Abstract
Granulomas are clusters of immune cells. These structures can be formed in reaction to infection and display signs of necrosis, such as in tuberculosis. Alternatively, in several immune disorders, such as sarcoidosis, Crohn's disease and common variable immunodeficiency, non-caseating granulomas are formed without an obvious infectious trigger. Despite advances in our understanding of the human immune system, the pathogenesis underlying these non-caseating granulomas in chronic inflammatory diseases is still poorly understood. Here, we review the current knowledge about the immunopathogenesis of granulomas, and we discuss how the involved immune cells can be targeted with novel therapeutics.
Introduction
Inflammation is a physiological response of the body to invading pathogens. However, if the inflammatory state is not transient and persists chronically, this can result in irreversible tissue damage.1 Typical non-infectious causes of chronic inflammation are autoimmune diseases, which are characterized by T-cell and antibody responses to self-antigens. Disorders that are characterized by innate immune responses without obvious autoantibodies are referred to as autoinflammatory diseases.2 In several autoinflammatory diseases, chronic inflammation can result in the formation of granulomas, which are clusters of immune cells in affected tissues.
The most common cause of all granuloma formation worldwide is tuberculosis.3 The formation of granulomas in tuberculosis is thought to be a physiological reaction to prevent the systemic spread of the causative pathogen, the mycobacterium.4 This immune response typically results in a caseating granuloma with signs of necrosis.5 Many other infectious agents can trigger granuloma formation (Table 1), as well as foreign body material such as beryllium, and inherited defects in neutrophil function (chronic granulomatous disease).3, 6, 7, 8, 9 In chronic inflammatory diseases and primary immunodeficiencies with chronic inflammation, the granulomas have not been associated with specific external agents. With the exception of granulomatosis with polyangiitis, these granulomas are non-caseating (Figure 1) and typically observed in patients with sarcoidosis,10 Crohn's disease11 and common variable immunodeficiency (CVID).12
Table 1. Overview of infectious and non-infectious diseases with granuloma formation.
| Category | Disease | Type of granuloma | Localization |
|---|---|---|---|
| Infectious | |||
| Bacterium | Tuberculosis | Caseating necrosis | Lung, extrapulmonary; disseminated |
| Brucellosis | Necrotizing and fibrotic | Liver, spleen | |
| Bartonellosis | Necrotizing | ||
| Actinomycosis | Non-caseating | Cervicofacial, abdominal, lung | |
| Fungus | Histoplasmosis | Necrotizing | Lung |
| Aspergillosis | Necrotizing | Lung | |
| Candidiasis | Necrotizing with abcesses | Skin | |
| Cryptococcal disease | Fibrotic with abcesses | Lung | |
| Parasitic | Leishmaniasis | Necrotizing | Skin |
| Dirofilariasis | Fibrotic and calcifying | Subcutaneous | |
| Schistomiasis | Non-caseating | Liver, intestines, bladder | |
| Viral | CMV | Unspecified | Spleen and liver |
| EBV | Unspecified | Skin | |
| Measles | Unspecified | Thyroid gland | |
| Non-infectious with known cause | |||
| Primary immunodeficiency | CGD | Non-caseating | Skin, intestines, liver |
| Malignancy | Lymphoma | Non-caseating | Lymphatic tissue |
| Foreign body | Non-caseating | Tissue with contact to foreign body particle; skin, lung, intestines | |
| Other | Berylliosis | Non-caseating | Lung |
| Non-infectious with unknown cause | |||
| Chronic inflammatory disease | GPA | Necrotizing | Lung, upper airways |
| Sarcoidosis | Non-caseating | Lung, skin, eye, lymph node, liver, CNS, heart | |
| Crohn's disease | Non-caseating | Intestines, skin, liver, lymph node | |
| Primary Immunodeficiency | CVID | Non-caseating | Lung, lymph node, liver, skin, spleen, intestines |
Abbreviations: CGD, chronic granulomatous disease; CMV, cytomegalovirus; CNS, central nervous system; CVID, common variable immunodeficiency; EBV, Epstein–Barr virus; GPA, granulomatosis with polyangiitis. This table provides a non-exhaustive list of causes of granuloma formation. The affected organs are listed from the most commonly involved organ on the left to less common. The information is derived from refs 3, 6, 7, 8, 9, 185, 186, 187, 188, 189, 190.
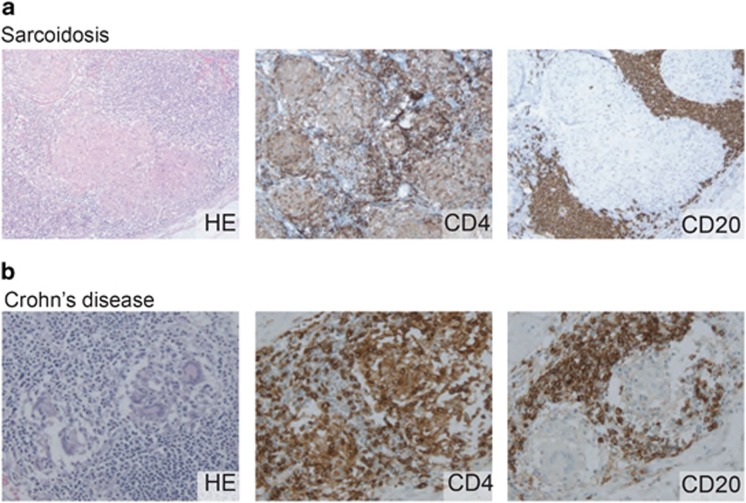
Figure 1.
Non-caseating granulomas in Crohn's disease and sarcoidosis. Hemotoxylin and eosin stainings reveal granulomatous structures in a lymph node biopsy of a patient with sarcoidosis (a) and a biopsy of the ileum of a patient with Crohn's disease (b). Typically, CD4-expressing Th cells are detected in and around the granulomas, whereas CD20-expressing B cells are found to accumulate around the granulomas.118, 130
In recent years, several new insights have been generated into granulomatous inflammation. These new insights might soon be translated to clinical care, as increasing numbers of therapeutic agents targeting various immune pathways are currently tested in clinical trials.13 Here, we review and discuss recent literature on granulomatous inflammation in sarcoidosis, Crohn's disease and CVID, all chronic inflammatory disorders with similar types of granulomas without a known trigger. We will specifically address the immune components involved in granuloma formation and how these can be used as disease markers and targeted by new therapeutic approaches for chronic autoinflammatory diseases with granuloma formation.
Chronic autoinflammatory diseases with granuloma formation
Sarcoidosis
Sarcoidosis is a multisystem granulomatous disease of unknown etiology. The hallmark of this disease is the presence of non-caseating granulomas affecting multiple organs. It is a rare disease with a worldwide prevalence ranging from 1 to 40 per 100 000 and a peak incidence at 20–39 years of age.14 The clinical presentation of sarcoidosis is highly variable and dependent on the organs involved. Systemic complaints of fever, weight loss and fatigue are common. About 90% of patients have pulmonary granulomas with frequent involvement of other organs such as lymph nodes, skin, liver, eye, central nervous system and heart.10 Owing to the high variability in clinical manifestations, it can be challenging to diagnose sarcoidosis. There is no definite test and diagnosis of sarcoidosis is based on three elements: (1) clinical and radiographic manifestations; (2) exclusion of diseases that may present similarly; (3) identification of non-caseating granulomas by histological analysis of tissue.15 Chest X-ray and computed tomography are the most common used visualization techniques. Radiographic pulmonary manifestations can vary from bihilar lymphadenopathy, pulmonary infiltration or fibrosis.16 Nuclear techniques, such as the fluorine-18 fluorodeoxyglucose positron emission tomography, can also be used to evaluate extrapulmonary manifestations of sarcoidosis or to find a location for biopsy.17 Blood tests can provide supportive information for making the diagnosis through detection of high serum levels of angiotensin-converting enzyme or soluble interleukin 2 receptor (sIL-2R), which is a marker for increased activation of T cells.14, 18
Fortunately, treatment is not necessary in over 50% of patients in whom the disease will resolve in 3 years without medication.10, 14 Patients are only given medication when inflammation leads to organ damage. First-line therapy for sarcoidosis is based on corticosteroids such as prednisone. Second-line treatment comprises immunosuppressive medication such as methotrexate and azathioprine. For refractory cases, third-line medication is available in the form of biologicals that block tumor necrosis factor-α (TNF-α): infliximab or adalimumab.19 This approach is successful in ~50% of treated patients in whom the granulomas resolve with no or little remaining organ damage. However, 20–25% of all diagnosed patients develop chronic disease with pulmonary fibrosis.14 Current therapies target inflammatory pathways and have little effect on fibrosis. This is a major limitation because fibrosis results in increased morbidity and mortality and the need for lung transplantation.20 The lack of a cure for sarcoidosis underlines the need to find new, effective drugs.10, 14
Crohn's disease
Crohn's disease is an inflammatory bowel disease.11 In recent years, the worldwide prevalence of Crohn's disease has been reported to increase, with current estimates in Western countries of 25 to 318 per 100 000.21 Similar to sarcoidosis, Crohn's disease typically affects young adults, but with a 10-fold higher prevalence. The chronic inflammation in the intestinal tract is thought to result from an interplay of the genetic background, environmental factors, intestinal microbiota and a dysregulated immune system.22 In Crohn's disease, chronic inflammation can manifest throughout the gastrointestinal tract, mainly affecting the ileum and the colon resulting in abdominal pain and diarrhea with passage of mucus or blood.11 In addition, subsets of patients show inflammation of the skin, eyes or joints. Diagnosis of Crohn's disease is based on clinical assessment and physical examination of the patient in conjunction with imaging and histopathology of inflamed tissues and with blood tests.11 Crohn's disease has many overlapping features with ulcerative colitis,23 the other major variant of inflammatory bowel disease. In contrast to Crohn's disease, inflammation in ulcerative colitis is restricted to the colon and does not result in granuloma formation. When inflammatory bowel disease is suspected, a colonoscopy is performed during which biopsies are taken. The histological finding of a non-caseating granuloma is the most discriminating factor for Crohn's disease.24 Supporting evidence from laboratory analyses include high C-reactive protein, low hemoglobin and high fecal calprotectin.11 Furthermore, the majority of patients has detectable serum levels of anti-Saccharomyces cerevisiae antibodies,25 or antibodies to the outer membrane porin C of Escherichia coli (anti-OmpC).26 Despite granulomas being a discriminating factor with ulcerative colitis, these structures are only identified in ~37% of patients with Crohn's disease.23 The presence of granulomas is associated with higher rates for surgical bowel resection, indicating that these are an indicator for severe disease.23 Treatment of Crohn's disease is similar to sarcoidosis and includes corticosteroids, immunosuppressive and biologicals. In spite of the introduction of infliximab, treatment outcomes remain suboptimal with disease control being achieved in only 60% of Crohn's patients,27 and intestinal complications and the requirement for surgery remain.11
