Abstract
The concept of immune memory forms the biological basis for vaccination programs. Despite advancements in the field of immune memory and vaccination, most current vaccines are evaluated by magnitude of antigen-specific antibody titers in serum or mucosa after vaccination. It has been shown, however, that antibody-mediated humoral immune memory is established regardless of the magnitude and duration of immune reactions, suggesting that assessment of vaccine efficacy should be performed for several years after vaccination. This long-term investigation is disadvantageous for prevalent and pandemic infections. Long-lived memory plasma cells and memory helper T cells which contribute to humoral immune memory are generated in the bone marrow after migration of memory cell precursors through bloodstream. Thus, it may be a novel evaluation strategy to assess the precursors of memory cells in the blood in the early phase of the immune reaction(s). We here review recent advances on the generation and maintenance of immune memory cells involved in humoral immunity and introduce a current concept of direct and short-term assessment of humoral immune memory formation upon vaccination as a correlate of protection.
Introduction
One of the key features of the adaptive immune system is the formation of immune memory upon infection. When Edward Jenner back in 1796 observed that milkmaids were protected against smallpox due to their exposure to the pus in the blisters from cowpox, he did not know that his discovery would lead to one of the first global immunization programs and thus the eradication of a severe human infectious disease as certified by the World Health Assembly on 8th May 1980.1, 2, 3 Later on, Louis Pasteur, Robert Koch and Paul Ehrlich moved the vaccination era forward by developing inactivated and attenuated agents from highly virulent pathogens and showed that only a small fraction of the pathogen, a toxin or its inactivated derivate, a toxoid, was sufficient to induce immunity.4 The development of vaccines has substantially reduced the threats associated with smallpox, poliomyelitis, rabies, diphtheria, tetanus, pertussis, Haemophilus influenzae type b, measles, mumps, and rubella. Although these infectious diseases are nowadays controlled in the greatest part of the world,5, 6 we still face uncontrolled virulent pathogens without access to effective vaccines.
Vaccination ultimately aims at the generation of immune memory to avoid expansion of pathogens by dual inhibitory mechanisms; providing antibodies continuously and maintaining memory cells which induce rapid recall responses. A vast majority of recent efficacious vaccines successfully allow the immune system to generate affinity-matured class-switched antibodies, leading to neutralization or clearance of the pathogen. For major infectious diseases caused by pathogens such as human immunodeficiency virus (HIV) or hepatitis C virus (HCV), however, effective vaccines are still required.
Recent evaluation systems for vaccines point toward the measurement of T-cell quality with regards to cytokine secretion as a protective correlate7 in addition to antibody titers in serum during the course of an immune response. Even though the generation of immune memory provides the basis for the concept of vaccination, direct assessment of immune memory cells and their precursors has not yet been established as a correlate of protection. With growing knowledge about the phenotype, function and localization of immune memory cells in the body, these cells may provide a novel correlate of protection for evaluation of more efficacious vaccines. We here review current advances on the generation, maintenance and roles of immune memory cells involved in humoral immunity, suggesting that the assessment of memory cell precursors in blood is more beneficial as potential correlates of protection, compared with conventional approaches based on the magnitude and persistence of immune responses.
Generation and maintenance of immune memory
Immune memory is characterized by the ability of the immune system to constantly provide antibodies, and also respond more rapidly and effectively to pathogens that have been encountered previously. After clearance of pathogens, the generated long-lived plasma cells, which are also called memory plasma cells,8 continuously provide pathogen-specific antibodies for protection up to a lifetime. First insights into the existence of lifelong protection against a specific pathogen were provided by a measles outbreak on the Faroe Islands in 1781.9, 10 Sixty-five years later, the islands were again affected by a second outbreak of measles. Interestingly, no elderly population that was already infected in 1781 was measles-stricken at that time.9 On the other hand, when the immune system is re-challenged with the same pathogen, memory T helper (Th) cells and memory B cells that have been generated in the course of the primary immune response, elicit a more rapid recall response at stronger magnitude than naive cells do. Thus, the protective immunity is accomplished in a consequence of immune memory, i.e. by the persistence of memory cells.
