Abstract
Although the liver's function as unique immune organ regulating immunity has received a lot of attention over the last years, the mechanisms determining hepatic immune surveillance against infected hepatocytes remain less well defined. Liver sinusoidal cells, in particular, liver sinusoidal endothelial cells (LSECs) and Kupffer cells (KCs), serve as physical platform for recruitment and anchoring of blood-borne immune cells in the liver. Liver sinusoidal cells also function as portal of entry for infectious microorganisms targeting the liver such as hepatotropic viruses, bacteria or parasites. At the same time, liver sinusoidal cells actively contribute to achieve immune surveillance against bacterial and viral infections. KCs function as adhesion hubs for CD8 T cells from the circulation, which initiates the interaction of virus-specific CD8 T cells with infected hepatocytes. Through their phagocytic function, KCs contribute to removal of bacteria from the circulation and engage in cross talk with sinusoidal lymphocyte populations to achieve elimination of phagocytosed bacteria. LSECs contribute to local immune surveillance through cross-presentation of viral antigens that causes antigen-specific retention of CD8 T cells from the circulation. Such cross-presentation of viral antigens activates CD8 T cells to release TNF that in turn triggers selective killing of virus-infected hepatocytes. Beyond major histocompatibility complex (MHC)-restricted T-cell immunity, CD1- and MR1-restricted innate-like lymphocytes are found in liver sinusoids whose roles in local immune surveillance against infection need to be defined. Thus, liver sinusoidal cell populations bear key functions for hepatic recruitment and for local activation of immune cells, which are both required for efficient immune surveillance against infection in the liver.
Infectious microorganisms targeting the liver
The liver is target of several pathogens, including bacteria derived from the gastrointestinal tract, parasites like Plasmodium spp. and hepatitis viruses, such as hepatitis A virus (HAV), hepatitis B virus (HBV) or hepatitis C virus (HCV). Bacteria derived from the gut lumen reach the liver via the portal vein that drains blood from the gastrointestinal tract. Pathogenic bacteria can actively traverse the gut wall and enter the body, but also gut microbiota may translocate once integrity of the gut wall is impaired, for instance, during increased venous pressure or chronic gut inflammatory diseases, and gain access to the bloodstream. Upon entering the bloodstream, bacteria are delivered via the portal vein to the liver where they encounter the liver's macrophage defense system.1 Parasites like Plasmodium spp. gain access to the bloodstream through mosquito bites and reach the liver via the bloodstream. The infection process in the liver involves transit of Plasmodium sporozoites through various liver cell populations, including Kupffer cells (KCs) before infecting their final target cell, the hepatocyte.2 Viruses targeting the liver like HAV, HBV or HCV may reach the liver after crossing mucosal surfaces in the gastrointestinal or genitourinary tract, or by directly gaining access to the bloodstream. Once circulating in the blood, hepatitis viruses show a remarkable liver tropism that is often mediated by high-jacking physiological transport pathways that converge in the liver.3 By this way, hepatitis viruses not only exit the bloodstream in the correct organ, but also efficiently achieve a tropism for hepatocytes. The high blood volume passing through the liver, that is, 20% of the total cardiac output, together with the slow blood flow and low shear forces in liver sinusoids together facilitate to hepatic clearance of the blood from molecules requiring metabolic degradation, but at the same time also allow pathogens to target the liver and establish infection of hepatocytes if they manage to escape immune-mediated destruction by sinusoidal cell populations.
Common to those viruses and parasites that target the liver and establish persistent infection, is the ability to circumvent the induction of strong innate immunity. RNA viruses like HCV are detected by helicases like RIG-I recognizing viral RNA in the cytosol. RIG-I activates the adapter molecule, MAVS, which is localized in the outer mitochondrial membrane. Activation of MAVS induces several transcription factors leading ultimately to the production of type I interferons. The HCV-encoded protease NS3/4A cleaves MAVS at Cys508 preventing anchoring to mitochondria and therefore inhibiting RIG-I signaling.4 A similar mechanism has been shown for HAV, where the HAV encoded serine protease 3 cleaves MAVS at Gln428, thereby preventing RIG-I signaling and type I interferon induction.5 As HAV is always cleared by the immune response, further research is required to identify the molecular mechanisms that determine the failure of the immune response to eliminate HCV-infected hepatocytes. In contrast, HBV infection is characterized by an almost complete lack of innate immunity during the acute infection and the rapid release of large amounts of viral antigens after infection in the absence of inflammation.6 The combination of lack of inflammation and large amounts of circulating viral antigens has been shown to be involved in the development of T cells with an exhausted phenotype,7 and is believed to be responsible for the exhaustion of HBV-specific immunity that facilitates persistent infection.8 Also Plasmodium spp. can evade innate immunity by remodeling of phagolysosomal compartments in infected cells.9 Thus, infection in the liver often occurs in the absence of strong innate immunity, which impedes pathogen-specific effector T cells that express CXCR3 to relocate via chemokines to sites of infection. As the liver sinusoids are a maze-like structure, finding infected hepatocytes in the absence of an inflammation-dependent localizing signal is a difficult task and likely requires high numbers of pathogen-specific CD8 T cells. Notwithstanding the ability of certain pathogens to evade innate immunity, the liver is an organ with predominant innate immunity that can mount appropriate inflammatory responses upon infection.10