CVID with granulomatous complications
CVID is a primary immunodeficiency. It is a rare, heterogeneous disease with a prevalence of 2 to 4 per 100 000 and mean age of diagnosis between 30 and 40 years.28 Patients suffer from recurrent sinopulmonary infections and to a lesser extent from gastrointestinal infections. The hallmark of CVID is a B-cell defect leading to low or absent levels of immunoglobulins, and can be accompanied by abnormal T-cell responses and cytokine defects. Diagnosis of CVID is made when a patient has severely reduced levels of serum immunoglobulin G (IgG) with low IgM and/or IgA, and fulfills all of the following criteria: (1) onset after 2 years of age; (2) poor or absent vaccination response and (3) exclusion of other causes of hypogammaglobinemia.29 Despite these commonalities in immunological defects and recurrent infections, CVID represents a heterogeneous group of patients with ranging clinical features that include autoimmunity, granuloma formation and hematological malignancies. These non-infectious complications are associated with high morbidity and early mortality.30 Previously, only in 2–10% of patients a molecular cause of disease was identified in genes such as ICOS, CD19, CD81, TNFRSF13C (encodes BAFFR) and TNFRSF13B (encodes TACI).31, 32, 33, 34, 35 However, none of these correlated with the incidence of granulomatous complications in 8–22% of CVID patients.12 With the recent identification of autosomal-dominant causes of complex antibody deficiencies and incomplete penetrance of some mutations (e.g. CTLA4, PIK3CD, PIK3R1 and NFKB1),36, 37, 38, 39 it will become possible to relate granulomas to a genetic cause.
In CVID patients, granulomas most prevalently affect the lungs, followed by lymph nodes, liver, skin and spleen. The presence of granulomas can precede the diagnosis of CVID for years resulting in a potential misdiagnosis of sarcoidosis. However, sarcoidosis patients do not present with recurrent infections or low/absent immunoglobulins, because serum IgG levels are normal or even elevated in sarcoidosis.40 CVID patients can also present with abdominal complaints, such as chronic diarrhea, weight loss and histological evidence of intestinal inflammation, resulting in an overlap of clinical features with Crohn's disease.41 CVID patients with granulomas are more frequently affected by other autoimmune manifestations and have a higher morbidity and mortality rate than non-granulomatous patients.12, 42 The primary treatment of CVID is intravenous or subcutaneous immunoglobulin substitution, which is highly effective in reducing the infectious burden.43 However, this treatment does not ameliorate the non-infectious complications. Conversely, granulomatous inflammation in CVID is treated with similar types of immunosuppressive agents that are used for sarcoidosis and Crohn's disease. The combination of immunodeficiency with inflammation highlights the complicated processes involved in CVID, because it appears contrasting to treat immunocompromised patients with immunosuppressive medication.
While granulomas are the hallmark of disease in sarcoidosis, these are only detected in subgroups of patients with Crohn's disease and CVID. However, the exact incidence of granulomas in these disorders remains unclear and might be underestimated because of sampling errors.23 Furthermore, granulomas in CVID are often poorly recognized by physicians or upon discovery the patient is misdiagnosed with sarcoidosis.12 As granulomatous complications are a predictor for poor disease outcome in CVID12, 42 and a pathognomic feature in Crohn's disease,44 detection of these inflammatory structures is important in diagnostic workup.
Key players in granuloma pathogenesis
Antigenic triggers
Granulomas are thought to be formed following by a foreign trigger. Therefore, in diseases thus far characterized by non-infectious granulomatous inflammation, the search for a causative agent is still ongoing. In sarcoidosis there is a particular interest in finding the responsible trigger. An increased number of sarcoidosis cases was reported in rescuers after the terrorist attack on the World Trade Center in New York,45 suggesting an external antigenic cause. Mycobacteria and Propionibacterium acnes are of specific interest, because DNA of these antigens was found in granuloma material from sarcoidosis patients with numbers ranging from 0 to 9% for Mycobacterium tuberculosis and 79 to 100% for Propionibacterium acnes.46 However, the causality of one single pathogen is debatable with such diverse pathogens being proposed.10 Antigenic agents have also been suggested to trigger granuloma formation in Crohn's disease, mainly because of the associated defective bacterial clearance by autophagy. Polymorphisms in genes involved in autophagy have been reported,47 the mechanism by which cells degrade and recycle of cellular components. In Crohn's disease this leads to the impaired capacity to handle pathogens by specialized intestinal epithelial cells, Paneth cells.48 Furthermore, the presence of anti-Saccharomyces cerevisiae antibodies and anti-OmpC antibodies are suggestive of fungal or bacterial triggers of granuloma formation.25, 26 Finally, the high prevalence of Mycobacterium avium in blood and tissue suggested that, similar to sarcoidosis, granulomas in Crohn's disease were formed in response to mycobacteria.49, 50 This theory is considered controversial, because M. avium is not typically pathogenic in humans and treatment of patients with anti-mycobacterial agents was proven ineffective.51 An antigenic driver for persistence of granulomas in CVID is unlikely, because these patients are regularly treated with antibiotic or anti-fungal drugs, and these do not effectively resolve this type of inflammation.12, 52 Yet, a high prevalence of human herpesvirus type 8 is reported in granulomatous or lymphocytic interstitial lung disease patients (67%) as compared with the low prevalence of 4.8% in patients with CVID without granulomatous or lymphocytic interstitial lung disease. Human herpesvirus type 8 infection might therefore contribute to the poor prognosis of patients with granulomatous CVID.53
In conclusion, there is no unambiguous evidence for specific causal factors that trigger non-infectious granulomatous inflammation. It is evident that the immune system drives tissue-destructive inflammation, but it remains to be determined if certain infectious or non-infectious particles are prone to trigger formation or persistence of granulomas.
Macrophages
Macrophages are immune cells that are specialized in clearing of degraded extracellular substances through phagocytosis. These specialized immune cells are derived from circulating monocytes and are typically found in granulomas (Figure 2). Macrophages are thought to be one of the first cell types to migrate into affected tissue to clear debris and recruit other immune cells.54 An important cytokine produced by macrophages is TNF-α, which induces vasodilation and thereby facilitates the infiltration of monocytes and lymphocytes. Macrophages also release other proinflammatory cytokines such as IL-1, IL-6, IL-12 and IL-23. Together with TNF-α, these cytokines promote leukocyte infiltration and T-cell activation, while inhibiting regulatory T cells (Tregs) and T-cell apoptosis.54 These activated macrophages are important in cell-mediated inflammation seen in granulomas, yet they also induce tissue damage. Polarization of macrophages mirrors the T-helper immune response status. Macrophages can acquire different functionalities in response to local triggers.55 One definition to describe the activated state of macrophages is the classical M1 and alternative M2 activation. M1 macrophages are activated by Toll-like receptors and interferon-γ (IFN-γ) produced by Th1 cells.56, 57 M2 macrophages are activated through IL-4 and IL-13 and secrete extracellular matrix components promoting tissue remodeling.56, 57 Inflamed tissue in patients with Crohn's disease predominantly contain M1 macrophages,58 and these contribute to the intestinal inflammation by disrupting the epithelial barrier in Crohn's disease.59 A similar M1 polarization was seen in alveolar macrophages of patients with sarcoidosis.60 Interestingly, an M2 polarization has been reported in other interstitial lung diseases with fibrosis. This is in line with an M2 polarization in a Th2 environment that has been observed in neurosarcoidosis with myofibrisosis,61 and in fibrotic intestinal lesions of patients with Crohn's disease.62 These studies suggest an M1 activation predominantly in the acute proinflammatory granulomatous inflammation with a possible shift towards M2 macrophages in fibrotic processes.