Memory plasma cells continuously providing high-affinity antibodies
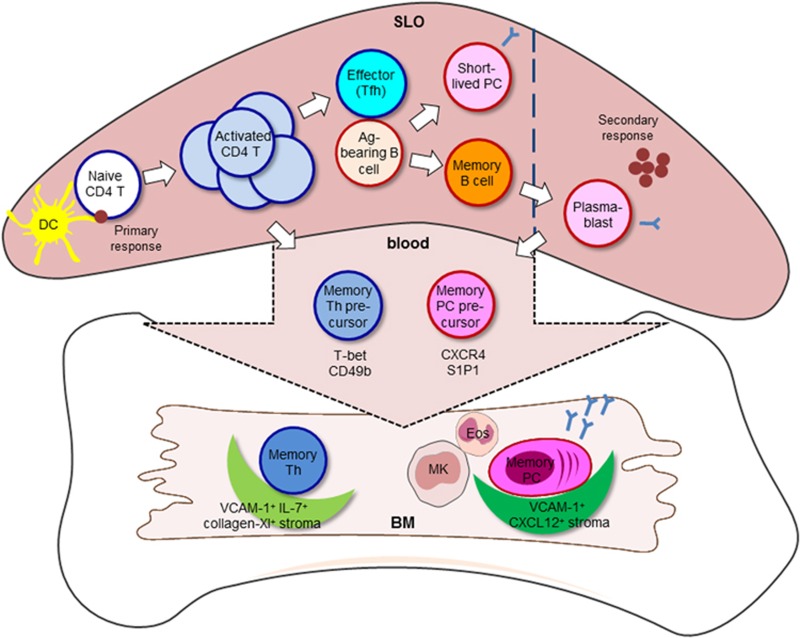
During an immune response, some antigen-specific activated B cells differentiate into memory B cells and short-lived plasma cells (Figure 1).8 Furthermore, in the course of a recall response, memory B cells differentiate into plasma blasts, migrate from the spleen into the bone marrow (BM) in a CXCR4-CXCL12 and S1P1-S1P dependent manner and eventually become memory plasma cells.8, 11, 12 The chemotactic responsiveness to CXCL12 and S1P in migratory plasma cells in the blood in the early phase of recall response may enable us to evaluate the vaccination efficacy as an assessment of long-term production of pathogen-specific antibodies. The BM-resident memory plasma cells are a major source of circulating IgG antibody.13, 14 They secrete several thousand antibodies per second for years, although they are resting in terms of proliferation.15, 16 Within the BM, antibody-secreting memory plasma cells are maintained in the absence of antigen,17 but rely on factors such as CXCL12, integrin alpha4, a proliferation inducing ligand (APRIL) and interleukin (IL)-6 provided by stromal cells, megakaryocytes and eosinophils.18, 19, 20, 21
Figure 1.
Generation and maintenance of immune memory. During the course of an infection or immunization, antigen-incorporated dendritic cells prime naive CD4 T cells in SLOs. Within the pool of activated CD4 T cells, a small population of memory precursor cells preferentially migrate into the BM, further differentiate into memory Th cells and survive on VCAM-1+ collagen XI+ IL-7+ reticular stromal cells. The other small population of activated CD4 T cells interacts with bystander B cells as follow-on antigen-presenting cells through cognate interaction and further differentiates into effector Tfh cells. Some activated B cells differentiate into memory B cells and short-lived plasma cells. In the course of an antigen-persistent or recall response, memory B cells differentiate into plasma blasts including memory plasma cell precursors which eventually move to the BM in a CXCR4-CXCL12 dependent manner. BM reticular stromal cells expressing VCAM-1 and CXCL12 provide a niche for memory plasma cells. Additionally, megakaryocytes (MK) and eosinophils (Eos), both expressing the survival factors APRIL and IL-6, are in close vicinity to the memory plasma cell survival niche. PC: plasma cell.