The role of cross-priming and cross-presentation for immune surveillance
The initiation of protective immunity against infectious pathogens that reside within cells, such as viruses, intracellular parasites and intracellular bacteria, requires cytotoxic CD8 T-cell responses.11 For their activation, CD8 T cells are stimulated by peptides presented on major histocompatibility complex (MHC) I molecules. Such peptide-loaded MHC I molecules are typically derived from proteins endogenously expressed in cells, cleaved by the proteasome and followed by TAP-mediated transport of peptides into the endoplasmic reticulum for chaperone-assisted loading onto MHC I molecules. This pathway, however, does not allow to present exogenous antigens on MHC I molecules, which is required for professional antigen-presenting cells to activate a CD8 T-cell response. The process of presentation of exogenous antigens to naive CD8 T cells has been termed cross-priming12 and is required for induction of protective immunity against those pathogens that evade expression of their antigens in professional antigen-presenting cells.11, 13
Cross-priming is a complex process that almost exclusively occurs within secondary lymphoid organs. Among the specialized immune cells, a subpopulation of dendritic cells (DCs) is capable of cross-priming naive CD8 T cells. This DC population is characterized in mice by expression of CD24, CD8, CD103 and XCR1 in mice, and is dependent on the transcription factors IRF8 and BATF3.14 In humans, cross-priming capacity is also restricted to CD11c+CD141+ DC population with XCR1 expression.15, 16 Within secondary lymphoid tissues, cross-priming-competent DCs that have received appropriate activation signals and matured into immunogenic DCs, interact with CD8 and CD4 T cells in a spatiotemporally controlled manner to generate long-lasting and potent antiviral immunity.17 The relevance of cross-priming for successful immune surveillance has been shown for numerous infectious microorganisms.11 As cross-priming does not require antigen expression, it also allows to circumvent immune evasion strategies of pathogens that aim at limiting antigen presentation.11 As the highly organized microarchitecture of lymphoid tissues is optimized to facilitate interaction of antigen (cross)-presenting cells with the low number of antigen-specific T cells,17 cross-priming in lymphoid tissues appears to be most suited to generate effector as well as memory CD8 T cells that recognize and control infectious microorganisms.
After generation of pathogen-specific CD8 T-cell immunity within lymphoid tissues, such CD8 T cells need to relocate to peripheral organs and recognize their target cells within infected tissue. As immune evasion from antigen presentation may also occur in infected cells,18 cross-presentation of pathogen-derived antigens during the effector phase in infected organs may help the immune response to achieve immune surveillance.
The liver as immune organ
The liver is recognized as an organ that bears unique immune functions and can skew immune responses.19 Apart from the liver microenvironment that constitutes a tolerogenic milieu rich in immune-regulatory mediators such as prostaglandins, transforming growth factor (TGF)β or interleukin (IL)-10, the liver harbors many antigen-presenting cell populations that have been suggested to engage in priming of naive T cells and thereby contribute to skewing of immune responses.20
Hepatocytes not only function as metabolic units, but participate also in shaping of immune responses by presenting antigens on MHC I to CD8 cells. Hepatocytes can prime naive CD8 T cells, which results in clonal T-cell deletion in a BIM-dependent manner leading to T-cell apoptosis, thus establishing antigen-specific tolerance.21, 22 Hepatocytes also engulf and remove naive CD8 T cells following priming by a process called suicidal emperipolesis.23 The relevance of hepatocyte-mediated deletion of CD8 T cells for liver tolerance became clear through the discovery that initial (auto-)antigen presentation by hepatocytes leads to clonal deletion, whereas initial antigen presentation in lymphoid tissues causes autoimmune hepatitis, once CD8 T cells recognize again antigen presented on hepatocytes.24 The relevance of hepatocyte-restricted priming of virus-specific CD8 T cells during viral infection of the liver has not been thoroughly addressed so far.
Liver sinusoidal endothelial cells (LSECs) comprise the most prominent non-parenchymal cell population in the liver that line the liver sinusoids. They possess extraordinary scavenger functions for efficient uptake of molecules from the bloodstream.25 The endocytosed antigens are used for antigen (cross)-presentation on MHC class I and II molecules to CD8 and CD4 T cells, respectively. As LSEC do not provide co-stimulatory signals via CD80/86 or IL-12 during priming of naive T cells, these cells do not undergo maturation into effector CD4 or CD8 T cells.26 Priming of naive CD4 T cells by LSECs rather leads to generation of regulatory T cells that can suppress organ-specific autoimmunity even in the central nervous system demonstrating the relevance of hepatic immune functions for systemic immune responses.27, 28 Cross-priming of CD8 T cells by LSECs has a different outcome. Priming by immature DCs in the absence of inflammatory signals leads to a nonresponsive, anergic state or clonal deletion.29 In contrast, CD8 T-cell priming by cross-presenting LSECs leads to development into a distinct memory-like state where CD8 T cells can be reactivated by combinatorial stimuli involving T-cell receptor (TCR) signaling, CD28 signaling and signaling through the IL-12 receptor.30 Induction of this memory-like phenotype does not require an inflammatory stimulation and maturation step of LSEC.31 Rather, this unique memory T-cell programing occurs in the absence of CD28 or IL-12 signals, but involves IL-6 trans-signaling, which identifies a for unknown direct T cell adjuvant critical for induction of memory T cells.32 Thus, antigen-presenting LSECs cross-prime memory CD8 T cells with proliferative potential or induce differentiation of regulatory CD4 T cells, which shows a unique functional diversity of this liver-resident antigen-presenting cell population in skewing of immune responses.
