ABSTRACT
Under an NIH priority to identify new drugs to treat class B parasitic agents, we performed high-throughput screens, which identified the activity of auranofin (Ridaura) against Entamoeba histolytica and Giardia intestinalis, major causes of water- and foodborne outbreaks. Auranofin, an orally administered, gold (Au)-containing compound that was approved by the FDA in 1985 for treatment of rheumatoid arthritis, was effective in vitro and in vivo against E. histolytica and both metronidazole-sensitive and -resistant strains of Giardia. We now report the results of an NIH-sponsored phase I trial to characterize the pharmacokinetics (PK) and safety of auranofin in healthy volunteers using modern techniques to measure gold levels. Subjects received orally 6 mg (p.o.) of auranofin daily, the recommended dose for rheumatoid arthritis, for 7 days and were followed for 126 days. Treatment-associated adverse events were reported by 47% of the subjects, but all were mild and resolved without treatment. The mean gold maximum concentration in plasma (Cmax) at day 7 was 0.312 μg/ml and the half-life (t1/2) 35 days, so steady-state blood levels would not be reached in short-term therapy. The highest concentration of gold, 13 μM (auranofin equivalent), or more than 25× the 50% inhibitory concentration (IC50) for E. histolytica and 4× that for Giardia, was in feces at 7 days. Modeling of higher doses (9 and 21 mg/day) was performed for systemic parasitic infections, and plasma gold levels of 0.4 to 1.0 μg/ml were reached after 14 days of treatment at 21 mg/day. This phase I trial supports the idea of the safety of auranofin and provides important PK data to support its potential use as a broad-spectrum antiparasitic drug. (This study has been registered at ClinicalTrials.gov under identifier NCT02089048.)
KEYWORDS: auranofin, phase I trial, antiparasitic agents
INTRODUCTION
The identification of new drugs to treat parasitic infections of global health import is a major priority. Treatment for the three major anaerobic protozoal infections, amebiasis, giardiasis, and trichomoniasis, relies primarily on imidazoles, particularly metronidazole, which was introduced in 1959. Metronidazole resistance has been generated in the laboratory for Entamoeba histolytica (1), and clinical failures have been linked to two other metronidazole-resistant protozoa, Giardia intestinalis and Trichomonas vaginalis (2). Metronidazole is also ineffective against cysts, resulting in treatment regimens of up to 20 days for amebic colitis, making compliance difficult (3). Nitazoxanide, a thiazolide drug, has been approved for short-course treatment of Giardia (4), but cross-resistance occurs with metronidazole (5). Albendazole is another alternative therapy for giardiasis, but resistance has also been reported (5). Therefore, an alternative, effective therapy is needed.
Following a high-throughput, whole-cell screen of amebic trophozoites against more than 13,000 bioactive compounds, including 910 FDA-approved drugs, we found that auranofin, an orally administered gold (Au)-containing compound, had a 10-fold-lower 50% inhibitory concentration (IC50) (0.5 μΜ auranofin) than metronidazole (5.2 μM) (6). Oral auranofin was also effective in rodent models of amebic colitis and liver abscess (6) and in in vitro and in vivo models with metronidazole-sensitive and -resistant strains of Giardia intestinalis (7). In addition, auranofin has the potential to be a broad-spectrum antiparasitic agent, as in vitro and/or in vivo efficacy has been demonstrated for trichomoniasis (Kirk Land, University of the Pacific, personal communication), Cryptosporidium infections (8), filariasis (9), Toxoplasma gondii infections (10), Trypanosoma brucei infections (11), Leishmania infantum infections (12), and schistosomiasis (13, 14).