Memory Th cells inducing recall responses
CD4 T cells play a crucial role in protection against a variety of pathogens. Upon infection, dendritic cells take up pathogenic antigens and prime naive T cells (Figure 1). Activated CD4 T cells clonally expand and differentiate into effector Th cell subsets,22 which are able to provide help to B cells in secondary lymphoid organs (SLOs). Several studies have shown that a lack of CD4 T cells impairs the generation of high-affinity memory B cells and plasma cells23, 24, 25 indicating that CD4 T cell help is crucial for the subsequent establishment of humoral immune memory. After the clearance of the pathogen, the majority of activated CD4 T cells undergo apoptosis, leaving behind a minor population of memory CD4 T cells that are heterogeneous and conventionally divided into distinct subsets based on their expression of surface molecules, ultimately defining their localization in the body as well as their ability of cytokine secretion.
Tissue-resident memory Th cells (Trm cells) are known to reside in epithelial barrier tissue such as the gut, skin or the respiratory tract,26 and are poised to rapidly respond upon local pathogen re-challenge. Central memory Th cells (Tcm cells) are defined by their expression of a chemokine receptor CCR7 and an adhesion molecule l-selectin (CD62L), which enable the passage from the blood vessel into SLOs and the migration to periarteriolar lymphoid sheaths (PALS). On the other hand, effector memory Th cells (Tem) are predominantly found in peripheral tissues to provide immediate protection and express only marginal levels of CD62L and CCR7.27 It has been shown that Tem cells can be replenished from the pool of Tcm cells,28 while the type and site of infection (systemic or local) defines the relative role of Tcm to Tem during recall responses.29 The existence of memory T follicular helper (Tfh) cells has also been postulated and characterized by their ability to rapidly provide help to memory B cells in SLOs.30, 31, 32, 33, 34, 35, 36
Recent studies by our group demonstrate that in mice, some activated CD4 T cells preferentially migrate into the BM during primary immune responses, and reside and rest there as professional memory Th cells characterized as CD44hiCD62L−CCR7− CD4 T cells (Figure 1).37, 38, 39, 40, 41, 42 In the memory phase of an immune reaction, about 90% of antigen-specific memory Th cell are located in the BM.37 This quiescent population is distinct from aforementioned conventional memory Th cell subsets and can be identified by high expression of Ly-6C, and the activation markers CD69 and CD49b (integrin α2) whose expression is required for homing to the BM.37, 38, 39, 42 Interestingly, it has been reported that also in humans, memory Th cells reactive to systemic pathogens, such as cytomegalovirus (pp65), tetanus toxoid, measles, mumps and rubella, are significantly enriched in the BM in comparison to peripheral blood.43 When naive mice are adoptively transferred with BM or splenic memory Th cells, BM memory Th cells induce recall response more efficiently.37 In case of ex vivo stimulation, BM memory Th cells respond faster in terms of production of cytokines and co-stimulatory molecules when compared to splenic cells.37 These results suggest that BM memory Th cells rapidly travel into the SLOs to provide B cell help and promote a high-affinity antibody response during recall response.