KCs are the largest population of organ-resident macrophages. Strategically located within the sinusoids, KCs remove particulate antigens from the blood circulating through the liver.33 Simultaneously, KCs come in close contact with circulating T cells as well as liver-resident NK cells and NKT cells. Under steady-state, noninflammatory conditions, KCs possess a tolerogenic phenotype and influence the liver micromilieu by secretion of IL-10.34 However, under inflammatory circumstances, KCs may activate T cells and NKT cells leading to immunity against bacterial infection and causing liver immunopathology.35
Liver DCs compared with DCs from lymphoid tissues show a tolerogenic phenotype. Upon stimulation with toll like receptor (TLR) ligands, liver myeloid DCs produce less IL-12, but produce more IL-10 and IL-27 maintaining the livers unique tolerogenic milieu.20 Also liver plasmacytoid DCs are characterized by reduced expression of MHC II and impaired type I interferon expression upon stimulation. Both, the tolerogenic phenotype of KCs and DCs may result from the continuous presence of pathogen-associated molecular patterns derived from bacteria and antigens from the gut.36 The close link between the gut and the liver where gut-derived bacterial degradation products or even entire bacteria at low concentrations reach the liver via the portal vein, necessitates a containment of innate immunity toward these inflammatory signals.10 Notwithstanding the tolerance in responding to continuous exposure to low dose of pathogen-associated molecular patterns,37 the liver can mount innate immunity in response to infection, thereby demonstrating that innate defense mechanisms are intact.
Hepatic stellate cells (HSC) are located in the space of Dissé and control the sinusoidal diameter through contraction, thereby achieving dynamic regulation of blood flow. HSCs express MHC class I and II molecules as well as the lipid-presenting CD1 molecule together with co-stimulatory molecules, like CD86, and co-inhibitory molecules like B7H1. Although well positioned to interact with CD8 T cells, their participation in local T-cell responses by antigen cross-presentation is controversially discussed. Several reports indicate HSCs capable for cross-presentation to CD8 T cells.38, 39, 40 Yet, FACS-sorted HSCs devoid of any contaminating LSEC are not able to cross-present exogenous antigen to CD8 T cells.41
Together, liver antigen-presenting cells contribute to the liver's unique immune functions through local activation and differentiation of naive T cells. However, little is known on the relevance of local antigen (cross)-presentation for the local induction of effector functions, that is, immune surveillance in the liver.
Immune surveillance in the liver
Infectious microorganisms reaching the liver have first contact with sinusoidal cell populations, but hepatitis viruses and Plasmodia spp. target the hepatocyte and use this cell population for massive expansion, as well as for establishing a state of persistence. Although innate immune responses generated by infected cells, that is, hepatocytes, or by immune cells detecting microbial-associated molecular patterns may help to contain infection and hinder the replication of pathogens, CD8 T-cell immunity is required to achieve control of infection in the liver, exemplified for control of HBV infection through CD8 T cells in chimpanzees.42 The sentinel function of sinusoidal cell populations allows them to mount innate immunity and inflammation,43 which may help through expression of interferons to contain pathogen infection in the liver.44
Successful immune surveillance against intracellular infections requires CD8 T-cell immunity. The induction of effector functions from pathogen-specific CD8 T cells through their local activation in the infected organ is necessary to achieve control of infection. Such local activation of effector CD8 T cells is believed to occur mainly by direct recognition of pathogen-derived peptides on MHC I molecules expressed by the infected cell itself, but may also result from MHC I-restricted cross-presentation of pathogen-specific antigens on noninfected cells or by non-MHC-restricted activation of innate-like lymphocytes resident to the liver.