Auranofin was FDA approved in 1985 for the treatment of rheumatoid arthritis (RA). Although it is still in use for RA, the majority of published pharmacokinetic data comes from studies in RA patients following long-term therapy in the 1980s. After oral administration, auranofin is rapidly metabolized to the active form of gold such that no intact drug can be detected. Methods to measure gold levels were less sensitive at that time and relied on the use of 195Au-labeled auranofin or atomic absorption spectrometry. Following an oral dose, 25% of the gold in auranofin was absorbed, 60% was plasma protein bound, and 85% was excreted in feces (15, 16). Steady-state levels were achieved in RA patients in 8 to 12 weeks, with an elimination half-life of 26 days (range, 21 to 31 days) (16, 17). Long-term (months to years) auranofin therapy was linked to side effects, including diarrhea (40% of subjects), skin rashes (2% to 5%), hematologic abnormalities (rare), and proteinuria (5%) (17). Because of the need to accurately determine the pharmacokinetics (PK) using modern techniques and evaluate the potential toxicity of short-course anti-infective versus long-course antiarthritic auranofin therapy in healthy volunteers, we now report the results of a Division of Microbiology and Infectious Diseases (DMID)-sponsored phase I clinical trial (ClinicalTrials.gov NCT02089048). This study characterized the PK and safety of gold administered orally as auranofin for 7 days in healthy subjects.
RESULTS
Inclusion of subjects.
Fifteen subjects were enrolled and included in the PK analysis population. The study population was composed of 13 men (86.7%) and 2 females (13.3%) with a racial mix of 9 white (60%), 5 black (33.3%), and 1 other (6.7%). The average (± standard deviation) age of the study population was 27 ± 5 years and the average weight 74.2 ± 8 kg. Two subjects were excluded from the noncompartmental PK analyses of plasma gold concentration because they had quantifiable day 1 predose plasma gold concentrations (0.044 and 0.046 μg/ml) exceeding 5% of the day 1 maximum plasma concentration (Cmax) for these subjects. All 15 subjects were included in the population PK analysis and in the summary presentation for fecal gold concentrations as all had predose fecal gold levels below the limits of quantitation.
Safety results.
Auranofin was generally well tolerated in the healthy-subject population studies. Twelve treatment-emergent adverse events (TEAE) were reported by 7 (46.7%) subjects during the study. Gastrointestinal symptoms, including abdominal discomfort, constipation, diarrhea, flatulence, and nausea, were reported in 3 (20%) patients. Two (13.3%) subjects reported a headache. No treatment was given, and their symptoms resolved. All five of the subjects who reported symptoms were male, but only 2 of the 15 volunteers were female. Two (13.3%) patients had decreased levels of platelets: one patient had a platelet count decrease from 150 on day −1 to 128 (103/μl) on day 14. A second patient had a decrease from 150 on day −1 to 123 (103/μl) on day 2. Both values returned to normal. No other symptoms or abnormalities of vital signs, physical exams, or laboratory testing were found. No subjects were withdrawn from the study because of TEAEs, and there were no significant adverse events (SAE) or deaths.
Plasma gold concentration-time data.
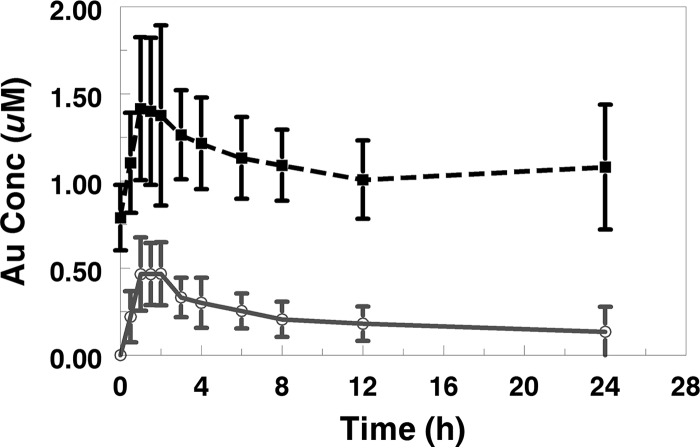
Following a single dose on day 1, 11 of the 13 subjects evaluable for plasma PK had quantifiable (>0.020 μg/ml) plasma gold concentrations at 30 min postdose, 12 subjects had quantifiable plasma gold levels 12 h postdose, and 7 subjects had quantifiable plasma gold levels 24 h postdose (mean area under the plasma concentration-time curve from time zero to 24 h postdose [AUC0–24], 0.971 μg · h/ml; Cmax, 0.102 μg/ml; time to Cmax [Tmax], 1.46 h). Following 7 days of once-daily dosing, all 13 subjects had quantifiable plasma gold levels throughout the 24-h dosing interval (mean AUC0–24, 5.05 μg · h/ml; Cmax, 0.312 μg/ml; Tmax, 1.65 h). Of those subjects, 9 had quantifiable plasma gold levels at 63 days postdose (day 70) and 4 had quantifiable plasma gold levels 119 days postdose (day 126). Mean plasma gold concentration-time profiles for day 1 and day 7 throughout the 24-h dosing interval are presented graphically in Fig. 1.
