Introduction
Chronic actinic dermatitis (CAD) is an uncommon inflammatory dermatosis characterized by dermatitis involving ultraviolet (UV) light–exposed skin with notable sparing of sun-protected areas. Rarely, spread of the exanthema to UV-protected areas of skin and even erythroderma with palmoplantar hyperkeratosis occurs.1 CAD typically afflicts men in the fifth decade of life or older. Although the pathophysiology remains unknown, it is thought that lymphocyte recognition of UV-induced neoantigens in the skin underlies this process. Furthermore, cross-reactivity to exogenous contact antigens may also be contributing, as allergic contact dermatitis to numerous allergens has been reported in more than 50% of affected individuals.2 Management involves strict photoprotection, topical corticosteroids, topical calcineurin inhibitors, prednisone, and immunomodulatory agents, often with only limited success. Herein, we report a case of severe CAD refractory to all common immunomodulatory agents that was successfully remitted with the Janus kinase (JAK)1/3 inhibitor tofacitinib citrate.
Case report
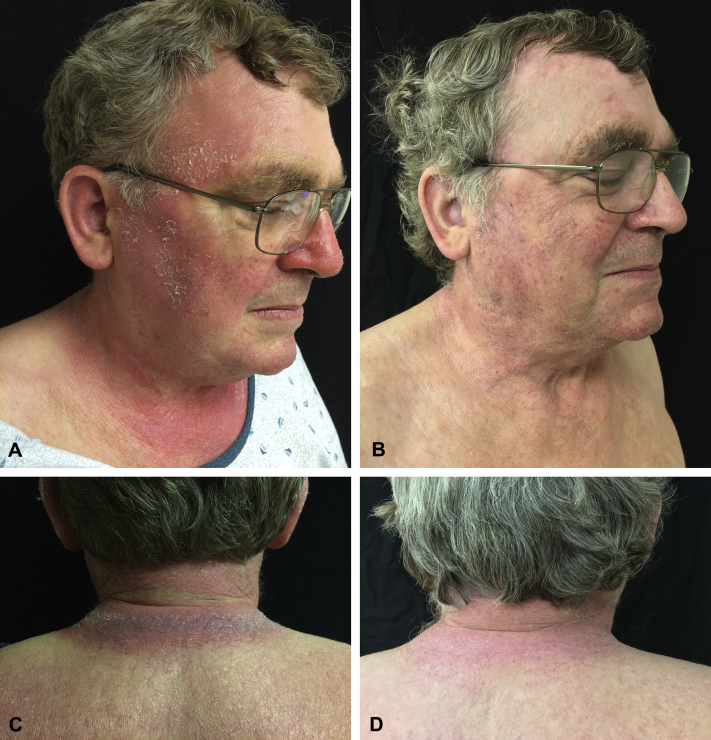
A man in his 60s presented with near erythroderma that began 4 years previously as pruritic, pink, well-marginated, lichenified plaques involving the face, upper chest, posterior neck, wrists, and dorsal hands. There was sparing of sun-protected areas including retroauricular folds, upper eyelids, upper and lower cutaneous lip, submental chin, and skin creases (eg, posterior neck crease; Fig 1, A and B). He experienced a burning sensation of photo-exposed skin on sunlight exposure, and, consequently, he avoided being outdoors and wore a hat and long-sleeve jacket throughout the entire year. Subsequently, involvement of the back, abdomen, and lower extremities occurred as well as fissuring of palms and soles. He denied a history of asthma or atopic dermatitis. He did not take any chronic prescription or over-the-counter medications or herbal supplements. A total of 11 skin biopsies over 2 years found a lichenoid lymphocytic infiltrate with exocytosis, mild spongiosis, and a perivascular lymphohistiocytic dermal cellular infiltrate. Direct immunofluorescence results were normal. T-cell receptor (TCR) γ gene amplification by polymerase chain reaction of skin biopsies twice showed a polyclonal pattern. Peripheral blood flow cytometry, blood TCR γ gene rearrangement, TCR V-β, antinuclear antibody, Ro, La, serum protein electrophoresis, urine protein electrophoresis, and HIV tests were repeatedly normal. Phototesting and photopatch testing were not performed because of widespread skin involvement (ie, lack of uninvolved skin). In light of the characteristic clinical and histopathologic features, a diagnosis of CAD was made.
