patients from three unrelated kindreds presenting with complex infectious phenotypes, with CMC and severe mycobacterial infections (OMIM ID: 616622).12 Six of seven patients developed mild mucocutaneous Candida infections, and mycobacterial infection was severe and observed in all patients. Four of the seven patients developed disseminated mycobacterial infection and one died because of BCG meningoencephalitis. The patients presented with mild T-cell lymphopenia, thymic hypoplasia, lack of palpable axillary and cervical lymph nodes, and absence of MAIT and iNKT cells that were consistent with the phenotype of Rorc−/− mice.12 Moreover, Rorc−/− mice were also susceptible to mycobacterial infection, suggesting that host susceptibility to mycobacteria was not a human-specific finding.12 All three homozygous mutations (S17L, Q308* and Q411*) in RORC identified in these patients were LOF and impaired DNA-binding ability of the target sequence of RORγT in the promoter region of IL-17A. CD3+ T cells from the patients displayed severe impairment in the production of IL-17A, IL-17F and IL-22, and impaired IFN-γ production in response to mycobacterial challenge. These clinical and experimental observations demonstrate the essential role of RORγT not only for the development of IL-17-producing lymphocytes to protect the mucocutaneous barriers against Candida, but also for the activation of IFN-γ-producing T cells required for systemic protection against Mycobacterium.
CMCD: molecular defects and patient management
AD IL-17F deficiency
The first identification of AD IL-17F deficiency (OMIM ID: 613956) was in a multiplex family from Argentina in 2011.38 A heterozygous missense mutation, S95L (c.284C>T), in IL17F was identified in this family. The S95L mutation was found in four patients with CMC, as well as two asymptomatic family members (aged 9 months and 21 years), suggesting incomplete clinical penetrance. All four patients developed CMC from the first year of life. In addition to CMC, recurrent furunculosis and recurrent upper respiratory tract infections were observed in one patient. One sibling, who lacked genetic testing for IL17F, died at the age of 6 years from encephalopathy of unclear etiology associated with extensive oral candidiasis. The S95L IL-17F mutant (IL-17FS95L) was normally expressed and formed homo- and heterodimers with IL-17F, IL-17FS95L and IL-17A. However, IL-17FS95L was severely hypomorphic and exerted a dominant-negative effect by impairing the binding of its complexes to the receptor. Curiously, Il17f−/− mice do not show susceptibility to experimental infection with intravenously administered Candida.69 In contrast, Il17a−/− mice are susceptible only to cutaneous and systemic candidiasis.69, 70 Possible explanations for this discrepancy could be a different function of IL-17F between mice and humans, or the dominant-negative effect of IL-17FS95L on IL-17A signaling. A subsequent study identified a second multiplex family with AD IL-17F deficiency.71 The proband and his mother, carrying an undescribed heterozygous IL17F variation, developed CMC. Although no functional validation was performed, this might be the second family reported with AD IL-17F deficiency.
AR IL-17RA deficiency
The first patient reported with AR IL-17RA deficiency (OMIM ID: 613953) was born to consanguineous Moroccan parents.38 A homozygous nonsense mutation, Q284*, in IL17RA that was inherited from asymptomatic consanguineous parents was identified. The patient developed recurrent CMC, and was resistant to local antifungal treatment from the first month of life. He was also susceptible to S aureus, presenting with skin abscess and folliculitis on the buttocks. Although the patient had several episodes of conjunctivitis, acute media otitis, lower respiratory tract infections and folliculitis, he never developed severe bacterial infection. Analysis of peripheral blood mononuclear cells and patient-derived fibroblasts showed no IL-17RA protein expression on their surface. Moreover, no response to homo- and heterodimeric IL-17A and IL-17F was observed in fibroblasts38 and no response to IL-17E (IL-25) was observed in peripheral blood mononuclear cells from the patient39 (Figure 2). A subsequent study identified a multiplex family with the combination of AR IL-17RA and adenosine deaminase 2 deficiency.72 Two siblings with CMC identified in this study shared a homozygous large deletion including entire regions of IL17RA and CECR1 (encoding adenosine deaminase 2). The absence of IL-17RA surface protein expression was confirmed with flow cytometry on patient neutrophils, monocytes and CD4+ T cells. Overall, the clinical observations in patients with AR IL-17RA deficiency are comparable to those in Il17ra−/− mice that show susceptibility to mucocutaneous pathogens, such as Candida and Staphylococcus.5, 73 Together with the original case of AR IL-17RA deficiency, complete clinical penetrance was observed in this disorder.