FIG 1.
Mean plasma gold (Au) concentration-time profiles over a 24-dose interval on study days 1 and 7.
Plasma gold pharmacokinetic parameters.
The mean terminal half-life (t1/2) following 7 days of once-daily dosing was 844 h or approximately 35 days. As expected with this long t1/2 and once-daily dosing, plasma gold levels had increased significantly from day 1 to day 7 and had not reached the steady state. Based on the geometric mean day 7/day 1 accumulation ratio for AUC(0–24) (RAUC) and accumulation ratio for Cmax (RCmax), plasma gold AUC0–24 values increased approximately 6-fold from day 1 to day 7 and geometric mean gold Cmax values increased approximately 3-fold over this same time period. Plasma gold noncompartmental PK parameters are summarized by day in Table 1.
TABLE 1.
Noncompartmental PK resultsa
| Value(s) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Day 1 (n = 13) |
Day 7 (n = 13) |
||||||
| Mean | SD | Median | Range | Mean | SD | Median | Range | |
| AUC0–24 (μg · h/ml) | 0.971 | 0.51 | 1.03 | 0.205–1.94 | 5.05 | 1.07 | 5.00 | 3.47–6.48 |
| AUC0–last (μg · h/ml) | 0.918 | 0.557 | 1.03 | 0.184–1.94 | 156.0 | 83.7 | 130.0 | 67.8–353 |
| Cmax (μg/ml) | 0.102 | 0.039 | 0.098 | 0.0446–0.167 | 0.312 | 0.0823 | 0.31 | 0.168–0.457 |
| Tmax (h) | 1.46 | 0.43 | 1.50 | 1.00–2.00 | 1.65 | 0.75 | 1.50 | 0.50–3.00 |
| t1/2 (h) | ND | ND | ND | ND | 844 | 576 | 681 | 381–2580 |
| RAUC | ND | ND | ND | ND | 6.88 | 4.84 | 4.74 | 3.21–21.3 |
| RCmax | ND | ND | ND | ND | 3.38 | 1.30 | 2.84 | 2.30–6.17 |
ND, not determined; RAUC, accumulation ratio for AUC, calculated as [AUC0–24 (day 7)/AUC0–24 (day 1)]; RCmax, accumulation ratio for Cmax, calculated as [Cmax (day 7)/Cmax (day 1)].
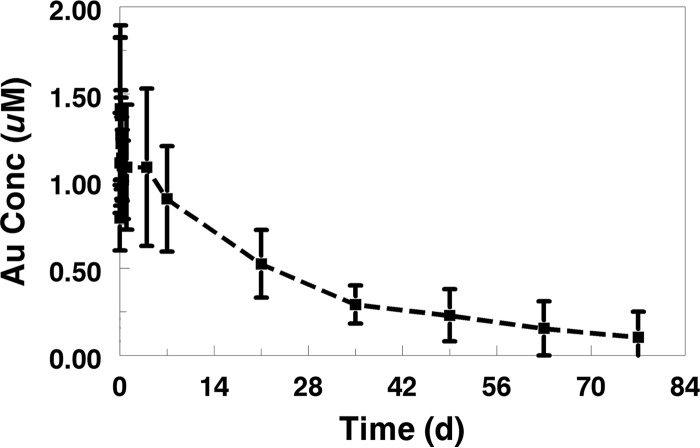
Mean concentrations were below the limit of quantitation (BLQ) after 77 days after the final dose (day 84). The entire plasma gold concentration-time profile for serial samples collected after dosing on day 7 is presented in Fig. 2.