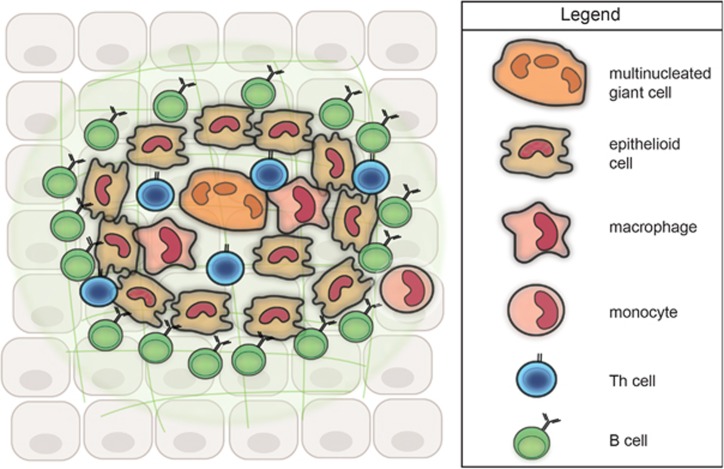
Figure 2.
Model of the cellular organization of a non-caseating granuloma. Histology of granulomatous tissue (e.g. in Figure 1) display the presence of macrophages, epithelioid cells and multinucleated giant cells in the core of the granuloma. Th cells are localized in and around the granuloma. B cells are rarely seen in granulomatous structures; however, they are abundantly present around granulomas.118, 130
Stimulated macrophages can further mature into epithelioid cells that are elongated and resemble epithelial cells. Epithelioid cells appear to lose their phagocytic function and shift to more secretory capacities.63, 64 However, to our knowledge, it remains unclear what soluble factors these epithelioid cells produce. Epithelioid cells can fuse together and create compact aggregations, which are called multinucleated giant cells.65 In contrast to epithelioid cells, these multinucleated cells are capable of phagocytosis and cytokine secretion, especially IL-1, TNF-α and tumor growth factor-β.66
Our understanding of TNF-α and its role in granuloma integrity is mostly based on tuberculosis animal models.67, 68 In the absence of TNF, primary granulomas can still be formed. However, granulomas appeared disorganized.67, 68 Furthermore, a loss of TNF signaling disrupts already formed granulomas. This could, in part, be due to impaired lymphocyte recruitment and activation, in which TNF-α also has a major role.67
Several abnormalities in monocyte and macrophage function have been reported in sarcoidosis, CVID and Crohn's disease, and these might contribute to the chronic inflammation and granuloma formation. Specifically, monocytes in patients with sarcoidosis and Crohn's disease have an increased ability to form multinucleated cells.69, 70 Furthermore, cultured alveolar macrophages of patients with sarcoidosis spontaneously produce more proinflammatory cytokines, including TNF-α, compared with controls,71 and these higher levels were associated with progressive disease.72 TNF-α production was also found to be increased in monocytes of patients with CVID.73 The TNF 488A allele leads to higher TNF production and is strongly positively associated with granulomatous CVID.74 Furthermore, 82% of TNF 488A allele-negative patients were IL-10 a-t-a allele positive, leading to lower IL-10 production resulting in a more proinflammatory TNF environment. Taken together, these two genetic variants seem to promote a cytokine shift contributing to an inflammatory environment leading to granulomatous complications.75 The intestinal microbiota can also affect the inflammatory environment. Intestinal macrophages of patients with Crohn's disease produced more proinflammatory cytokines such as TNF-α after stimulation with commensal bacteria,76 whereas reduced levels of proinflammatory cytokines were reported in response to E. coli.77, 78, 79 Furthermore, E. coli is able to survive and replicate in intestinal macrophages in patients with Crohn's disease, is present in granulomas and can induce granuloma formation in vitro.80, 81, 82 Owing to this apparent decreased macrophage function, it has been proposed that Crohn's disease should also be considered a primary immunodeficiency.83, 84
T cells
The inflammatory mediators produced by macrophages in affected tissue trigger the recruitment of additional immune cells, especially CD4+ Th cells (Figures 1 and 2). Th cells are important mediators of immune responses and are thought to organize the granulomatous structure together with the already present macrophages. Traditionally, Th cells were divided in Th1 and Th2 subsets, and the Th cells in granulomatous tissue were assumed to be type 1 cells that produce IL-2 to induce T-cell proliferation and the accumulation of effector T cells. However, with the more recent detection of other subsets such as Th17 cells and Tregs, the concepts of Th-mediated inflammation have changed.85
Naive Th cells have the ability to differentiate in a particular subset through a specific cytokine milieu. The major subsets are Th1, Th2, Th17 and Tregs that are defined by their cytokine profiles and distinct effector functions (Figure 3). Th1 cells develop in the presence of IFN-γ and IL-12 and protect against intracellular pathogens through the production of IFN-γ and the resulting macrophage activation.54, 85 Upregulation of cytokines promoting Th1 differentiation have been reported in sarcoidosis: IL-2, IL-12, IL-15 and IL-18.86 Th cell involvement in sarcoidosis is underpinned by the typical CD4 T-cell lymphopenia in peripheral blood in combination with CD4 T-cell infiltrates at the site of inflammation, such as in bronchoalveolar lavage fluid.87, 88 Despite these signs of local T-cell hyperactivity, the typical diminished cutaneous response to tuberculin is suggestive of T-cell anergy in non-granulomatous tissue.89 CD4 T-cell anergy in these patients is likely due to chronic stimulation and results from reduced availability of G proteins,90 and reduced nuclear factor-κB capacity of these cells.91
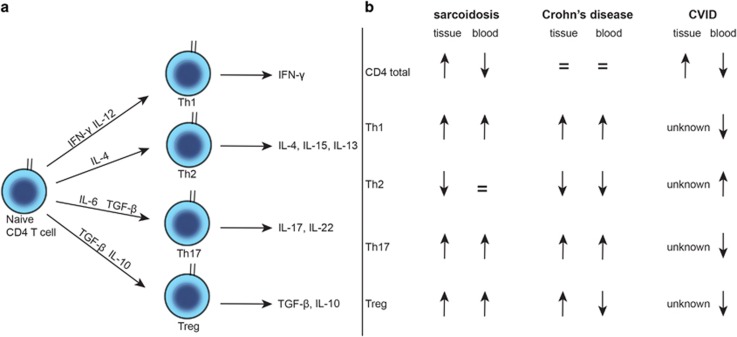
Figure 3.
Involvement of CD4+ Th cell subsets in three chronic granulomatous inflammatory diseases. (a) Model of Th cell maturation into Th1, Th2, Th17 and Treg subsets. Key cytokines are depicted. (b) Summary of observations on total CD4+ Th as well as Th1, Th2, Th17 and Treg subsets in tissue and blood of patients with sarcoidosis, Crohn's disease and CVID.87, 88, 111, 112, 177, 178, 179, 180, 181, 182, 183, 184
Patients with Crohn's disease show overexpression of IL-12 in intestinal tissue leading to increased production of IFN-γ.92, 93 Still, total blood CD4 T-cell numbers as normal, and even an expansion of CD4 memory T cells has been observed in patients with active Crohn's disease.54 The hyperactive state of inflammation in Crohn's disease is further illustrated by mucosal T-cell proliferation and expansion with resistance to apoptosis.94 Unlike sarcoidosis and Crohn's disease, patients with granulomatous CVID have low levels of total T cells and naive CD4 T cells.74 This decrease could be related to the immunodeficiency and result from increased T-cell turnover and apoptosis or decreased thymic output. It remains unclear whether this decrease also distinguishes granulomatous inflammation from Crohn's disease and sarcoidosis or it is the result of migration of T cells from circulation to the affected tissue.