AR IL-17RC deficiency
So far, three unrelated CMCD patients, one from Argentina and the others from Turkey, have been reported with AR IL-17RC deficiency (OMIM ID: 616445).40 Three different nonsense homozygous mutations, Q138*, R376* and R378*, in IL-17RC that were inherited from asymptomatic parents, were identified in the patients. All patients with biallelic mutations in IL-17RC developed CMC, suggesting complete clinical penetrance for this disorder. Unlike AR IL-17RA and ACT1 deficiencies, patients with AR IL-17RC deficiency did not have recurrent staphylococcal infections. Moreover, none of the patients suffered from severe or recurrent bacterial infections. All mutations were shown to be loss-of-expression, with a lack of IL-17RC cell surface expression in HEK293T-transfected cells and normal IL-17RA expression on SV-40-immortalized fibroblasts obtained from the patients. The specific IL-17RC defect in these patients was demonstrated by a lack of cellular responses to homo- and heterodimers of IL-17A and IL-17F, but normal responses to IL-17RC-independent signaling via IL-25 (Figure 2). Staphylococcal disease is frequently observed in patients with AR IL-17RA and ACT1 deficiency (described below), whereas it is not obvious in patients with AD IL-17F or AR IL-17RC deficiency. The infectious phenotype of patients with AR IL-17RC deficiency resembled that of/ observed in patients with AD IL-17F deficiency, and was consistent with that of Il17rc−/− mice.74 This clinical observation supports the contribution of an additional defect in the signaling pathway, downstream of IL-17E, in patients with AR IL-17RA and ACT1 deficiency.
AR ACT1 deficiency
AR ACT1 deficiency was first reported in 2013 in two siblings born to consanguineous Algerian parents.39 A homozygous mutation, T536I, in the SEF/IL-17 receptor (SEFIR) domain of TRAF3IP2 (encodes ACT1) that was inherited from asymptomatic parents was identified. Both patients developed CMC, suggesting complete clinical penetrance for this disorder. One patient also had recurrent episodes of folliculitis decalvans and bilateral blepharitis caused by S. aureus. ACT1 is an adaptor molecule that interacts with multiple partners, including members of the IL-17R family.39, 41 Upon stimulation, ACT1 is recruited to IL-17RA, IL-17RB and/or IL-17RC (and probably IL-17RE) by homotypic dimerization of two SEFIR domains, and activates the NFκB, mitogen-activated protein kinase and CCAAT enhancer-binding protein pathways (Figure 2).39 ACT1 also has an inhibitory role in B-cell survival by negatively regulating CD40 and B-cell-activating factor receptor through interaction with TRAF3.75 The T536I ACT1 mutation does not disturb its protein expression. However, this mutation specifically impairs the homotypic interaction of ACT1 with IL-17RA, IL-17RB and IL-17RC, abolishing responses to IL-17A and IL-17F in fibroblasts and to IL-17E in leukocytes 39 (Figure 2). In contrast, the T536I mutation does not affect SEFIR domain-independent interactions. This mutant normally interacts with CD40 and other SEFIR-independent interaction partners such as heat-shock proteins 70 and 90.39 The selective defect in the IL-17 signaling due to T536I-specific ACT1 mutation in the SEFIR domain may explain the phenotypic discrepancy between identified human AR ACT1 deficiency and Act1−/− mice. Unlike human AR ACT1 deficiency, Act1−/− mice display enhanced B-cell responses to CD40L and BAFF, resulting in hypergammaglobulinemia.75 In conclusion, the specific TRAF3IP2 mutation that selectively impairs the function of ACT1 SEFIR domain is responsible for CMCD.