FIG 2.
Mean plasma gold (Au) concentration-time profile after final dose on study day 7 through week 12.
Fecal gold concentration.
Fecal gold levels were not detected in the day 1 fecal sample from any of the 15 subjects after a single dose. On day 7 following once-daily dosing, 11 of 15 subjects had quantifiable fecal gold concentrations (mean, 8.80 μg/g; median, 7.63 μg/g; range, BLQ to 25.7 μg/g). Based on an average level of fecal output in high-income countries of 128 g/day (18), the estimated mean fecal gold output on day 7 was 1.01 mg or approximately 64% of the ingested gold dose. Seven days after the final dose (day 14), fecal gold levels were BLQ in all subjects and remained undetectable throughout the remainder of the study.
Population pharmacokinetic model and Monte Carlo (MC) simulation.
The final population PK model was able to estimate typical PK parameters as well as the between-subject variability (BSV) for clearance/bioavailability (CL/F), apparent central compartment volume (V1/F), and apparent rapidly equilibrating peripheral compartment volume (V2/F) without encountering significant (>20%) BSV shrinkage. The BSV could not be estimated for the acid dissociation constant (KA) or for the apparent slowly equilibrating peripheral compartment volume (V3/F) or the rapid and slow intercompartmental clearances (Qrapid and Qslow) without encountering poor estimation and significant BSV shrinkage, so these BSV values were fixed to 0. Estimated population PK parameters and variances are described in Table 2. The typical values for CL/F and volume of distribution at the steady state (Vss)/F were 0.055 liters/h and 122 liters, with BSV values for CL/F, V1/F, and V2/F of 51%, 23%, and 21%, respectively. The mean post hoc individual-subject CL/F and Vss/F values were similar to the typical population estimates. While the median bootstrap (BS) parameter and BSV estimates were similar to the final model values, the small sample size (15 subjects) and high body surface area (BSA) variability led to a broad BS 95% confidence interval (CI) (2.5th to 97.5th percentile).
TABLE 2.
Population PK resultsa
| Parameter | Value(s) |
|||
|---|---|---|---|---|
| PopPK parameter estimate | PopPK BS median (2.5th–97.5th percentile) | Bayesian estimate geometric mean | Bayesian estimate SD | |
| CL (liters/h) | 0.055 | 0.050 (0.0035–0.0735) | 0.056 | 0.024 |
| V1 (liters) | 13.5 | 13.5 (9.42–18.30) | 13.6 | 2.8 |
| Qrapid (liters/h) | 4.71 | 5.51 (4.00–7.94) | 4.71b | NA |
| V2 (liters) | 39.3 | 40.80 (33.60–48.20) | 39.7 | 7.4 |
| Qslow (liters/h) | 0.035 | 0.0435 (0.0287–0.0616) | 0.035b | NA |
| V3 (liters) | 69.2 | 92.5 (46.19–372.10) | 69.2b | NA |
| KA (h−1) | 1.16 | 1.06 (0.75–1.50) | 1.16b | NA |
| Vss (liters) | 122 | 146.85 (101.20–426.22) | 123.1 | 7.8 |
| BSV-CL | 51% | 66% (44%–236%) | ||
| BSV—V1 | 23% | 20.3% (5.8%–35%) | ||
| BSV—V2 | 20% | 17.5% (4.9%–27%) | ||
| IOV-F | 41% | 62% (35%–89%) | ||
| Residual error | 16% | 17% (15%–20%) | ||
Values for 95% confidence intervals were determined from the bootstrap (BS) parameter. Vss values represent the sum of V1 + V2 + V3 estimates. PopPK, population PK.
No BSV variability was estimated, so all subjects were assigned empirical Bayesian values that were equal to the population PK values for these parameters.