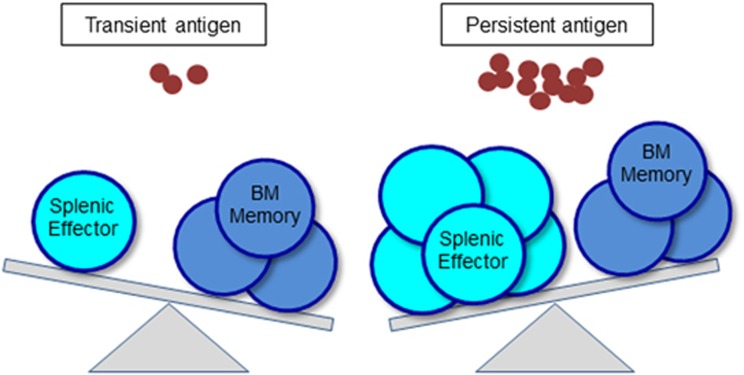
How BM memory Th cells are generated is current focus of investigation. Several studies shed light on the important role of B cells for the generation of memory Th cells in different compartments. The relation between B cells and Tfh cells seems to be interdependent for effective humoral immunity. Tfh cells are crucial providers of T cell help to B cells for germinal center formation, affinity maturation and the generation of high-affinity antibodies and memory B cells.44 In turn, cognate B cells typically become the primary source of antigen in germinal center reactions and thus are critical for Tfh cell differentiation that relies on continuous antigen presentation.45 Additionally, cognate B cells have been shown to support the expansion of Tfh cells but are not required for the induction of Tfh-lineage specific transcription factor Bcl6.46 In the spleen, B cells have also been reported to be essential for the generation of memory Th cells.47, 48, 49, 50, 51 Interestingly, our recent study suggests a negative role of B cells on the generation of BM memory Th cells.52 In the course of an immunization, the ratio of CD49b- and T-bet (a master transcription factor for Th1 differentiation)- co-expressing cells in activated antigen-specific CD4 T cells is increased according to their migratory process from spleen (~14%) via bloodstream (~34%) to BM (~53%), suggesting that this population contains the potential precursors for BM memory Th cells. Accordingly, the fluctuation of T-bet+CD49b+ antigen-specific Th cells in the periphery correlates with the number of accumulating antigen-specific Th cells in the BM and thus their detection in the periphery may function as a correlate of protection after vaccination. In the absence of B cells (in B-cell depleted and B-cell deficient mice), we found an enhanced generation of CD49b+T-bet+ memory Th cell precursors in the spleen and blood and their accumulation in the BM during the early phase of an immune response. This may suggest that B cells control the numerical balance of antigen-specific Th cells in the spleen and BM (Figure 2).52
Figure 2.
The balance of splenic effector Tfh cells and BM memory Th cells is determined by the quantity and duration of antigen. By antigen persistence, the generation of BM resting memory cells is not affected, while the numbers of splenic effector Tfh cells are enhanced.
Interestingly, although persistent antigen emulsified in adjuvants of oil and aluminum hydroxide augments the expansion of antigen-specific CD4 T cells and the retention of effector Tfh cells in SLOs compared to antigen with soluble adjuvants, e.g. LPS,42, 53 no adjuvant affects the ultimate number of BM memory Th cells.52 These data suggest that antigen persistence is an important factor for the magnitude and duration of an immune response, but not for the generation of BM memory Th cells. Thus, a strong and long-lasting immune response is not always a direct factor to determine the proportion of protective immune memory.
BM as a central tissue for immune memory
The maintenance of immune memory cells in the BM depends on cytokine signals provided in distinct stromal niches.54 The chemokine CXCL12 guides the migration of memory plasma cell precursors to their niches comprised of reticular VCAM-1+ stromal cells.11, 18, 55 Furthermore, the prominent plasma cell survival factors, IL-6 and APRIL, secreted by megakaryocytes and eosinophils in the direct vicinity of VCAM-1+ CXCL12+ stromal cells, provides an optimized environment to maintain the survival niche for memory plasma cells in the BM.19, 20
BM memory Th cells reside in the near vicinity of IL-7- and collagen XI-co-expressing reticular stromal cells that comprise about 1% of the BM cells, thus defining their pool size (Figure 1).37, 54 Some reports have shown that memory B cells reside in the spleen.56 Interestingly, a dynamic population of memory B-cell clones which have undergone somatic hypermutation, has been found in the human BM.57 Although the exact localization of memory B cells still remains controversial, the BM should be considered as a potential tissue for the residence of memory B cells. Thus, the knowledge about the whereabouts of immune memory cells will greatly contribute to the understanding of how immune memory cells are generated upon vaccination.