Direct MHC I-restricted recognition of virus-infected hepatocytes by CD8 T cells
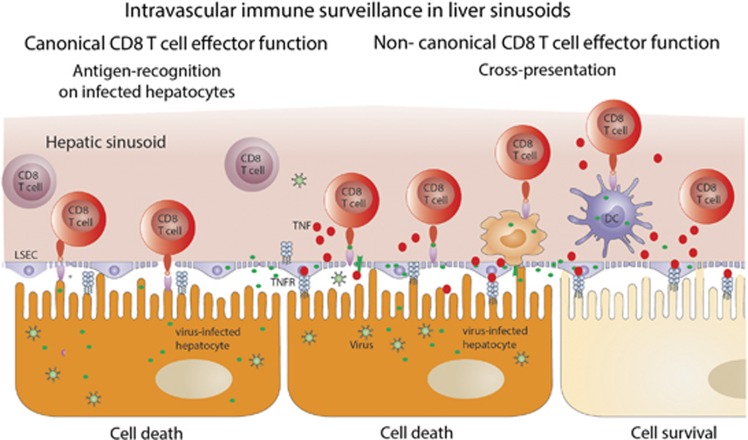
Immune surveillance in the liver is believed to occur mainly through recruitment of circulating pathogen-specific effector CD8 T cells. Adhesion of circulating CD8 T cells to sinusoidal cell populations in the liver is facilitated by the narrow sinusoidal diameter that together with the low perfusion pressure leads to low shear forces.45 As a consequence, lymphocyte adhesion to sinusoidal cells does not require expression of selectins to slow down circulating lymphocytes and narrow diameter within the sinusoids.46 As sinusoidal lining LSEC possess the so-called fenestrae,45 holes within the sinusoidal endothelial barrier of about 200 nm in size, and a lack of a basal membrane separating hepatocytes from the sinusoidal lumen, circulating CD8 T cells may establish direct contact with hepatocytes. Such direct contact with T cells may result from hepatocytes extending their protrusions through endothelial fenestrae47 or T cells passing through endothelial fenestrae. Thereby, T cells can recognize virus-derived antigens presented by MHC class I molecules directly on hepatocytes without the need for transmigration. Remarkably, adhesion of CD8 T cells within the liver can occur without inflammation in the infected area. In this case, during adhesion, T cells do not adhere directly to the endothelial cell layer, but rather to platelet aggregations within the sinusoids. These platelets in turn adhere to LSEC even under steady-state conditions creating a platform for CD8 T-cell adhesion even under under noninflammatory conditions.48 This is of interest as HBV acts as a stealth virus and does not induce inflammation in the liver.6 Interestingly, HBV-specific CD8 T cells do not arrest in the liver upon antigen-specific contact with hepatocytes expressing HBV antigens, but are randomly recruited to platelet aggregates forming on LSEC.48 The molecular mechanisms governing this stochastic interaction of T cells with platelets still await clarification. Such stochastic recruitment of CD8 T cells from the circulation may not be very efficient and may further add to the necessity for large numbers of T CD8 T cells to control infection in the liver. Once arrested in the sinusoid, T cells do not need to transmigrate across LSECs, but rather establish firm contact with HBV-expressing hepatocytes through fenestrae.48 This suggests that hepatic immune surveillance functions as intravascular immune surveillance that is facilitated by the unique characteristics of sinusoidal structure and blood flow.
The establishment of persistent viral infections of the liver, in particular, chronic hepatitis B or chronic hepatitis C, suggests that antiviral immune surveillance in the liver can be circumvented by these hepatotropic viruses. Various mechanisms have been identified that contribute to the development of persistent HBV or HCV infection.3, 8, 49 Yet, control of HBV-infected hepatocytes occurs to a significant extent through non-cytolytic mechanisms. In a HBV-transgenic mouse model, it was shown that tumor necrosis factor (TNF) and interferons control HBV gene expression and replication in a non-cytolytic manner.50 These results were confirmed in experiments using HBV-infected chimpanzees.51 Mechanistically, cytokine-induced activation of nucleic acid-degrading enzymes, that is, ApoBECs, were responsible for this non-cytolytic cytokine effect on HBV replication.52 However, HBV-specific CD8 T cells were eventually required for elimination of HBV-infected hepatocytes through cytotoxic effector mechanisms.42 Notwithstanding of the cytokine-induced control of HBV-replication, HBV-specific CD8 T cells are required for elimination of HBV-infected hepatocytes to achieve control of HBV infection.
For efficient immune surveillance against Plasmodium-infected hepatocytes, a strong initial priming period is required to generate high numbers of pathogen-specific CD8 T cells.53 Direct recognition of Plasmodium-infected hepatocytes by pathogen-specific CD8 T cells is then required to induce death of the infected hepatocytes.54
Recently, a further mechanism has been found that identifies the need for several CD8 T cells recognizing and attacking infected epithelial target cells to elicit killing. Recognition of an infected cell by a single CD8 T cell may not be sufficient to induce apoptosis, but rather two or more CD8 T cells are required to cooperatively achieve killing of target cells. Within a pool of CD8 T cells, killing capacity of a single CD8 T cell may range from 2 to 16 infected cells per day.55 The relevance of this swarm hunting behavior for clearance of virus-infected hepatocytes has not been addressed yet. In general, killing of hepatocytes requires delivery of death-inducing signals via perforin/granzyme B and FasL.
Cross-presentation and non-canonical MHC class I-mediated CD8 T-cell effector function
Although the role of cross-priming for generation of virus-specific immunity is widely recognized, the role of cross-presentation of viral antigens to virus-specific CD8 T cells during the effector phase is much less characterized. Obviously, immune escape from MHC class I-restricted antigen presentation in infected cells poses a hurdle to CD8 T-cell immune surveillance and may result in the failure to control viral infection despite the presence of virus-specific CD8 T cells.18 In the liver with its particular intravascular immune surveillance, cross-presentation may therefore benefit the recruitment of circulating T cells. Indeed, cross-presentation of soluble antigens by LSECs leads to antigen-specific recruitment of CD8 T cells to the liver even in the absence of inflammation.56 Using bioluminescence imaging allows for longitudinal detection of antiviral immunity in the liver within the same animals after infection with hepatotropic adenoviruses encoding for immunological relevant antigens combined with the marker protein luciferase.57 Detection of a decrease in luciferase expression, by reduced bioluminescence signal, in adenovirus-infected hepatocytes is superior to serological biomarkers of antiviral immunity, the hepatocellular enzymes ALT or AST, as it reflects in real time the number of living infected hepatocytes.57 This experimental setup allowed to investigate the link between the extraordinary scavenger function, and the ability to cross-present circulating antigens by LSECs and hepatic immune surveillance.