In addition to Th1 responses, other Th subsets have been implicated in chronic inflammation. It is thought that the initial Th1 response during acute granulomatous inflammation shifts to a Th2 response in response when this becomes chronic. The production of Th2 cytokines can activate and stimulate fibroblasts and thereby contribute to fibrosis.20
More recently, Th17 have been shown to be disruptive in chronic inflammatory diseases.95 Th17 cells are generated in the presence of IL-6 and tumor growth factor-β, and in turn produce IL-17 and IL-22 that are major factors in responses against extracellular pathogens and fungi (Figure 3). IL-17 was proposed to be a key mediator of inflammation in rheumatoid arthritis, yet anti-IL-17 therapy with secukinumab was not effective.96 Therefore, the exact role of Th17 cells in inflammatory disorders is not clear and information is mostly based on animal models. IL-17 overexpression leads to tissue damage in different organs such as lungs, intestines, joints and brain.97 Th17 cells have the ability to change to a Th1 phenotype enabling cells to produce both IFN-γ and IL-17 referred to as Th1/Th17 cells.98 The plasticity of Th17 cells enables to further enhance inflammation either directly through the coproduction of IL-17 and IFN-γ or through providing help in the generation of new pathogenic Th1 cells.99 Moreover, Th17 cells in mouse models have recently been shown to adapt into a regulatory phenotype with a change in transcriptional profile and regulatory capacities.100
In both sarcoidosis and Crohn's disease IL-17 expression is increased in inflammatory tissue, concomitant with an increase of Th17 cells in the peripheral blood.22, 101 In contrast, CVID patients have low Th17 cell numbers in their peripheral blood, which is associated with higher numbers of CD21low B cells and lower numbers of memory B cells.102 The presence of an expanded CD21low B-cell population in CVID patients is associated with higher incidence of non-infectious complications.103 The concomitant decrease in Th17 cells is suggestive of a combined defect in B and T cells in this subset of CVID patients. The nature of this defect remains to be determined and could be B- or T-cell intrinsic or arise from impaired regulation of Th maturation.102
Tregs are important to dampen immune responses and thereby maintain a physiological immune homeostasis and self-tolerance.104 Naive T cells can mature into Tregs through the expression of the transcription factor Forkhead box p3 (FoxP3) in the context of tumor growth factor-β, subsequently exerting immune regulatory functions through production of tumor growth factor-β and IL-10.85 Tregs became an intensively studied cell population after it was reported that CD4+CD25+ depletion in mice resulted in a variety of autoimmunity including gastrointestinal involvement.105 Furthermore, patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, a genetic disorder caused by mutation in the FOXP3 gene, are affected by excessive gastrointestinal autoimmunity.106
In patients with sarcoidosis, higher frequencies of Tregs have been reported in both peripheral blood and bronchoalveolar lavage fluid with accumulation of Tregs in the vicinity of granulomas.107 These Tregs inhibited T-cell proliferation, yet several groups confirmed a decreased suppressor function on CD4+ cells.107, 108, 109 Moreover, Tregs from patients with active sarcoidosis were not able to suppress granuloma formation in an in vitro model, whereas Treg cells from healthy controls were.110 However, it remains unclear whether Tregs were defective or were merely exhausted as a result of the continuous inflammation. Patients with active Crohn's disease have decreased Treg numbers in the blood, whereas these are increased in the intestinal mucosa.111, 112 The anti-inflammatory function of Tregs is likely to be intact as they have preserved suppressor function,112 and are able to inhibit effector T-cell responses.111 However, it has been postulated that effector T cells in the lamina propria are unresponsive to the inhibiting effects of Tregs, implicating a contributing factor to the chronic inflammatory response.111 Treg function in CVID has been less well documented, yet decreased levels of Tregs in blood of CVID patients were specifically seen in patients with autoimmune complications.113 Importantly, Tregs require the inhibitory receptor cytotoxic T-lymphocyte-antigen 4 (CTLA-4) for suppressive function, and mutations in CTLA4 underlie an immunodeficiency in which Tregs have reduced suppressive function. These patients often present with granulomatous inflammation and intestinal inflammation with similarities to Crohn's disease.39, 114, 115 As decreased CTLA-4 expression on Tregs has also been reported in patients with sarcoidosis, it is possible that defects in CTLA-4 and Treg function contribute to granuloma formation in autoinflammatory diseases.116
B cells
The main focus in granulomatous inflammation has previously been directed to macrophage and T-cell dysfunction. However, in addition to macrophages and T cells, B-cell infiltrates are present in granulomatous tissue of patients with tuberculosis.117 Furthermore, several studies showed that numerous B cells surround granulomas in affected tissues from patients with sarcoidosis as well as Crohn's disease (Figure 1).118, 119, 120 These B cells are likely to be essential for the development of granulomas as indicated by two findings: first, patients with CVID can develop granulomas, whereas patients with X-linked agammaglobulinemia do not.121 CVID and X-linked agammaglobulinemia patients both have an antibody deficiency due to B-cell dysfunction, but mature B cells are completely absent in X-linked agammaglobulinemia.122 Second, in a mouse model of oil granulomas the absence of T cells did not affect the ability of granuloma formation, whereas granulomas were not formed in the absence of B cells.123 Traditionally, B cells are regarded as the antibody-producing cells of the immune system. While this is a major function of B cells, it has become clear that B-cell development is a complicated process with many B-cell subsets and functions involved. Other B-cell functions include the ability to act as antigen-presenting cells, costimulate T cells, have regulatory effects and produce cytokines that direct Th subset maturation.124 These insights, together with novel possibilities to target B cells with biologicals, provide a strong rationale to investigate the role of B cells in granulomatous inflammation.
Some recent insights have been generated into B-cell abnormalities in sarcoidosis and Crohn's disease. Patients with sarcoidosis carry reduced numbers of IgM, IgG and IgA memory B cells and plasma cells in blood with the exception of CD27−IgA+ memory B cells.118, 125, 126 Despite their reduced numbers, levels of somatic hypermutations in Ig gene transcripts of these cells were increased, suggestive of chronic activation.118 This is potentially related to serum B-cell activating factor (BAFF), a critical factor for mature B-cell survival of which the levels are increased in sarcoidosis patients with active disease.125, 126 Furthermore, the levels of nuclear factor-κB transcription factors in B cells are reduced and potentially affect B-cell responses and proliferation.127 How these B-cell abnormalities affect the formation of persistence of granulomas still needs to be determined, yet combined these results do suggest a disturbed B-cell homeostasis.
Patients with Crohn's disease also display reductions in blood IgM memory B-cell numbers. In contrast to sarcoidosis, they have normal numbers of Ig class-switched memory cells plasma cells.128, 129 In addition, transitional B cells and anergic CD21low B cells were found to be expanded.130 Expansions of CD21low B cells are an indication of chronic activation,131 as were the observed increased levels of somatic hypermutions in Ig gene transcripts.130 It remains unclear if the decline in memory B cells is the result of impaired generation from, for example, the splenic marginal zone, or from their specific recruitment to granulomatous tissue.
CVID is characterized by hypogammaglobinemia and all patients have reduced blood plasma cells, which in many patients is accompanied by memory B-cell defects.103 Furthermore, in subgroups of patients, expansions of transitional B cells, as well as CD21low B cells, have been identified. Many of these abnormalities have formed the basis of flow cytometry-based classifications.103 However, B-cell phenotypes do not seem to correlate well with severity of disease or non-infectious complications. Yet, granulomatous complications are found to be associated with lower numbers of Ig-switched memory B cells.52, 103 Still, it remains unclear if these reductions are related to the immunodeficiency or the result of migration towards the sites of granulomatous inflammation. Patients with mutations in ICOS (inducible T-cell costimulator) and TACI gene can develop granulomatous complications and autoimmunity in general.132 These genes are involved in different pathways of B-cell survival and T-cell-dependent or -independent antibody responses. With the implementation of whole-exome sequencing, the genetics of CVID unravels rapidly. Possibly, this will provide better insights into affected processes and will help to dissect the mechanisms that, when impaired, result in granuloma formation.
In addition to a role in ongoing inflammation, B cells might also function to dampen or restrict inflammatory processes. Subsets of B cells are capable of production of IL-10, and these regulatory B cells could therefore dampen the ongoing immune response. This function is illustrated by mouse models of colitis, in which B cells were found to ameliorate intestinal inflammation.133
Although it remains unclear how B cells contribute to disease pathogenesis, the common signs of chronic activation of B cells in granulomatous autoinflammatory diseases is suggestive of their role in ongoing inflammation. The systemic B-cell abnormalities could provide good markers for disease and treatment monitoring. Moreover, disease-specific abnormalities could provide more insight into pathogenesis and starting points for novel therapeutic approaches.
Therapeutic implications
Remission or fibrosis?
In many patients granulomas persist and lead to organ damage due to fibrosis. Fibrosis is therefore a common problem in sarcoidosis and Crohn's disease,14,129 The impact of granulomas on permanent organ damage in CVID patients is currently unknown due to the complications of recurrent respiratory infections that lead to bronchiectasis in 23% of patients.28 Despite fibrosis leading to increased morbidity and mortality,20 to date, therapies targeting inflammatory pathways do not resolve or delay the process. Moreover, it is not yet possible to identify which patients will develop fibrotic complications.10 Therefore, exploring fibrotic pathways may lead to new and much needed therapies to prevent irreversible organ damage.
Mechanism of current therapies
First- and second-line medication to treat patients with chronic inflammatory disease are corticosteroids and immunosuppressives such as methotrexate and azathioprine. Corticosteroids have anti-inflammatory properties through the inhibition of leukocyte migration and proinflammatory cytokine production (esp. TNF-α and IFN-γ).134 Methotrexate inhibits the purine metabolism and azathioprine purine synthesis, which both lead to decreased lymphocyte proliferation and cytokine release.135 While these therapies are administered to suppress proinflammatory cytokines by inhibiting T-cell responses, these immunosuppressive drugs also affect the B-cell compartment.136, 137
With the introduction of biological therapies, a third line of treatment has become available, of which TNFα blockers are most notable. The most widely used TNFα blockers are antibodies against TNFα (infliximab and adalimumab), which have proven to be effective in Crohn's disease and sarcoidosis (Figure 4).138, 139 This treatment specifically disrupts the granuloma structure. As this can result in reactivation of latent tuberculosis, all patients need to be intensively screened for tuberculosis before treatment with TNFα blockers.140 TNF blockers infliximab and etanercept have proven to be beneficial in some patients with granulomatous CVID.52, 141 Etanercept is a recombinant TNFα receptor fused to an Ig constant region and is often used to treat RA.142 Importantly, etanercept is not effective in sarcoidosis and Crohn's disease, and can even lead to increased disease activity in these disorders.143, 144 This might be related to its different biological properties as opposed to anti-TNF antibodies: (1) etanercept binds only to soluble trimeric and not monomeric soluble TNF-α (2) etanercept has low affinity to transmembrane TNF;145 (3) etanercept binds to both TNF-α and lymphotoxin-α, a cytokine that is crucial for secondary lymphoid organ development, IgA regulation and T-cell gut homing.146 These abilities could explain the reduced effectivity of etanercept in Crohn's disease and sarcoidosis, as well as observed disease complications. Treatment with TNFα blockers also affects the blood B-cell compartment in patients with Crohn's disease and sarcoidosis.129, 130, 147 It remains to be determined if this is an indirect effect following modulation of inflammation or if this is through direct binding to TNFRII that is expressed on B cells.