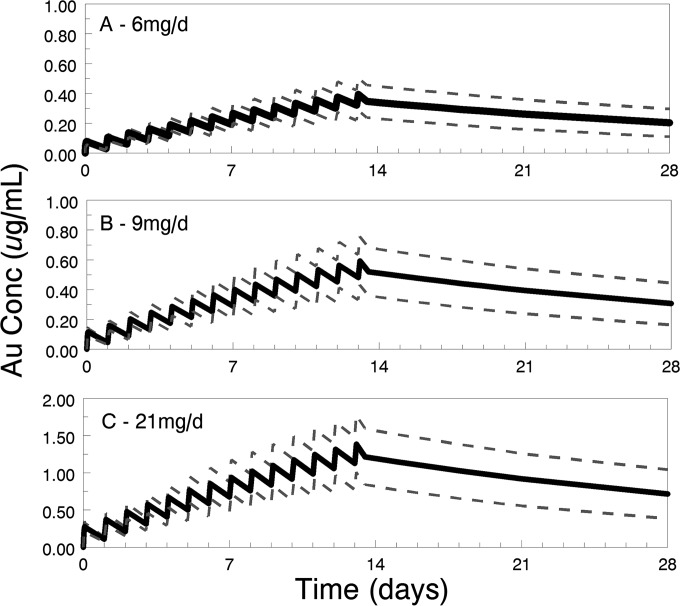
Based on the long terminal half-life, it is clear that the concentrations on day 7 were unlikely to be close to the steady state. Thus, while administration of 6 mg daily for 7 days results in an average plasma gold concentration of approximately 0.2 μg/ml (∼1.0 μM), higher systemic gold exposures may be beneficial for the treatment of less-sensitive organisms. Therefore, higher and more prolonged dosing was assessed using the population PK model and MC simulations. The predicted exposures of 6 mg daily for 14 days along with 9 or 21 mg daily for 14 days are presented in Fig. 3. These simulations demonstrate that gold concentrations continue to accumulate between day 7 and day 14 of auranofin administration.
FIG 3.
Predicted median (with 10th and 90th percentiles) plasma gold (Au) concentrations following treatment at 6 (A), 14 (B), and 21 (C) mg/day for 14 days.
DISCUSSION
The repurposing of existing drugs can save significant time and money in the development of new therapeutics, particularly for neglected tropical diseases, which are not a major interest of pharmaceutical companies. We and others (6, 8–14) have shown that auranofin holds promise for treating both protozoal and helminthic diseases. When the use of auranofin in therapy was approved by the FDA in 1985, safety and pharmacokinetics/pharmacodynamics (PK/PD) data requirements were much less stringent. Because a large number of side effects in long-term therapy of patients with rheumatoid arthritis had been reported (17), the National Institutes of Health sponsored a phase I study in healthy volunteers for the first time.
Subjects received a daily oral dose of 6 mg, the recommended dose for rheumatoid arthritis (19), for 7 days and were followed until day 126 for significant adverse events (SAE). Treatment-emergent adverse events (TEAE) were reported by 46.7% of the subjects and for 26.7% of subjects by the investigator, and the most frequently reported TEAE was headache (13.3% of subjects); all TEAEs were assessed as mild and resolved without treatment. Two subjects had a slight decrease in platelet counts; the decrease was rated as mild and resolved. Thus, for short-term therapy, auranofin was safe and well tolerated.
Since only the single gold molecule in each molecule of auranofin (29% of mass) can be detected following ingestion, levels were determined in plasma and feces to help predict levels appropriate for treatment of systemic infections, such as filariasis and onchocerciasis, or gastrointestinal infections, such as intestinal amebiasis and giardiasis.