Similar to circulating antigens, LSEC also cross-present hepatocyte-derived antigens. Infection of hepatocytes with recombinant adenoviruses coding for ovalbumin, which shows high hepatocyte tropism, led to ovalbumin cross-presentation to ovalbumin-specific CD8 T cells. Such cross-presentation led to stimulation of circulating ovalbumin-specific CD8 T cells and to secretion of TNF by such activated CD8 T cells. Surprisingly, in transgenic mouse models where MHC class I-restricted interaction of virus-infected hepatocytes with CD8 T cells was excluded, virus-specific CD8 T cells still induced viral hepatitis, indicating that other mechanisms that do not rely on direct target-cell recognition allow locally activated CD8 T cells to achieve immune surveillance in the liver.58 Conversely, exclusive MHC class I-restricted presentation of virus-derived antigens on hepatocytes led only to 40–50% of antiviral CD8 T-cell effector function, suggesting that cross-presentation of viral antigens released from infected hepatocytes was operative in at least 50% of the total antiviral CD8 T-cell activity. This was termed noncanonical CD8 T-cell effector function, as it does not require direct MHC class I-restricted recognition of virus-infected target cells with virus-specific CD8 T cells to elicit antiviral effector functions (Figure 1—noncanonical CD8 T-cell effector function). Mechanistically, this noncanonical CD8 T-cell effector function is initiated by LSECs cross-presenting hepatocyte-derived viral antigens to CD8 T cells. TNF released from such CD8 T cells activated through cross-presenting LSECs than acts on hepatocytes to initiate a TNFR1-restricted induction of caspase-mediated apoptosis.58 Neither IFNγ nor type I interferons contribute to this noncanonical CD8 T-cell effector function against virus-infected hepatocytes, which demonstrates a unique role for TNF in the local immune surveillance in the liver. The noncanonical CD8 T-cell effector function was important to obtain rapid control over viral infection of the liver,58 which is particularly important to rapidly gain control over the spread of viral infection among hepatocytes in the liver. It is of interest to note that LSECs fail to cross-present HBV-derived peptides to HBV-specific CD8 T cells,48 indicating that HBV may escape from this noncanonical CD8 T-cell effector function, and that this may have a role in the establishment of persistent infection or even in the failure to terminate persistent infection. Taken together, MHC class I-restricted antigen (cross)-presentation is operational in immune surveillance against viral infection of the liver.
Figure 1.
Schematic drawing illustrating of MHC class I-restricted intravascular immune surveillance within hepatic sinusoids against viral infection. Direct MHC class I-restricted recognition of virus-infected hepatocytes by intravascular virus-specific CD8 T cells and noncanonical CD8 T-cell effector function facilitated by cross-presentation of hepatocyte-derived viral antigens through LSECs.
Furthermore, sinusoidal liver cells engage in mutual exchange of MHC class I molecules to improve local immune surveillance. Transfer of MHC class I molecules from HSCs resulted in improved cross-presentation by LSECs.41 Such trogocytosis of MHC class I molecules is distinct from cross-dressing of peptide-loaded MHC class I molecules that has been shown to be involved in cross-priming.59 Continuous supply of MHC class I molecules from other liver cell populations may ensure optimal performance of LSECs in cross-presentation and local intrahepatic immune surveillance.
Non-MHC-mediated immune surveillance through CD1 and MR1
In addition to cross-presentation of peptides generated by proteasomal digestion or endosomal degradation from viral proteins on MHC class I molecules and subsequent recognition by CD8 T cells bearing α/β T-cell receptors, other antigen-presentation pathways are increasingly recognized in their importance for local, organ-specific immune surveillance. The CD1 and MHC-like protein 1 (MR1) function to present lipid antigens or bacterial metabolites to NKT cells or mucosal-associated invariant T cells (MAIT cells), respectively, and thereby cause their activation as well as effector function.