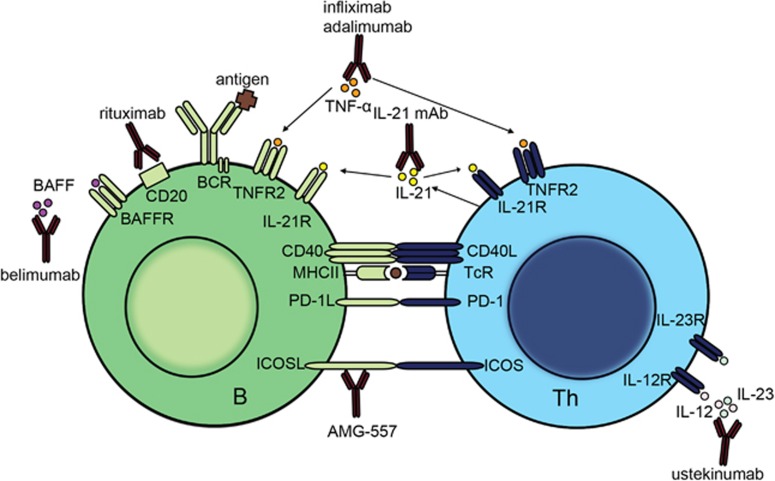
Figure 4.
Novel therapeutics that are currently used or in trial for treatment of granulomatous autoinflammatory diseases that target B and/or T cells. Indicated are monoclonal antibodies that specifically target T cells (ustekinumab), or B cells (rituximab and belimumab). Anti-TNFα (infliximab, adalimumab) and anti-IL-21 monoclonal antibodies block cytokines that affect both B and T cells. Finally, AMG-557 targets ICOSL and affects the B–T-cell interaction: BCR, B-cell receptor; mAb, monoclonal antibody; MHCII, major histocompatibility complex class 2; TcR, T-cell receptor.
Targeting of T cells in granulomatous diseases has yielded mixed results. A clinical trial for the treatment of patients with Crohn's disease with abatacept was ineffective.148 Abatacept is a recombinant fusion protein of CTLA4 with an immunoglobulin. CTLA-4 inhibits T-cell activation by binding to CD28 on T cells. Abatacept has shown beneficial effects in RA patients,149 and in animal models of intestinal inflammation. These results illustrate that, in spite of unraveling underlying immune mechanisms, translation into effective therapies for human autoinflammatory disease remains challenging.
Targeting of Th17 responses have also been studied. However, blocking IL-17 with secukinumab was ineffective in patients with Crohn's disease,150 whereas treatment with brodalumab, an anti-IL-17 receptor monoclonal antibody, even resulted in exacerbation of Crohn's disease.151
Ustekinumab, a monoclonal antibody against both IL-12 and IL-23, resulted in a clinical response in patients with refractory Crohn's disease152 and is currently implemented in patients who are resistant to TNFα blockers.153 However, ustekinumab did not show therapeutic efficacy in sarcoidosis patients.154
Patients with Crohn's disease do show a good response to treatment with vedolizumab, a humanized monoclonal antibody that binds to integrin α4β7.155 As α4β7 specifically mediates gut homing, it can selectively inhibit intestinal inflammation. Because granulomas in patients with sarcoidosis and CVID more frequently present in other tissues than the gut, vedolizumab is likely to have limited effects in these diseases.
Targeting of B cells with rituximab has shown promising results in granulomatous CVID.52 Rituximab is a humanized anti-CD20 antibody that depletes all naive and memory B cells.156 The efficacy of rituximab in sarcoidosis is still unclear: several case reports show proven effectivity; however, in one small prospective study with 10 patients, only 5 of them showed a marginal (>5%) improvement of respiratory function.157 In contrast, a patient with Crohn's disease displayed disease exacerbation following treatment with rituximab, implying a protective role for B cells in Crohn's disease.158 These different outcomes of rituximab treatment highlight the complexity of the underlying inflammatory processes.
New targets for treatment with biologicals
New therapies are in high demand for refractory patients with chronic inflammatory disorders. As new therapeutic targets become evident and new biologicals may become available in the coming years, we propose therapeutic candidates involving the B and/or T cells (Figure 4).
IL-21 is a cytokine produced by Th cells and stimulates B and T cells through the IL-21 receptor. Increased levels of IL-21 have been reported in inflamed tissue of Crohn's disease patients, with infliximab inducing a downregulation of IL-21.159 IL-21 is also implicated in the immunopathogenesis of RA and therefore treatment with a monoclonal antibody binding to IL-21, NNC0114-0005, is currently being tested in these patients.160 When safety and efficacy is proven in RA, this treatment could be translated into other inflammatory disorders such as Crohn's disease. However, caution should be taken because genetic defects in IL-21 gene were recently reported to cause a severe CVID-like disorder161 that manifests with early-onset inflammatory bowel disease.162
Interestingly, the key in therapy might lie in improving T-cell function by targeting of the inhibitory receptor programmed death-1 (PD-1).163 In patients with sarcoidosis, PDL-1 expression is increased on T cells in granulomatous tissue, and the number of PD-1-expressing Th cells in blood are increased.164 As a downregulation of PD-1 on CD4 cells was seen in patients with spontaneous clinical resolution, blocking the PD-1/PD-1L pathway could be a therapeutic target.164 A variety of malignancies also show upregulation of the PD-1/PD-1L pathway and currently several antibodies against PD-1, such as pembrolizumab and nivolumab, are being tested in the treatment of solid and hematological malignancies.165 Still, a cautious approach is warranted as sarcoidosis-induced disease also has been reported by the use of pembrolizumab in a patient with sarcoma.166 This case could be explained by the enhanced CD4 T-cell proliferation that was reported when PD-1 was blocked, which could lead to a Th1 proinflammatory response that is also observed in sarcoidosis.
ICOS and ICOSL are important factors in adaptive immunity through B–T-cell interaction and genetic defects in ICOS have been reported to result in adult onset CVID.31 Moreover, ICOSL gene polymorphisms are associated with Crohn's disease,167 and increased ICOS expression was reported on Tregs in patients with sarcoidosis.168 A monoclonal antibody targeting ICOSL, AMG-557, has been developed and is currently undergoing the first trial in systemic lupus erythematosus.169 While treatment targeting the ICOS/ICOSL pathway is still under development, it is a potential target of interest for granulomatous inflammatory diseases, because ICOS/ICOSL is an implicated pathway in both sarcoidosis and Crohn's disease. Moreover, targeting this costimulatory pathway affects both T- and B-cell activation without their cellular depletion.169
Finally, alternative approaches to target B cells are promising therapeutics. Currently, belimumab, a monoclonal antibody targeting BAFF, is currently implemented in the treatment of systemic lupus erythematosus. BAFF is a cytokine produced mainly by macrophages, and it is essential for mature B-cell survival.170 Especially autoreactive B cells are dependent on high BAFF levels.171 As patients with active sarcoidosis and CVID display increased BAFF levels, it likely contributes to disease pathogenesis.102, 125, 126 Thus, belimumab might be more effective than rituximab through stronger effects on pathogenic B cells.170
Treatment monitoring
With the increasing possibilities for biological therapies, it becomes important to determine which patient should benefit most from which drug. While several agents can be efficacious in patients, there are still subgroups of patients that have refractory disease.138, 139 For example, only 60% of patients with Crohn's disease achieved short-term disease control.27 Starting a patient on ineffective therapy can be expensive and will delay the start of a potentially effective treatment with the possibility of disease exacerbation. Thus, there is a need for biomarkers that can predict therapy outcome before the start of treatment or shortly after.
Therapeutic drug monitoring for infliximab has been extensively studied. Measurements of serum trough levels of infliximab have become standard in diagnostics because low drug levels resulting from the formation of antibodies against infliximab can hamper therapy success.172 Treatment monitoring through immunological tests are another option. Specifically, quantification of serum soluble IL-2R levels is routinely used for patients with sarcoidosis,18 because it correlates with pulmonary function tests and with local disease activity as visualized by fluorine-18 fluorodeoxyglucose positron emission tomography scan.173, 174
Quantification of serum BAFF levels could be a good biomarker as it is elevated in chronic active sarcoidosis.125, 126 Specific lymphocyte subsets could also act as potential biomarkers for therapy. A restoration of the relative numbers of peripheral blood Tregs has been reported in both sarcoidosis and Crohn's disease in patients responding well to infliximab.175, 176 Furthermore, successful infliximab therapy in Crohn's disease resulted in a normalization of IgM memory B-cell numbers.129, 147 Therefore, with increasing knowledge about the specific immune dysregulation seen in these inflammatory diseases, immune monitoring by analysis of specific lymphocyte subsets are potential biomarkers and improve treatment response rates for patients.