Fig 1.
CAD before and after treatment with tofacitinib. A and C, Before treatment with tofacitinib, there were edematous and lichenified pink-red and violaceous plaques photodistributed on the face, chest, and posterior neck, with sharp demarcation at the shirt collar. B and D, Marked improvement in the rash visible 10 months after starting tofacitinib (photographs taken during the spring).
Numerous treatments were ineffective, including class 1 topical steroids, tacrolimus 0.1% ointment, prednisone (months-long courses at doses up to 60 mg/d), hydroxychloroquine, methotrexate, azathioprine, mycophenolate mofetil, cyclosporine, omalizumab, acitretin, oral bexarotene, extracorporeal photopheresis, and various combinations of these treatments.
In light of a recent report showing the successful treatment of moderate-to-severe atopic dermatitis using tofacitinib,3 we initiated treatment with this agent in our patient. Within days of starting tofacitinib, 5 mg twice daily, the patient noted reduced burning sensation during sun exposure. By 2 months of treatment, there was near complete remission of signs and symptoms of disease (Fig 1, C and D), and the patient was able to spend hours outdoors in a short-sleeve shirt during the summer for the first time since the exanthem began 4 years earlier.
The patient developed uncomplicated herpes zoster after 6 months of treatment with tofacitinib, which was held during treatment with valacyclovir and then for another 2 weeks while he continued to be free of signs and symptoms of CAD. The rash and burning sensation started to recur 21 days after tofacitinib discontinuation but again remitted with restarting the medication. He remains symptom free with near complete resolution of CAD on tofacitinib monotherapy for the past 12 months. Laboratory monitoring every 3 months, including complete blood count and differential, hepatic function panel, serum creatinine, and fasting lipids has not revealed abnormalities, and annual QuantiFERON-TB Gold testing has been negative.
Discussion
A case of refractory, severe CAD responded dramatically to tofacitinib, a JAK 1/3 inhibitor with activity in helper T cell 1– and helper T cell 2–mediated cutaneous diseases including psoriasis,4 atopic dermatitis,3 dermatomyositis,5 and alopecia areata.6 Although the pathogenesis of CAD remains unclear, there is evidence of a delayed-type hypersensitivity reaction to a photo-induced cutaneous endogenous antigen.2
The response of our patient to tofacitinib, especially considering the failure of numerous immunomodulatory agents to control his disease previously, highlights the unique mechanism of action of JAK inhibition. As with other immunomodulatory agents, the potential adverse effects of JAK inhibitors, such as cancer and herpes zoster and other infections, warrant careful consideration. The successful treatment of refractory, severe CAD adds to the growing list of inflammatory dermatoses that may be effectively treated with JAK inhibition and may offer a clue into the pathogenesis of this disease.
Footnotes
Funding sources: This study was supported in part by The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research (Dr. King). The funding sponsor was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Conflicts of interest: Dr King has served on advisory boards or is a consultant for Aclaris Therapeutics Inc, Pfizer Inc, Eli Lilly and Company, and Concert Pharmaceuticals Inc. These organizations were not involved in the design and conduct of the study; collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication. Drs Vesely and Imaeda have no conflict of interest.
References
- 1.Lim H.W., Buchness M.R., Ashinoff R., Soter N.A. Chronic actinic dermatitis: study of the spectrum of chronic photosensitivity in 12 patients. Arch Dermatol. 1990;126:317–323. doi: 10.1001/archderm.126.3.317. [DOI] [PubMed] [Google Scholar]
- 2.Paek S.Y., Lim H.W. Chronic actinic dermatitis. Dermatol Clin. 2014;32(3):355–361. doi: 10.1016/j.det.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Bachelez H., van de Kerkorf P.C., Strohal R., OPT Compare Investigators Tofactinib versus etancercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):522–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 4.Levy L.L., Urban J., King B.A. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73(3):395–399. doi: 10.1016/j.jaad.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Kurtzman D.J., Wright N.A., Lin J. Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol. 2016;152:944–955. doi: 10.1001/jamadermatol.2016.0866. [DOI] [PubMed] [Google Scholar]
- 6.Craiglow B.G., King B.A. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134(12):2988–2990. doi: 10.1038/jid.2014.260. [DOI] [PubMed] [Google Scholar]