Assessment of immune memory as correlate of protection
The majority of commercially applied vaccines induce the production of IgG and/or IgA in serum or mucosa in order to neutralize or block pathogens, or interfere with pathogenic invasion via the bloodstream.5, 6 The neutralizing antibodies serve as a correlate of protection and are regarded as a hallmark of vaccine efficacy. In order to measure the type and magnitude of an immune response to either infection or immunization, Ig detection assays such as ELISA are fully established for all Ig classes in mice, other laboratory rodents and all large-animal species which are used for vaccine development (for example, pig, cattle etc.).58 Classical examples are smallpox vaccines,59 which contain live attenuated pathogens that elicit a strong humoral immune response leading to protection. Serum neutralizing antibody titers against intracellular pathogens (for example, hepatitis A and B) have been also defined as correlates of protection.60, 61 As the measurement of neutralizing antibodies in the early phase of an immune reaction reflects the secretion of Ig by short-lived plasma cells rather than the establishment of protective memory by memory plasma cells, it may be important to assess antibody titers at later time points, i.e. several years after vaccination. However, monitoring the antibody titers for several years is disadvantageous to determine the efficacy of a potent vaccine in clinical studies. We here propose that assessment of memory precursor cells in peripheral blood speeds up the process for evaluation of vaccine candidates and supports a quick admission from bench to bedside. Assessing long-lived memory B cells and memory plasma cells by multicolor flow cytometry or ELISPOT assay can be a good indicator for appropriate immune memory against infections.62 It is already reported that the functional evaluation of memory B cells by ELISPOT assay correlates with protection against hepatitis B virus (HBV) infection.63 However, insignificant correlations between memory B cell numbers and protection against infection have been also found after vaccination against human rotavirus64 or measles.65 Further investigation about memory B cells should be performed.
In mice, antigen-specific plasma blasts migrate from the spleen into the BM within the short time frame of 48 h between days 4 and 6 post-secondary immunization.11, 13, 16 Interestingly, even though few in numbers, antigen-specific murine plasma blasts in the peripheral blood also peak between days 4 and 6, and characteristically express the chemokine receptor CXCR4.11 In humans, migratory CXCR4+ plasma blasts are also found in the peripheral blood between days 6 and 8 after secondary immunization with tetanus antigen.66 Kabashima et al.12 have indicated that migratory plasma cells in the blood on day 3 after challenge significantly express S1P1 mRNA and attract to S1P ex vivo. Hence, the assessment of migrating memory plasma cell precursors in the human blood can correctly provide a correlate of protection.
Major hurdles in analyzing protective T-cell responses in humans are technical limitations due to the vast HLA polymorphism and the wide variety of functions and populations of T cells generated during an immune response. However, the detection of cytokine-producing cells by ex vivo stimulation with antigen may facilitate the detection of antigen-specific CD4 T cells. The magnitude of a CD4 T-cell response after vaccination can be measured by a single parameter such as IFN-γ production.67 However, it does not always reflect the precise functional potentials. Only complex combinations of numbers, proliferative activity, abilities for secretion of multiple cytokines or expression of mobilization-related molecules (chemokine receptors and adhesion molecules) of antigen-specific CD4 T cells should be considered as correlates of protection. Okhrimenko et al.43 have shown that human BM enriches memory Th cells in comparison with peripheral blood. However, considering that human BM from vaccinated patients cannot be easily assessed for evaluation of vaccine efficacy during the clinical phase of vaccine development, the peripheral blood of vaccinated patients can be a major resource to analyze antigen-specific memory cell precursors that are destined to reside in the BM. We have recently shown in mice that antigen-specific CD49b+T-bet+ Th cells in the periphery are enriched in the BM.52 Hence, the enumeration of CD49b+T-bet+ memory Th cell precursors in the blood can be beneficial to estimate the prospective generation of resting Th cell memory following vaccination. Further investigation to define the full characteristics and lifestyle of memory Th cell precursors will be crucial for developing a new evaluation system for vaccine effectiveness.