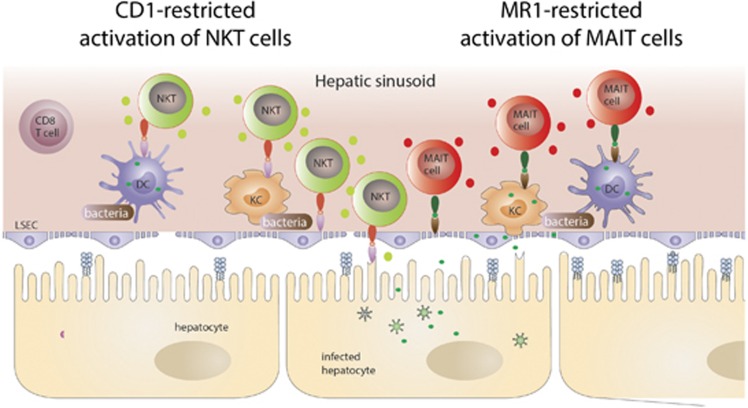
MAIT cells have been first described in 1999 and gained increasing attention over the last years for their prominent role in antibacterial defense.60 In humans, MAIT cell represent the most abundant innate-like T-cell population with about 5% of the total T-cell repertoire and that can reach up to 45% of the total liver lymphocytes.61, 62 They express a semi-invariant T-cell receptor (Vα7.2-Jα33/12/20) recognizing antigen presented by the MHC-like protein 1 (MR1).60, 63 MR1 is highly conserved molecule across many species, including mouse and human, sharing >90% homology at the protein level.64, 65 The identity of antigens presented in the context of MR1 remain largely unknown, but structural analysis of the MR1 antigen-binding cleft revealed that bacterial metabolites are presented by MR1. Kjer-Nielsen et al.65 showed that a photodegradable product of folic acid (vitamin B9) stabilized the MR1/β2-microglobulin complex, and that this complex sufficed to activate MAIT cells. A derivative of riboflavin (vitamin B2) that binds to MR1 is responsible for MAIT-cell activation, and as these vitamins are selectively produced by bacteria, this supports the notion that MAIT cells have an important role in antibacterial defense. Under physiological conditions, the MR1 protein is almost absent from the cell surface owing to lack of stabilization by its ligand.63, 66 Upon infection of cells by bacteria producing riboflavin, however, the MR1 complex is stabilized and transported to the surface.66 Yet, infection is not an absolute prerequisite for MR1-dependent activation of MAIT cells, suggesting that uptake of bacterial constituents also suffice for MR1-mediated MAIT-cell activation.67 In particular, biliary epithelial cells but very likely also liver sinusoidal cells are involved in MAIT-cell activation upon contact with bacteria68 (Figure 2—MR1-restricted activation of MAIT cells). Besides direct antigen recognition, MAIT cells can also be activated in an MR1-independent manner. It has been shown that the cytokines IL-12 and IL-18 activate MAIT cells, and lead to expression and release of IFNγ.61, 69 Thereby, MAIT cells may contribute to antiviral defense in the liver, where secreted IFNγ participate in the non-cytolytic elimination of HBV.70, 71 Along this line, TCR-independent stimulation of MAIT cells leads to IFNγ-dependent reduction of HCV replication in infected hepatocytes,72 suggesting that MAIT cell-derived IFNγ operates in antiviral defense in the liver.
Figure 2.
Illustration of possible CD1/MR-restricted intravascular immune surveillance in the liver sinusoid that may contribute to antibacterial immunity through activation of liver NKT cells and MAIT cells.
NKT cells belong to the so-called innate-like T cells and can be divided into two subpopulations: the type I or invariant NKT cells (iNKT) or the type II or diverse NKT cells. iNKT cells express like MAIT cells an invariant T-cell receptor α chain (Vα14-Jα18 in mice, Vα24-Jα18 in humans) with a limited number of T-cell receptor β chains.73, 74 This invariant TCR recognizes antigens in the context of the MHC class I like molecule CD1 that presents glycolipids.75 In contrast, type II NKT cells express a relatively broad range of TCRs. Unlike for MR1, glycolipid antigens presented by CD1 molecules are not restricted, but rather a broad variety of endogenous and exogenous lipids can be presented on CD1.76 Therefore, NKT cells acts as interface bridging innate and adaptive immunity.77 NKT cells comprise only about 0.1% of the peripheral T-cell pool, but are specifically enriched in the liver making up to 40% of all lymphocytes in the murine liver and up to 25% of lymphocytes in the human liver.10 Besides being stimulated through the CD1 molecule presenting glycolipids, iNKT cells are also stimulated by activation of Toll-like receptors, or by the cytokines IL-12, IL-18 and type I interferons.78 The outcome of NKT cell stimulation varies on the type of stimulation, the localization of stimulation and the antigen-presenting cell. Upon stimulation with the lipid αGalCer presented by CD1 molecules, iNKT cells secrete IFNγ, IL-4 and IL-17, but produce IFNγ only by stimulation through IL-12 and IL-18, or by following ischemia or toxin-induced liver injury.79 A prominent role of CD1 and iNKT cells in control of bacterial infection of the liver has been described80 (Figure 2—CD1-restricted activation of NKT cells).
The role of iNKT during viral hepatitis is so far not well investigated. However, reports indicated that iNKT cells inhibit viral replication of HCV in hepatocytes via the secretion of IFNγ.81 During early HBV infection, high numbers of activated iNKT are found that may contribute to viral defense by secreting IFNγ inhibiting HBV replication.82, 83 A recent report shows that NKT cells contribute to the antiviral response during HBV infection. Hepatocytes infected with HBV present endoplasmic reticulum-associated endogenous lipids generated by HBV-induced secretory phospholipases leading to activation of NKT cells. Absence of NKT cells, CD1 molecules or a defect in transferring endoplasmic reticulum-associated lipids to CD1 molecules leads to failure of inducing a strong T- and B-cell immunity and control of viral infection.84 Thus, liver NKT cells appear to contribute to both, antibacterial as well as antiviral immunity.