Conclusions
Granulomatous inflammation is a complex interplay between mature macrophages, Th cells and B cells. Generally, our understanding of the immune system is improving and fortunately biologicals that block TNF-α have become available. Yet, the complexity in chronic inflammatory diseases is illustrated by numerous failed drug trials, while refractory disease make new therapeutics for chronic inflammation much needed. A translational approach towards basic immunology and advances in other immune-mediated diseases remain necessary to improve treatment options for refractory patients. Specifically, cross-disciplinary studies into granulomatous inflammation in various disorders could yield new insights. Studies into granuloma formation in genetically defined immunodeficiencies can provide candidate pathways, whereas insights into immune dysregulation in sarcoidosis and Crohn's disease can provide immunological markers to identify CVID patients at risk for granulomatous complications. Recent insights into disease pathogenesis and the potential involvement of B cells open new avenues for treatment and treatment monitoring. In particular, patients with granulomatous inflammatory disease could benefit from targeting B cells or B–T-cell interactions with new therapeutics.
Acknowledgments
We thank Dr LSJ Kamphuis and Dr KH Lam for their support with immunohistochemistry of biopsy samples.
The authors declare no conflict of interest.
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008; 454: 428–435. [DOI] [PubMed] [Google Scholar]
- Doria A, Zen M, Bettio S, Gatto M, Bassi N, Nalotto L et al. Autoinflammation and autoimmunity: bridging the divide. Autoimmun Rev 2012; 12: 22–30. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Farver CF, Vaszar LT, Dempsey OJ, Popper HH, Mani H et al. Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol 2012; 65: 51–57. [DOI] [PubMed] [Google Scholar]
- Williams GT, Williams WJ. Granulomatous inflammation—a review. J Clin Pathol 1983; 36: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme IM, Basaraba RJ. The formation of the granuloma in tuberculosis infection. Semin Immunol 2014; 26: 601–609. [DOI] [PubMed] [Google Scholar]
- Woodard BH, Rosenberg SI, Farnham R, Adams DO. Incidence and nature of primary granulomatous inflammation in surgically removed material. Am J Surg Pathol 1982; 6: 119–129. [DOI] [PubMed] [Google Scholar]
- Levine S, Smith VV, Malone M, Sebire NJ. Histopathological features of chronic granulomatous disease (CGD) in childhood. Histopathology 2005; 47: 508–516. [DOI] [PubMed] [Google Scholar]
- Rossman MD. Chronic beryllium disease: a hypersensitivity disorder. Appl Occup Environ Hyg 2001; 16: 615–618. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Gal AA. Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med 2010; 134: 667–690. [DOI] [PubMed] [Google Scholar]
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet 2014; 383: 1155–1167. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012; 380: 1590–1605. [DOI] [PubMed] [Google Scholar]
- Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol 2009; 133: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas SW, Baeten DL. Recent advances in the treatment of immune-mediated inflammatory diseases. Methods Mol Biol 2016; 1371: 143–155. [DOI] [PubMed] [Google Scholar]
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–2165. [DOI] [PubMed] [Google Scholar]
- Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R et al. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160: 736–755. [DOI] [PubMed] [Google Scholar]
- Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. BMJ 1961; 2: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treglia G, Taralli S, Giordano A. Emerging role of whole-body 18F-fluorodeoxyglucose positron emission tomography as a marker of disease activity in patients with sarcoidosis: a systematic review. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: 87–94. [PubMed] [Google Scholar]
- Grutters JC, Fellrath JM, Mulder L, Janssen R, van den Bosch JM, van Velzen-Blad H. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest 2003; 124: 186–195. [DOI] [PubMed] [Google Scholar]
- Drent M, Cremers JP, Jansen TL, Baughman RP. Practical eminence and experience-based recommendations for use of TNF-alpha inhibitors in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31: 91–107. [PubMed] [Google Scholar]
- Patterson KC, Hogarth K, Husain AN, Sperling AI, Niewold TB. The clinical and immunologic features of pulmonary fibrosis in sarcoidosis. Transl Res 2012; 160: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015; 50: 942–951. [DOI] [PubMed] [Google Scholar]
- Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 2014; 13: 3–10. [DOI] [PubMed] [Google Scholar]
- Heresbach D, Alexandre JL, Branger B, Bretagne JF, Cruchant E, Dabadie A et al. Frequency and significance of granulomas in a cohort of incident cases of Crohn's disease. Gut 2005; 54: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feakins RM. Ulcerative colitis or Crohn's disease? Pitfalls and problems. Histopathology 2014; 64: 317–335. [DOI] [PubMed] [Google Scholar]
- Sendid B, Colombel JF, Jacquinot PM, Faille C, Fruit J, Cortot A et al. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol 1996; 3: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Targan SR, Landers CJ, Dutridge D, Ippoliti A, Vasiliauskas EA et al. Familial expression of anti-Escherichia coli outer membrane porin C in relatives of patients with Crohn's disease. Gastroenterology 2006; 130: 1078–1085. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- Gathmann B, Mahlaoui N, Ceredih, Gerard L, Oksenhendler E, Warnatz K et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014; 134: 116–126. [DOI] [PubMed] [Google Scholar]
- Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol 1999; 93: 190–197. [DOI] [PubMed] [Google Scholar]
- Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012; 119: 1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol 2003; 4: 261–268. [DOI] [PubMed] [Google Scholar]
- Pan-Hammarstrom Q, Salzer U, Du L, Bjorkander J, Cunningham-Rundles C, Nelson DL et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet 2007; 39: 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med 2006; 354: 1901–1912. [DOI] [PubMed] [Google Scholar]
- van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest 2010; 120: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA 2009; 106: 13945–13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science 2013; 342: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest 2014; 124: 3923–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Bryant VL, Frede N, Slade C, Woon ST, Lehnert K et al. Haploinsufficiency of the NF-kappaB1 subunit p50 in common variable immunodeficiency. Am J Hum Genet 2015; 97: 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014; 345: 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvry D, Mouthon L, Brillet PY, Kambouchner M, Ducroix JP, Cottin V et al. Granulomatosis-associated common variable immunodeficiency disorder: a case–control study versus sarcoidosis. Eur Respir J 2013; 41: 115–122. [DOI] [PubMed] [Google Scholar]
- Mannon PJ, Fuss IJ, Dill S, Friend J, Groden C, Hornung R et al. Excess IL-12 but not IL-23 accompanies the inflammatory bowel disease associated with common variable immunodeficiency. Gastroenterology 2006; 131: 748–756. [DOI] [PubMed] [Google Scholar]
- Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol 2004; 114: 415–421. [DOI] [PubMed] [Google Scholar]
- Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency: an update. Arthritis Res Ther 2012; 14: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K, Genta RM, Lujan G, Robiou C, Sonnenberg A. Significance of the epithelioid granuloma in biopsies of Crohn's colitis. Inflamm Bowel Dis 2014; 20: 2271–2275. [DOI] [PubMed] [Google Scholar]
- Crowley LE, Herbert R, Moline JM, Wallenstein S, Shukla G, Schechter C et al. 'Sarcoid like' granulomatous pulmonary disease in World Trade Center disaster responders. Am J Ind Med 2011; 54: 175–184. [DOI] [PubMed] [Google Scholar]
- Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 2002; 40: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 2007; 39: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil E, Hugot JP, Arbeille B, Paris R, Grodet A, Peuchmaur M et al. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn's disease. Gastroenterology 2012; 142: 1097–1099 e4. [DOI] [PubMed] [Google Scholar]
- Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 2004; 364: 1039–1044. [DOI] [PubMed] [Google Scholar]
- Bull TJ, McMinn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P et al. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J Clin Microbiol 2003; 41: 2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza JL, Lana R, Diaz-Rubio M. Mycobacterium avium subspecies paratuberculosis and its relationship with Crohn's disease. World J Gastroenterol 2009; 15: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiquot JN, Gerard L, Malphettes M, Fieschi C, Galicier L, Boutboul D et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol 2013; 33: 84–95. [DOI] [PubMed] [Google Scholar]
- Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med 2005; 202: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014; 5: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013; 8: e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner D, Schumann M, Batra A, Kredel LI, Kuhl AA, Erben U et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis 2015; 21: 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtan P, Mierzejewski M, Osinska I, Domagala-Kulawik J. Macrophage polarization in interstitial lung diseases. Cent Eur J Immunol 2016; 41: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Heppner FL, Goebel HH, Stenzel W. M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis. Am J Pathol 2011; 178: 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharl M, Huber N, Lang S, Furst A, Jehle E, Rogler G. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn's disease associated intestinal fibrosis. Clin Transl Med 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector WG. Immunologic components of granuloma formation. Epithelioid cells, giant cells, and sarcoidosis. Ann NY Acad Sci 1976; 278: 3–6. [DOI] [PubMed] [Google Scholar]