Conclusions and perspectives
Vaccination is based on the concept of immune memory. Conventional approaches to evaluate vaccine-induced immune responses by measuring neutralizing antibody titers and T-cell responses in the effector phase do not always represent the gain of immune memory. We here propose that the quantification of memory cell precursors in the blood, for example, CD49b+T-bet+ resting memory Th cell precursors and CXCR4+ S1P1+ memory plasma cell precursors,11, 12, 52 can be advantageous in order to determine the quality of protection upon the prospective establishment of humoral immune memory, since immune memory in the BM can be established regardless of the magnitude of the immune reaction. Additionally, memory precursor cells are detectable in peripheral blood within one week after vaccination, which offers an enormous time saving in comparison to the detection of neutralizing antibodies or antigen-specific memory cells that appear at much later time points of the immune reaction. The new evaluation system of immune memory will validate correlates of protection for the existing vaccines from different angles, and will establish a basis for new vaccine design strategies against pathogens for which vaccines have not yet been developed successfully.
Acknowledgments
This work is supported by grants from the Leibniz Association (International Leibniz Research Cluster ‘ImmunoMemory') and from the German Research Council (DFG, TO 944/2-1). J.S is supported by the Leibniz Association (Leibniz Graduate School for Rheumatology) and S.H. is supported by the Alexander von Humboldt-Foundation.
The authors declare no conflict of interest.
References
- Fenner F. A successful eradication campaign. Global eradication of smallpox. Rev Infect Dis 1982; 4: 916–930. [DOI] [PubMed] [Google Scholar]
- Loomis RJ, Johnson PR. Emerging Vaccine Technologies. Vaccines 2015; 3: 429–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis NJ. Edward Jenner and the eradication of smallpox. Scott Med J 1997; 42: 118–121. [DOI] [PubMed] [Google Scholar]
- Rappuoli R, Miller HI, Falkow S. Medicine. The intangible value of vaccination. Science 2002; 297: 937–939. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47: 401–409. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8: 247–258. [DOI] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 2006; 6: 741–750. [DOI] [PubMed] [Google Scholar]
- Panum PL. Beobachtungen über das Maserncontagium. Virchows Arch Pathol Med 1847; 1: 492–512. [Google Scholar]
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 1996; 272: 54–60. [DOI] [PubMed] [Google Scholar]
- Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol 2002; 169: 1277–1282. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL et al. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med 2006; 203: 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997; 388: 133–134. [DOI] [PubMed] [Google Scholar]
- McMillan R, Longmire RL, Yelenosky R, Lang JE, Heath V, Craddock CG. Immunoglobulin synthesis by human lymphoid tissues: normal bone marrow as a major site of IgG production. J Immunol 1972; 109: 1386–1394. [PubMed] [Google Scholar]
- Hibi T, Dosch HM. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur J Immunol 1986; 16: 139–145. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 1998; 8: 363–372. [DOI] [PubMed] [Google Scholar]
- Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV et al. Long-lived plasma cells are contained within the CD19(−CD38(hi)CD138(+ subset in human bone marrow. Immunity 2015; 43: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 2004; 20: 707–718. [DOI] [PubMed] [Google Scholar]
- Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood 2010; 116: 1867–1875. [DOI] [PubMed] [Google Scholar]
- Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 2011; 12: 151–159. [DOI] [PubMed] [Google Scholar]
- Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol 2003; 171: 1684–1690. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RK, Paul WE. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol 1971; 106: 872–874. [PubMed] [Google Scholar]
- Francus T, Francus Y, Siskind GW. Memory T cells enhance the expression of high-avidity naive B cells. Cell Immunol 1991; 134: 520–527. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature 2003; 421: 282–287. [DOI] [PubMed] [Google Scholar]
- Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 2015; 21: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N et al. Memory T cells and vaccines. Vaccine 2003; 21: 419–430. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22: 745–763. [DOI] [PubMed] [Google Scholar]
- Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood 2010; 115: 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. Eur J Immunol 2012; 42: 1981–1988. [DOI] [PubMed] [Google Scholar]