Conclusion
Taken together, immune surveillance in the liver is mainly triggered by sinusoidal liver cell populations, which establishes an intravascular immune surveillance paradigm for the liver. MHC class I-restricted antigen (cross)-presentation is not only important for cross-priming of CD8 T-cell immunity, but is also instrumental for efficient virus-specific immune surveillance in the liver through LSEC-dependent activation of noncanonical CD8 T-cell effector functions that eliminate virus-infected hepatocytes via TNF. Complemented is antiviral immune surveillance by antibacterial immune surveillance in the liver, which may similarly be initiated by sinusoidal liver cell populations, yet involves CD1- and MR1-restricted activation of NKT and MAIT cells rather than MHC class I-restricted activation of CD8 T cells. Thus, separate antigen-presentation pathways and distinct immune effector cell populations in the liver may serve to respond to viral and bacterial infections, and very likely cooperate to control infections locally in the liver.
Acknowledgments
We are grateful for funding by the DFG (SFB TR179, SFB TR36, SFB 704), DZIF and the EU FP7 project MIP DILI.
The authors declare no conflict of interest.
References
- Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 2014; 6: 237ra266. [DOI] [PubMed] [Google Scholar]
- Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS et al. Migration of Plasmodium sporozoites through cells before infection. Science 2001; 291: 141–144. [DOI] [PubMed] [Google Scholar]
- Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol 2012; 12: 201–213. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature 2006; 442: 39–44. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liang Y, Qu L, Chen Z, Yi M, Li K et al. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci USA 2007; 104: 7253–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA 2004; 101: 6669–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider DT, Alfei F, Roelli P, Barras D, Chennupati V, Darbre S et al. High antigen levels induce an exhausted phenotype in a chronic infection without impairing T cell expansion and survival. J Exp Med 2016; 213: 1819–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology 2014; 146: 1193–1207. [DOI] [PubMed] [Google Scholar]
- Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol 2002; 3: 1041–1047. [DOI] [PubMed] [Google Scholar]
- Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology 2008; 47: 729–736. [DOI] [PubMed] [Google Scholar]
- Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol 2010; 10: 403–414. [DOI] [PubMed] [Google Scholar]
- Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med 1997; 186: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 2002; 17: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008; 322: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A, Hartung E, Guttler S, Mora A, Zhou X, Hegemann A et al. Expression of XCR1 characterizes the Batf3-dependent lineage of dendritic cells capable of antigen cross-presentation. Front Immunol 2012; 3: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010; 207: 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 2015; 162: 1322–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels R, Podlech J, Pahl-Seibert MF, Julch M, Thomas D, Simon CO et al. Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J Exp Med 2004; 199: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009; 27: 147–163. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 2010; 10: 753–766. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol 1998; 28: 221–236. [DOI] [PubMed] [Google Scholar]
- Holz LE, Benseler V, Bowen DG, Bouillet P, Strasser A, O'Reilly L et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology 2008; 135: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci USA 2011; 108: 16735–16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest 2004; 114: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K et al. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol 2012; 303: R1217–R1230. [DOI] [PubMed] [Google Scholar]
- Lohse AW, Knolle PA, Bilo K, Uhrig A, Waldmann C, Ibe M et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 1996; 110: 1175–1181. [DOI] [PubMed] [Google Scholar]
- Carambia A, Freund B, Schwinge D, Bruns OT, Salmen SC, Ittrich H et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol 2015; 62: 1349–1356. [DOI] [PubMed] [Google Scholar]
- Carambia A, Frenzel C, Bruns OT, Schwinge D, Reimer R, Hohenberg H et al. Inhibition of inflammatory CD4 T cell activity by murine liver sinusoidal endothelial cells. J Hepatol 2013; 58: 112–118. [DOI] [PubMed] [Google Scholar]
- Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med 1996; 184: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher JP, Schanz O, Wohlleber D, Abdullah Z, Debey-Pascher S, Staratschek-Jox A et al. Liver-primed memory T cells generated under noninflammatory conditions provide anti-infectious immunity. Cell Rep 2013; 3: 779–795. [DOI] [PubMed] [Google Scholar]
- Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med 2000; 6: 1348–1354. [DOI] [PubMed] [Google Scholar]
- Böttcher JP, Schanz O, Garbers C, Zaremba A, Hegenbarth S, Kurts C et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8(+ T cell function. Cell Rep 2014; 8: 1318–1327. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Uhrig A, Protzer U, Trippler M, Duchmann R, Meyer zum Buschenfelde KH et al. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology 1998; 27: 93–99. [DOI] [PubMed] [Google Scholar]
- Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015; 62: 279–291. [DOI] [PubMed] [Google Scholar]
- Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology 1977; 72: 1268–1270. [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Wedel A, Schraut W, Strobel M, Wendelgass P, Sternsdorf T et al. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem 1994; 269: 17001–17004. [PubMed] [Google Scholar]
- Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 2007; 26: 117–129. [DOI] [PubMed] [Google Scholar]