Management and treatment of patients with CMCD and AD stat1-GOF
Most patients with CMCD are treated with topical and/or systemic antifungal agents.30, 31, 33, 38, 39, 40 Fluconazole is the main first-line oral therapy, followed by itraconazole, posaconazole and/or voriconazole. As for topical treatment, nystatin is a good alternative to triazoles.33 CMC in approximately one-third of patients with GOF-STAT1 mutations is successfully treated with azoles, whereas a partial response is observed in the others.33, 34 In general, long-lasting treatments and/or prophylaxis are required to treat persistent and prevent recurrence of CMC.30, 31, 33, 38, 39, 40 Patients with AR IL-17RA and ACT1 deficiency develop staphylococcal infections in addition to CMC. Antibiotic prophylaxis with sulfamethoxazole–trimethoprim seems to be effective to treat these patients.38, 39 Patients with GOF-STAT1 mutations present various clinical manifestations in addition to CMC. Many patients suffer from bacterial infections, such as lower respiratory infections (in 47% patients), ear-nose-and-throat infections (44%) and skin infections (28%), associate with infections of S. aureus (36%), Streptococcus spp. (20%), Pseudomonas aeruginosa (13%) and Haemophilus influenzae (9%).34 Thus, some patients are also considered for antibiotic prophylaxis, such as sulfamethoxazole–trimethoprim, to prevent bacterial infections. Moreover, patients with GOF-STAT1 mutations occasionally develop severe autoimmune disorders that require immunosuppressive treatment.57 The Janus kinase inhibitor, ruxolitinib, has been trialed in two patients with GOF-STAT1 mutations, leading to improvement of CMC and autoimmune syndrome, without significant adverse effects.62, 76 Hematopoietic stem cell transplantation might be considered as a treatment option for patients with GOF-STAT1 mutations, especially for those with severe clinical presentations, such as recurrent severe viral and/or bacterial infections, IPEX-like syndrome or hemophagocytic syndrome.66 Indeed, invasive infections, cerebral aneurysms and cancers are considered to be strong predictors of poor outcome.34 Hematopoietic stem cell transplantation seems to be an effective cure of CMC, but large case studies are required to validate the wider application of this treatment for all individuals with CMC.55
Conclusion
The recent identification of genetic etiologies of Syndromic CMC and CMCD has revealed the nonredundant role of IL-17 in mucocutaneous immunity to Candida in humans. These discoveries have improved our understanding of CMC, by revealing inheritance, clinical course and prognosis. Furthermore, clarification of the molecular pathogenesis potentially gives us the opportunity to find target molecules, such as Janus kinase inhibitors, which target signaling, to improve the clinical symptoms. It might also inform of the potential risk of increased susceptibility to Candida in patients treated with anti-IL-17-targeted immunotherapies.77 The discovery of GOF-STAT1 mutation as a molecular pathogenesis of Syndromic CMC was a breakthrough in this field. From the first identification, more than 300 of patients with GOF-STAT1 have been identified.34 However, there are still many patients with CMC who lack a genetic etiology. Further studies are required to reveal the entire picture of this congenital disorder.
Acknowledgments
We thank Miyuki Tsumura, Shiho Nishimura Sonoko Sakata and Reiko Kagawa for helpful discussions. This study was supported by JSPS KAKENHI Grant Numbers 16K15528 and 16H05355, and by the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and development, AMED. This research was also funded by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), University Paris Descartes, the French National Research Agency (ANR; Grant no ANR-11-BSV3-0005 to AP), the ‘Investments for the future' program (grant no ANR-10-IAHU-01), US National Institutes of Health (NIH; Grant no 5U01AI109697-02), the Rockefeller University and the St Giles Foundation.
The authors declare no conflict of interest.