- Ise W, Inoue T, McLachlan JB, Kometani K, Kubo M, Okada T et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci USA 2014; 111: 11792–11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 2013; 38: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 2011; 35: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J Immunol 2011; 186: 2889–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol 2013; 190: 4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yan X, Zhong B, Nurieva RI, Wang A, Wang X et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med 2012; 209: 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 2009; 30: 721–730. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Tokoyoda K, Hanazawa A, Hayashizaki K, Zehentmeier S, Hosokawa H et al. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci USA 2012; 109: 7409–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa A, Hayashizaki K, Shinoda K, Yagita H, Okumara K, Lohning M et al. CD49b-dependent establishment of T helper cell memory. Immunol Cell Biol 2013; 91: 524–531. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 2010; 10: 193–200. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Radbruch A. Signals controlling rest and reactivation of T helper memory lymphocytes in bone marrow. Cell Mol Life Sci 2012; 69: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa A, Lohning M, Radbruch A, Tokoyoda K. CD49b/CD69-dependent generation of resting T helper cell memory. Front Immunol 2013; 4: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von Roth P et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci USA 2014; 111: 9229–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010; 33: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Okada T, Ansel KM. Cutting Edge: distinct waves of BCL6 expression during T follicular helper cell development. J Immunol 2011; 187: 2089–2092. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol 2000; 165: 5558–5565. [DOI] [PubMed] [Google Scholar]
- Misumi I, Whitmire JK. B cell depletion curtails CD4+ T cell memory and reduces protection against disseminating virus infection. J Immunol 2014; 192: 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollo SB, Zajac AJ, Harrington LE. Temporal requirements for B cells in the establishment of CD4 T cell memory. J Immunol 2013; 191: 6052–6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. J Immunol 2000; 165: 3640–3646. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol 2009; 182: 1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo S, Sarkander J, Manne C, Mursell M, Hanazawa A, Zimmel D et al. B cells negatively regulate the establishment of CD49b(+T-bet(+ resting memory T helper cells in the bone marrow. Front Immunol 2016; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013; 38: 596–605. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Chang HD, Radbruch A. Organization and maintenance of immunological memory by stroma niches. Eur J Immunol 2009; 39: 2095–2099. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 2001; 194: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamani-Matsuda M, Cosma A, Weller S, Faili A, Staib C, Garcon L et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood 2008; 111: 4653–4659. [DOI] [PubMed] [Google Scholar]
- Paramithiotis E, Cooper MD. Memory B lymphocytes migrate to bone marrow in humans. Proc Natl Acad Sci USA 1997; 94: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts V, Wilson HL, Meurens F, van Drunen Littel-van den Hurk S, Wilson D, Walker S et al. Large animal models for vaccine development and testing. ILAR J 2015; 56: 53–62. [DOI] [PubMed] [Google Scholar]
- Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bull World Health Organ 1975; 52: 307–311. [PMC free article] [PubMed] [Google Scholar]
- Nalin DR, Kuter BJ, Brown L, Patterson C, Calandra GB, Werzberger A et al. Worldwide experience with the CR326F-derived inactivated hepatitis A virus vaccine in pediatric and adult populations: an overview. J Hepatol 1993; 18(Suppl 2): 51–55. [DOI] [PubMed] [Google Scholar]
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179: 489–492. [DOI] [PubMed] [Google Scholar]
- Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine 2012; 30: 4907–4920. [DOI] [PubMed] [Google Scholar]
- Tuaillon E, Tabaa YA, Petitjean G, Huguet MF, Pajeaux G, Fondere JM et al. Detection of memory B lymphocytes specific to hepatitis B virus (HBV) surface antigen (HBsAg) from HBsAg-vaccinated or HBV-immunized subjects by ELISPOT assay. J Immunol Methods 2006; 315: 144–152. [DOI] [PubMed] [Google Scholar]
- Rojas OL, Caicedo L, Guzman, Rodriguez LS, Castaneda J, Uribe L et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol 2007; 20: 300–311. [DOI] [PubMed] [Google Scholar]
- Leyendeckers H, Odendahl M, Lohndorf A, Irsch J, Spangfort M, Miltenyi S et al. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol 1999; 29: 1406–1417. [DOI] [PubMed] [Google Scholar]
- Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 2005; 105: 1614–1621. [DOI] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13: 843–850. [DOI] [PubMed] [Google Scholar]