- Schildberg FA, Wojtalla A, Siegmund SV, Endl E, Diehl L, Abdullah Z et al. Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology 2011; 54: 262–272. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Mucida D, Tyznik AJ, Kronenberg M, Cheroutre H. Hepatic stellate cells function as regulatory bystanders. J Immunol 2011; 186: 5549–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzel K, Schildberg FA, Welz M, Borner C, Geiger S, Kurts C et al. Transfer of MHC-class-I molecules among liver sinusoidal cells facilitates hepatic immune surveillance. J Hepatol 2014; 61: 600–608. [DOI] [PubMed] [Google Scholar]
- Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8(+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Loser E, Protzer U, Duchmann R, Schmitt E, zum Buschenfelde KH et al. Regulation of endotoxin-induced IL-6 production in liver sinusoidal endothelial cells and Kupffer cells by IL-10. Clin Exp Immunol 1997; 107: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering R, Wu J, Meng Z, Hilgard P, Lu M, Trippler M et al. Toll-like receptor-stimulated non-parenchymal liver cells can regulate hepatitis C virus replication. J Hepatol 2008; 48: 914–922. [DOI] [PubMed] [Google Scholar]
- Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol 2002; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest 1997; 99: 2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 2006; 44: 1182–1190. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L et al. Immunosurveillance of the liver by intravascular effector CD8(+ T cells. Cell 2015; 161: 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5: 215–229. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD et al. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA 1994; 91: 3764–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999; 284: 825–829. [DOI] [PubMed] [Google Scholar]
- Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014; 343: 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL et al. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog 2010; 6: e1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med 2007; 13: 1035–1041. [DOI] [PubMed] [Google Scholar]
- Halle S, Keyser KA, Stahl FR, Busche A, Marquardt A, Zheng X et al. In vivo killing capacity of cytotoxic T cells is limited and involves dynamic interactions and T cell cooperativity. Immunity 2016; 44: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 2009; 49: 1664–1672. [DOI] [PubMed] [Google Scholar]
- Stabenow D, Frings M, Truck C, Gartner K, Forster I, Kurts C et al. Bioluminescence imaging allows measuring CD8 T cell function in the liver. Hepatology 2010; 51: 1430–1437. [DOI] [PubMed] [Google Scholar]
- Wohlleber D, Kashkar H, Gartner K, Frings MK, Odenthal M, Hegenbarth S et al. TNF-induced target cell killing by CTL activated through cross-presentation. Cell Rep 2012; 2: 478–487. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 2011; 471: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 1999; 189: 1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher JE, Klenerman P, Willberg CB. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol 2014; 5: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011; 117: 1250–1259. [DOI] [PubMed] [Google Scholar]
- Huang S, Gilfillan S, Cella M, Miley MJ, Lantz O, Lybarger L et al. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem 2005; 280: 21183–21193. [DOI] [PubMed] [Google Scholar]
- Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol 1998; 161: 4066–4077. [PubMed] [Google Scholar]
- Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012; 491: 717–723. [DOI] [PubMed] [Google Scholar]
- Miley MJ, Truscott SM, Yu YY, Gilfillan S, Fremont DH, Hansen TH et al. Biochemical features of the MHC-related protein 1 consistent with an immunological function. J Immunol 2003; 170: 6090–6098. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog 2013; 9: e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery HC, van Wilgenburg B, Kurioka A, Parekh K, Stirling K, Roberts S et al. Bacteria exposed biliary epithelium and liver B cells activate intrahepatic MAIT cells in an MR1-dependent manner. J Hepatol 2016; 64: 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun 2012; 80: 3256–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2012; 61: 1754–1764. [DOI] [PubMed] [Google Scholar]
- Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hosel M et al. Interferon-gamma and tumor necrosis factor-alpha produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 2016; 150: 194–205. [DOI] [PubMed] [Google Scholar]
- van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C et al. MAIT cells are activated during human viral infections. Nat Commun 2016; 7: 11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 2013; 13: 101–117. [DOI] [PubMed] [Google Scholar]
- Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol 2014; 32: 323–366. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995; 268: 863–865. [DOI] [PubMed] [Google Scholar]
- Mori L, Lepore M, De Libero G. The immunology of CD1- and MR1-restricted T cells. Annu Rev Immunol 2016; 34: 479–510. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nat Immunol 2003; 4: 1164–1165. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 2005; 23: 877–900. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol 2010; 22: 79–86. [DOI] [PubMed] [Google Scholar]
- Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol 2010; 11: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokordelis P, Kramer B, Boesecke C, Voigt E, Ingiliz P, Glassner A et al. CD3(+CD56(+ natural killer-like T cells display anti-HCV activity but are functionally impaired in HIV(+ patients with acute hepatitis C. J Acquir Immune Defic Syndr 2015; 70: 338–346. [DOI] [PubMed] [Google Scholar]
- Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009; 58: 974–982. [DOI] [PubMed] [Google Scholar]
- Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 2000; 32: 1117–1124. [DOI] [PubMed] [Google Scholar]
- Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med 2012; 18: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]