- Williams WJ, James EM, Erasmus DA, Davies T. The fine structure of sarcoid and tuberculous granulomas. Postgrad Med J 1970; 46: 496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maarsseveen TC, Vos W, van Diest PJ. Giant cell formation in sarcoidosis: cell fusion or proliferation with non-division? Clin Exp Immunol 2009; 155: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pando R, Bornstein QL, Aguilar Leon D, Orozco EH, Madrigal VK, Martinez Cordero E. Inflammatory cytokine production by immunological and foreign body multinucleated giant cells. Immunology 2000; 100: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 2008; 29: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol 1999; 162: 3504–3511. [PubMed] [Google Scholar]
- Liu ZX, Noguchi M, Hiwatashi N, Toyota T. Monocyte aggregation and multinucleated giant-cell formation in vitro in Crohn's disease. The effect of cell adhesion molecules. Scand J Gastroenterol 1996; 31: 706–710. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Mizuno K, Horio T. Monocyte-derived multinucleated giant cells and sarcoidosis. J Dermatol Sci 2003; 31: 119–128. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Zissel G, Goldmann T, Tschernig T, Vollmer E, Pabst R et al. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur Respir J 2003; 21: 421–428. [DOI] [PubMed] [Google Scholar]
- Pueringer RJ, Schwartz DA, Dayton CS, Gilbert SR, Hunninghake GW. The relationship between alveolar macrophage TNF, IL-1, and PGE2 release, alveolitis, and disease severity in sarcoidosis. Chest 1993; 103: 832–838. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Lien E, Kristoffersen AK, Muller F, Haug CJ, Espevik T et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency—possible immunologic and clinical consequences. Blood 1996; 87: 674–681. [PubMed] [Google Scholar]
- Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol 1997; 159: 6236–6241. [PubMed] [Google Scholar]
- Mullighan CG, Marshall SE, Bunce M, Welsh KI. Variation in immunoregulatory genes determines the clinical phenotype of common variable immunodeficiency. Genes Immun 1999; 1: 137–148. [DOI] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest 2008; 118: 2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Rahman FZ, Hayee B, Graham SJ, Marks DJ, Sewell GW et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med 2009; 206: 1883–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TR, Hudspith BN, Rayment NB, Prescott NJ, Petrovska L, Hermon-Taylor J et al. Defective macrophage handling of Escherichia coli in Crohn's disease. J Gastroenterol Hepatol 2015; 30: 1265–1274. [DOI] [PubMed] [Google Scholar]
- Vazeille E, Buisson A, Bringer MA, Goutte M, Ouchchane L, Hugot JP et al. Monocyte-derived macrophages from Crohn's disease patients are impaired in the ability to control intracellular adherent-invasive Escherichia coli and exhibit disordered cytokine secretion profile. J Crohns Colitis 2015; 9: 410–420. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn's disease. Int J Med Microbiol 2002; 292: 185–193. [DOI] [PubMed] [Google Scholar]
- Ryan P, Kelly RG, Lee G, Collins JK, O'Sullivan GC, O'Connell J et al. Bacterial DNA within granulomas of patients with Crohn's disease—detection by laser capture microdissection and PCR. Am J Gastroenterol 2004; 99: 1539–1543. [DOI] [PubMed] [Google Scholar]
- Meconi S, Vercellone A, Levillain F, Payre B, Al Saati T, Capilla F et al. Adherent-invasive Escherichia coli isolated from Crohn's disease patients induce granulomas in vitro. Cell Microbiol 2007; 9: 1252–1261. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L. Revisiting Crohn's disease as a primary immunodeficiency of macrophages. J Exp Med 2009; 206: 1839–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DJ, Rahman FZ, Sewell GW, Segal AW. Crohn's disease: an immune deficiency state. Clin Rev Allergy Immunol 2010; 38: 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol 2010; 7: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ES, Moller DR. Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol 2011; 7: 457–467. [DOI] [PubMed] [Google Scholar]
- Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 1996; 156: 4952–4960. [PubMed] [Google Scholar]
- Inui N, Chida K, Suda T, Nakamura H. TH1/TH2 and TC1/TC2 profiles in peripheral blood and bronchoalveolar lavage fluid cells in pulmonary sarcoidosis. J Allergy Clin Immunol 2001; 107: 337–344. [DOI] [PubMed] [Google Scholar]
- Bianco A, Spiteri MA. Peripheral anergy and local immune hyperactivation in sarcoidosis: a paradox or birds of a feather. Clin Exp Immunol 1997; 110: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoz G, Prigent AF, Aloui R, Charpin G, Gormand F, Gallet H et al. Impaired G-proteins and cyclic nucleotide phosphodiesterase activity in T-lymphocytes from patients with sarcoidosis. Eur J Clin Invest 1993; 23: 18–27. [DOI] [PubMed] [Google Scholar]
- Lee NS, Barber L, Kanchwala A, Childs CJ, Kataria YP, Judson MA et al. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol 2011; 18: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology 1997; 112: 1169–1178. [DOI] [PubMed] [Google Scholar]
- Garcia de Tena J, Manzano L, Leal JC, San Antonio E, Sualdea V, Alvarez-Mon M. Active Crohn's disease patients show a distinctive expansion of circulating memory CD4+CD45RO+CD28null T cells. J Clin Immunol 2004; 24: 185–196. [DOI] [PubMed] [Google Scholar]
- de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016; 13: 13–27. [DOI] [PubMed] [Google Scholar]
- Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 and non-classic Th1 cells in chronic inflammatory disorders: two sides of the same coin. Int Arch Allergy Immunol 2014; 164: 171–177. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis 2013; 72: 863–869. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 2007; 13: 139–145. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci USA 2015; 112: 7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015; 523: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology 2012; 51: 37–46. [DOI] [PubMed] [Google Scholar]
- Barbosa RR, Silva SP, Silva SL, Melo AC, Pedro E, Barbosa MP et al. Primary B-cell deficiencies reveal a link between human IL-17-producing CD4 T-cell homeostasis and B-cell differentiation. PLoS ONE 2011; 6: e22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 2008; 111: 77–85. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490–500. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164. [PubMed] [Google Scholar]
- Gambineri E, Perroni L, Passerini L, Bianchi L, Doglioni C, Meschi F et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol 2008; 122: 1105–1112.e1. [DOI] [PubMed] [Google Scholar]
- Miyar M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 2006; 203: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald-Richter KA, Richmond BW, Braun NA, Isom J, Abraham S, Taylor TR et al. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J Immunol 2013; 190: 5446–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappl G, Pabst S, Riemann D, Schmidt A, Wickenhauser C, Schutte W et al. Regulatory T cells with reduced repressor capacities are extensively amplified in pulmonary sarcoid lesions and sustain granuloma formation. Clin Immunol 2011; 140: 71–83. [DOI] [PubMed] [Google Scholar]
- Taflin C, Miyara M, Nochy D, Valeyre D, Naccache JM, Altare F et al. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol 2009; 174: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol 2004; 173: 3119–3130. [DOI] [PubMed] [Google Scholar]
- Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology 2005; 128: 1868–1878. [DOI] [PubMed] [Google Scholar]
- Mouillot G, Carmagnat M, Gerard L, Garnier JL, Fieschi C, Vince N et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol 2010; 30: 746–755. [DOI] [PubMed] [Google Scholar]
- Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 2014; 20: 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S, Petersen BS, Tomczak M, Melum E, Huc-Claustre E, Dougan SK et al. Early-onset Crohn's disease and autoimmunity associated with a variant in CTLA-4. Gut 2015; 64: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos CE, van Nimwegen M, In 't Veen JC, Hoogsteden HC, Hendriks RW, van den Blink B et al. Decreased cytotoxic T-lymphocyte antigen 4 expression on regulatory T cells and Th17 cells in sarcoidosis: double trouble? Am J Respir Crit Care Med 2015; 192: 763–765. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Turner OC, Turner J, Marietta P, Brooks JV, Orme IM. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect Immun 2001; 69: 1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis LS, van Zelm MC, Lam KH, Rimmelzwaan GF, Baarsma GS, Dik WA et al. Perigranuloma localization and abnormal maturation of B cells: emerging key players in sarcoidosis? Am J Respir Crit Care Med 2013; 187: 406–416. [DOI] [PubMed] [Google Scholar]
- Geboes K, van den Oord J, De Wolf-Peeters C, Desmet V, Rutgeerts P, Janssens J et al. The cellular composition of granulomas in mesenteric lymph nodes from patients with Crohn's disease. Virchows Arch A 1986; 409: 679–692. [DOI] [PubMed] [Google Scholar]
- Fazel SB, Howie SE, Krajewski AS, Lamb D. B lymphocyte accumulations in human pulmonary sarcoidosis. Thorax 1992; 47: 964–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med 1993; 86: 31–42. [PubMed] [Google Scholar]
- Rosen FS, Cooper MD, Wedgwood RJ. The primary immunodeficiencies (1). N Engl J Med 1984; 311: 235–242. [DOI] [PubMed] [Google Scholar]
- Chen H, Liao D, Holl TM, Snowden P, Ueda Y, Kelsoe G. Genetic regulation of pristane-induced oil granuloma responses. Int J Exp Pathol 2010; 91: 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood 2008; 112: 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda-Hayakawa I, Tanimura H, Osawa M, Iwasaka H, Ohe S, Yamazaki F et al. Elevated serum BAFF levels in patients with sarcoidosis: association with disease activity. Rheumatology 2013; 52: 1658–1666. [DOI] [PubMed] [Google Scholar]