References
- Kirkpatrick CH. Chronic mucocutaneous candidiasis. Pediatr Infect Dis J 2001; 20: 197–206. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol 2012; 12: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatrics 2013; 25: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol 2010; 22: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009; 206: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypowyj S, Picard C, Marodi L, Casanova JL, Puel A. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol 2012; 42: 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 2009; 361: 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 2013; 121: 2385–2392. [DOI] [PubMed] [Google Scholar]
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010; 207: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C et al. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore) 2013; 92: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010; 89: 381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M et al. Immunodeficiencies. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015; 349: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010; 207: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28: 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 2009; 206: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor beta1 deficiency. Clin Infect Dis 2014; 58: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 2008; 205: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol 2015; 135: 1558–1568.e1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol 2008; 122: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008; 205: 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 2006; 25: 745–755. [DOI] [PubMed] [Google Scholar]
- Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med 2015; 212: 1641–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnish-German AC. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 1997; 17: 399–403. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M et al. Positional cloning of the APECED gene. Nat Genet 1997; 17: 393–398. [DOI] [PubMed] [Google Scholar]
- Kisand K, Lilic D, Casanova JL, Peterson P, Meager A, Willcox N. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol 2011; 41: 1517–1527. [DOI] [PubMed] [Google Scholar]
- Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med 2013; 369: 1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 1998; 280: 1432–1435. [DOI] [PubMed] [Google Scholar]
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 1998; 280: 1435–1438. [DOI] [PubMed] [Google Scholar]
- Altare F, Lammas D, Revy P, Jouanguy E, Doffinger R, Lamhamedi S et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J Clin Invest 1998; 102: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 2011; 208: 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011; 365: 54–61. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Tsumura M, Okada S, Hirata O, Minegishi S, Imai K et al. Simple diagnosis of STAT1 gain-of-function alleles in patients with chronic mucocutaneous candidiasis. J Leukoc Biol 2014; 95: 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner M, Fuchs S, Raabe J, Frede N, Glocker C, Doffinger R et al. The extended clinical phenotype of 26 patients with chronic mucocutaneous candidiasis due to gain-of-function mutations in STAT1. J Clin Immunol 2016; 36: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 2016; 127: 3154–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfiha A, Jeddane L, Al-Herz W, Ailal F, Casanova JL, Chatila T et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol 2015; 35: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. J Clin Immunol 2015; 35: 696–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DM. Chronic hyperplastic candidiasis and squamous carcinoma. Br J Dermatol 1969; 81: 125–127. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011; 332: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 2013; 39: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med 2015; 212: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 2011; 34: 149–162. [DOI] [PubMed] [Google Scholar]
- Davis SD, Schaller J, Wedgwood RJ. Job's Syndrome. Recurrent, ‘cold', staphylococcal abscesses. Lancet 1966; 1: 1013–1015. [DOI] [PubMed] [Google Scholar]
- Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine 2012; 91: e1–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Barbati E, Meinzer U, Liu L, Pedergnana V, Migaud M et al. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J Infect Dis 2015; 211: 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang W, Lin Z, Wang X, Li T, Yu J et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol 2014; 133: 905–908.e903. [DOI] [PubMed] [Google Scholar]
- Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis 2014; 59: 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 2014; 124: 590–597. [DOI] [PubMed] [Google Scholar]
- Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol 2014; 26: 454–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol 2009; 27: 485–517. [DOI] [PubMed] [Google Scholar]
- Maher CO, Dunne K, Comerford R, O'Dea S, Loy A, Woo J et al. Candida albicans stimulates IL-23 release by human dendritic cells and downstream IL-17 secretion by Vdelta1 T cells. J Immunol 2015; 194: 5953–5960. [DOI] [PubMed] [Google Scholar]
- Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L et al. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol 2012; 24: 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezaki S, Yamada M, Kato M, Park MJ, Maruyama K, Yamazaki Y et al. Chronic mucocutaneous candidiasis caused by a gain-of-function mutation in the STAT1 DNA-binding domain. J Immunol 2012; 189: 1521–1526. [DOI] [PubMed] [Google Scholar]
- Hori T, Ohnishi H, Teramoto T, Tsubouchi K, Naiki T, Hirose Y et al. Autosomal-dominant chronic mucocutaneous candidiasis with STAT1-mutation can be complicated with chronic active hepatitis and hypothyroidism. J Clin Immunol 2012; 32: 1213–1220. [DOI] [PubMed] [Google Scholar]
- Toth B, Mehes L, Tasko S, Szalai Z, Tulassay Z, Cypowyj S et al. Herpes in STAT1 gain-of-function mutation [corrected]. Lancet 2012; 379: 2500. [DOI] [PubMed] [Google Scholar]
- Aldave JC, Cachay E, Nunez L, Chunga A, Murillo S, Cypowyj S et al. A 1-year-old girl with a gain-of-function STAT1 mutation treated with hematopoietic stem cell transplantation. J Clin Immunol 2013; 33: 1273–1275. [DOI] [PubMed] [Google Scholar]
- Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 2013; 131: 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol 2013; 131: 1611–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans G, Moens L, Schaballie H, Van Eyck L, Borgers H, Wuyts M et al. Gain-of-function mutations in signal transducer and activator of transcription 1 (STAT1): chronic mucocutaneous candidiasis accompanied by enamel defects and delayed dental shedding. J Allergy Clin Immunol 2014; 134: 1209–1213.e1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic SS, Puel A, Casanova JL. Orf infection in a patient with Stat1 gain-of-function. J Clin Immunol 2014; 35: 80–83. [DOI] [PubMed] [Google Scholar]
- Kumar N, Hanks ME, Chandrasekaran P, Davis BC, Hsu AP, Van Wagoner NJ et al. Gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation-related primary immunodeficiency is associated with disseminated mucormycosis. J Allergy Clin Immunol 2014; 134: 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla F, Fox H, Davenport EE, Sadler R, Anzilotti C, van Schouwenburg PA et al. Chronic mucocutaneous candidiasis: characterization of a family with STAT-1 gain-of-function and development of an ex-vivo assay for Th17 deficiency of diagnostic utility. Clin Exp Immunol 2016; 184: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol 2015; 135: 551–553. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Muramatsu H, Okuno Y, Hayashi Y, Mizoguchi Y, Tsumura M et al. Extrapulmonary tuberculosis mimicking Mendelian susceptibility to mycobacterial disease in a patient with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol 2016; 137: 619–622.e611. [DOI] [PubMed] [Google Scholar]
- Zheng J, van de Veerdonk FL, Crossland KL, Smeekens SP, Chan CM, Al Shehri T et al. Gain-of-function STAT1 mutations impair STAT3 activity in patients with chronic mucocutaneous candidiasis (CMC). Eur J Immunol 2015; 45: 2834–2846. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kofod-Olsen E, Spaun E, Larsen CS, Christiansen M, Mogensen TH. A STAT1-gain-of-function mutation causing Th17 deficiency with chronic mucocutaneous candidiasis, psoriasiform hyperkeratosis and dermatophytosis. BMJ Case Rep 2015. [DOI] [PMC free article] [PubMed]
- Sharfe N, Nahum A, Newell A, Dadi H, Ngan B, Pereira SL et al. Fatal combined immunodeficiency associated with heterozygous mutation in STAT1. J Allergy Clin Immunol 2014; 133: 807–817. [DOI] [PubMed] [Google Scholar]
- Liu H, Rohowsky-Kochan C. Interleukin-27-mediated suppression of human Th17 cells is associated with activation of STAT1 and suppressor of cytokine signaling protein 1. J Interferon Cytokine Res 2011; 31: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010; 32: 681–691. [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 2010; 185: 5453–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader O, Weig MS, Gross U, Schon MP, Mempel M, Buhl T. Photo quiz. A 32-year-old man with ulcerative mucositis, skin lesions, and nail dystrophy. Chronic mucocutaneous candidiasis by multidrug-resistant Candida albicans. Clin Infect Dis 2012; 54 972 1035–1036. [DOI] [PubMed] [Google Scholar]
- Fellmann F, Angelini F, Wassenberg J, Perreau M, Arenas Ramirez N, Simon G et al. IL-17 receptor A and adenosine deaminase 2 deficiency in siblings with recurrent infections and chronic inflammation. J Allergy Clin Immunol 2016; 137: 1189–1196.e1182. [DOI] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010; 120: 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P et al. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 2010; 184: 4307–4316. [DOI] [PubMed] [Google Scholar]
- Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF et al. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity 2004; 21: 575–587. [DOI] [PubMed] [Google Scholar]
- Mossner R, Diering N, Bader O, Forkel S, Overbeck T, Gross U et al. Ruxolitinib induces interleukin 17 and ameliorates chronic mucocutaneous candidiasis caused by STAT1 gain-of-function mutation. Clin Infect Dis 2016; 62: 951–953. [DOI] [PubMed] [Google Scholar]
- Huppler AR, Bishu S, Gaffen SL. Mucocutaneous candidiasis: the IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther 2012; 14: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007; 448: 1058–1062. [DOI] [PubMed] [Google Scholar]