- Saussine A, Tazi A, Feuillet S, Rybojad M, Juillard C, Bergeron A et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS ONE 2012; 7: e43588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Barber L, Akula SM, Sigounas G, Kataria YP, Arce S. Disturbed homeostasis and multiple signaling defects in the peripheral blood B-cell compartment of patients with severe chronic sarcoidosis. Clin Vaccine Immunol 2011; 18: 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A, Rosado MM, Ciccocioppo R, Cazzola P, Morera R, Corazza GR et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 2005; 100: 1788–1795. [DOI] [PubMed] [Google Scholar]
- Lawrance IC, Rogler G, Bamias G, Breynaert C, Florholmen J, Pellino G et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis (e-pub ahead of print 2 November 2015; doi:10.1016/j.crohns.2014.09.008). [DOI] [PMC free article] [PubMed]
- Timmermans WM, van Laar JA, van der Houwen TB, Kamphuis LS, Bartol SJ, Lam KH et al. B-cell dysregulation in Crohn's disease is partially restored with infliximab therapy. PLoS ONE 2016; 11: e0160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorarinsdottir K, Camponeschi A, Gjertsson I, Martensson IL. CD21−/low B cells: a Snapshot of a unique B cell subset in health and disease. Scand J Immunol 2015; 82: 254–261. [DOI] [PubMed] [Google Scholar]
- Bogaert DJ, Dullaers M, Lambrecht BN, Vermaelen KY, De Baere E, Haerynck F. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet 2016; 53: 575–590. [DOI] [PubMed] [Google Scholar]
- Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol 2000; 12: 597–605. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Saag KG, Cutolo M, da Silva JA, Bijlsma JW. The molecular basis for the effectiveness, toxicity, and resistance to glucocorticoids: focus on the treatment of rheumatoid arthritis. Scand J Rheumatol 2005; 34: 14–21. [DOI] [PubMed] [Google Scholar]
- Joshi P, Dhaneshwar SS. An update on disease modifying antirheumatic drugs. Inflamm Allergy Drug Targets 2014; 13: 249–261. [DOI] [PubMed] [Google Scholar]
- Glaesener S, Quach TD, Onken N, Weller-Heinemann F, Dressler F, Huppertz HI et al. Distinct effects of methotrexate and etanercept on the B cell compartment in patients with juvenile idiopathic arthritis. Arthritis Rheumatol 2014; 66: 2590–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickenberg S, Mickholz E, Jung E, Nofer JR, Pavenstadt HJ, Jacobi AM. Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res Ther 2012; 14: R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006; 174: 795–802. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345: 1098–1104. [DOI] [PubMed] [Google Scholar]
- Franxman TJ, Howe LE, Baker JR Jr. Infliximab for treatment of granulomatous disease in patients with common variable immunodeficiency. J Clin Immunol 2014; 34: 820–827. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997; 337: 141–147. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001; 121: 1088–1094. [DOI] [PubMed] [Google Scholar]
- Louie GH, Chitkara P, Ward MM. Relapse of sarcoidosis upon treatment with etanercept. Ann Rheum Dis 2008; 67: 896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis 2005; 41 (Suppl 3): S199–S203. [DOI] [PubMed] [Google Scholar]
- Ruddle NH. Lymphotoxin and TNF: how it all began—a tribute to the travelers. Cytokine & growth factor reviews 2014; 25: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A, Rosado MM, Cazzola P, Biancheri P, Tinozzi FP, Laera MR et al. Splenic function and IgM-memory B cells in Crohn's disease patients treated with infliximab. Inflamm Bowel Dis 2008; 14: 591–596. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R et al. Abatacept for Crohn's disease and ulcerative colitis. Gastroenterology 2012; 143: 62–69 e4. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 2003; 349: 1907–1915. [DOI] [PubMed] [Google Scholar]
- Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Feagan BG, Vermeire S, Panaccione R, Melmed GY, Blosch C et al. A randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and efficacy of AMG 827 in subjects with moderate to severe Crohn's disease. Gastroenterology 2012; 143: E26. [Google Scholar]
- Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med 2012; 367: 1519–1528. [DOI] [PubMed] [Google Scholar]
- Kopylov U, Afif W, Cohen A, Bitton A, Wild G, Bessissow T et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn's disease—the McGill experience. J Crohns Colitis 2014; 8: 1516–1522. [DOI] [PubMed] [Google Scholar]
- Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J 2014; 44: 1296–1307. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83: 435–445. [PubMed] [Google Scholar]
- Sweiss NJ, Lower EE, Mirsaeidi M, Dudek S, Garcia JG, Perkins D et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J 2014; 43: 1525–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA, Rosenbloom B, Targan SR. Anti-CD2O chimeric monoclonal antibody (rituximab) treatment of immune-mediated thrombocytopenia associated with Crohn's disease. Gastroenterology 2003; 124: 583. [DOI] [PubMed] [Google Scholar]
- Liu C, Xia X, Wu W, Wu R, Tang M, Chen T et al. Anti-tumour necrosis factor therapy enhances mucosal healing through down-regulation of interleukin-21 expression and T helper type 17 cell infiltration in Crohn's disease. Clin Exp Immunol 2013; 173: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatenko S, Skrumsager BK, Mouritzen U. Safety PK and PD of recombinant anti-interleukin-21 monoclonal antibody in a first-in-human trial. Int J Clin Pharmacol Ther 2016; 54: 243–252. [DOI] [PubMed] [Google Scholar]
- Kotlarz D, Zietara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med 2013; 210: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer E, Kansu A, Sic H, Majek P, Ikinciogullari A, Dogu FE et al. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. J Allergy Clin Immunol 2014; 133: 1651–9 e12. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+ T-cell proliferative capacity. Am J Respir Crit Care Med 2014; 190: 560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 2015; 27: 39–46. [DOI] [PubMed] [Google Scholar]
- Cousin S, Toulmonde M, Kind M, Cazeau AL, Bechade D, Coindre JM et al. Pulmonary sarcoidosis induced by the anti-PD1 monoclonal antibody pembrolizumab. Ann Oncol 2016; 27: 1178–1179. [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 2010; 42: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivel P, Grunewald J, Eklund A, Bruder D, Wahlstrom J. Pulmonary sarcoidosis is associated with high-level inducible co-stimulator (ICOS) expression on lung regulatory T cells—possible implications for the ICOS/ICOS-ligand axis in disease course and resolution. Clin Exp Immunol 2016; 183: 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Tsuji W, Kivitz A, Peng J, Arnold GE, Boedigheimer MJ et al. Inducible T-cell co-stimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus. Lupus Sci Med 2016; 3: e000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 2014; 10: 365–373. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999; 190: 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert F, Noman M, Vermeire S, Van Assche G, G DH, Carbonez A et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- Keijsers RG, Verzijlbergen JF, van Diepen DM, van den Bosch JM, Grutters JC. 18F-FDG PET in sarcoidosis: an observational study in 12 patients treated with infliximab. Sarcoidosis Vasc Diffuse Lung Dis 2008; 25: 143–149. [PubMed] [Google Scholar]
- Vorselaars AD, van Moorsel CH, Zanen P, Ruven HJ, Claessen AM, van Velzen-Blad H et al. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med 2015; 109: 279–285. [DOI] [PubMed] [Google Scholar]
- Verwoerd A, Hijdra D, Vorselaars AD, Crommelin HA, van Moorsel CH, Grutters JC et al. Infliximab therapy balances regulatory T cells, TNFR2 expression and sTNFR2 in sarcoidosis. Clin Exp Immunol 2016; 185: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Vermeire S, Bullens D, Ferrante M, Van Steen K, Noman M et al. Restoration of Foxp3+ regulatory T-cell subsets and Foxp3− Type 1 regulatory-like T cells in inflammatory bowel diseases during anti-tumor necrosis factor therapy. Inflamm Bowel Dis 2015; 21: 2418–2428. [DOI] [PubMed] [Google Scholar]
- Kakazu T, Hara J, Matsumoto T, Nakamura S, Oshitani N, Arakawa T et al. Type 1 T-helper cell predominance in granulomas of Crohn's disease. Am J Gastroenterol 1999; 94: 2149–2155. [DOI] [PubMed] [Google Scholar]
- Senju M, Hulstaert F, Lowder J, Jewell DP. Flow cytometric analysis of peripheral blood lymphocytes in ulcerative colitis and Crohn's disease. Gut 1991; 32: 779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser SA, Romero C, Urbina P, Naser N, Valentine J. Cellular infiltration and cytokine expression correlate with fistulizing state in Crohn's disease. Clin Vaccine Immunol 2011; 18: 1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011; 66: 144–150. [DOI] [PubMed] [Google Scholar]
- Rezaei N, Aghamohammadi A, Kardar GA, Nourizadeh M. Pourpak Z. T- helper 1 and 2 cytokine assay in patients with common variable immunodeficiency. J Invest Allergol Clin Immunol 2008; 18: 449–453. [PubMed] [Google Scholar]
- Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol 1997; 150: 823–832. [PMC free article] [PubMed] [Google Scholar]
- Karttunnen R, Breese EJ, Walker-Smith JA, MacDonald TT. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol 1994; 47: 1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiss NJ, Salloum R, Gandhi S, Alegre ML, Sawaqed R, Badaracco M et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS ONE 2010; 5: e9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valour F, Senechal A, Dupieux C, Karsenty J, Lustig S, Breton P et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 2014; 7: 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen HJ, Smith AM. The role of the innate immune system in granulomatous disorders. Front Immunol 2013; 4: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, Pallone F. Inability of normal human intestinal macrophages to form multinucleated giant cells in response to cytokines. Gut 1995; 37: 798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos AC, Wildenberg ME, Duijvestein M, Verhaar AP, van den Brink GR, Hommes DW. Anti-tumor necrosis factor-alpha antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology 2011; 140: 221–230. [DOI] [PubMed] [Google Scholar]
- Vos AC, Wildenberg ME, Arijs I, Duijvestein M, Verhaar AP, de Hertogh G et al. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis 2012; 18: 401–408. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61: 1619–1635. [DOI] [PubMed] [Google Scholar]