The majority of the gold was found in feces by day 7, which agrees with earlier studies using radiolabeled gold (15, 16). The mean concentration of gold in feces at day 7, 8.80 μg/g, would be equivalent to a gold concentration of approximately 44.7 μM or an auranofin concentration of 13.0 μM. This is significantly above the in vitro IC50 for E. histolytica of 0.5 μM auranofin (6) and three times the IC50 for Giardia of 4.0 μM (7). These findings support the use of 6 mg daily for 7 days for treatment of E. histolytica infections and 5 days for treatment of Giardia infections in the clinical trial of auranofin for amebiasis and/or giardiasis (ClinicalTrial.gov NCT02736968).
We found that the terminal half-life of gold in plasma after the final dose was an average of 35 days versus the 26 days reported in the package insert. While most gold concentrations were BLQ at the final week 12 measurement, there were a few subjects with very long terminal half-life results that were imprecisely estimated due to the extreme flatness of their PK profiles. The prolonged elimination and extensive accumulation of gold with repeat dosing are important in planning clinical trials. Auranofin is a category C drug whose use is not recommended in pregnant women on the basis of results of studies in pregnant rabbits and rats, who had decreased maternal and fetal weight and litter size using 4× to 50× the human dose (20). In the current clinical trial, we required the use of highly effective birth control for four half-lives or 4 months.
Higher doses of auranofin will clearly be required for some infections. At a 6 mg/day dose of auranofin, the Cmax of gold in plasma at day 7 was 1.58 μM (equivalent to 0.46 μM auranofin) whereas the in vitro auranofin IC50 for treatment of infections by Onchocerca adult female worms, the proposed target of a much-needed macrofilaricide, was 0.3 μM (9). As discussed below, higher daily doses of auranofin would likely be required for an effective macrofilaricide but would likely be achievable. On the other hand, the in vitro IC50 for treatment of Leishmania infantum infections was 9.68 μM and that for L. major infections 15.66 μM, or 21× to 34× higher than the achievable plasma levels with this dose or even with an approved higher dose (12). To determine the concentrations achieved with higher and more prolonged dose regimens, we performed population PK modeling and performed a Monte Carlo simulation for doses of 6 mg, 9 mg, and 21 mg per day for 14 days. As expected with the long terminal half-life, there was significant accumulation beyond day 7. Trough concentrations nearly doubled between day 7 and day 14. Giving the dose proposed in the current study for an additional 7 days would be expected to raise the trough concentration to 0.2 to 0.4 μg/ml gold (0.29 to 0.58 μM auranofin). The highest dose evaluated in the simulation, 21 mg, generated trough concentrations ranging from 0.8 to 1.5 μg/ml gold (1.18 to 2.21 μM auranofin), with levels remaining at 0.4 to 1.0 μg/ml gold (0.58 to 1.5 μM auranofin) at 2 weeks after the final dose (Table 3). The FDA has approved clinical trials using auranofin at up to 21 mg/day for treatment of relapsed chronic lymphocytic leukemia after daily doses of 9 and 12 mg for at least 28 days were well tolerated (Clinical Trails registration no. NCT01419691). A daily dose of 9 to 12 mg for 7 days would be required for auranofin to act as a macrofilaricide for onchocerciasis. The safety profile for the chronic lymphocytic leukemia (CLL) study (NCT0419691) suggests that this should be achievable.
TABLE 3.
Simulation results from final population PK model using 6 mg/day, 9 mg/day, and 21 mg/day for 14 daysa
| Parameter | Concn (μg/ml) at indicated dose |
|||||
|---|---|---|---|---|---|---|
| 6 mg |
9 mg |
21 mg |
||||
| Median | 5th–95th percentile | Median | 5th–95th percentile | Median | 5th–95th percentile | |
| D7—C24 (trough) | 0.17 | 0.12–0.22 | 0.25 | 0.18–9.33 | 0.59 | 0.42–0.77 |
| D7—C2 (Cmax) | 0.24 | 0.19–0.31 | 0.25 | 0.18–9.33 | 0.85 | 0.66–1.07 |
| D14—C24 (trough) | 0.32 | 0.22–0.42 | 0.25 | 0.18–9.33 | 1.13 | 0.78–1.48 |
| D14—C2 (Cmax) | 0.40 | 0.29–0.51 | 0.25 | 0.18–9.33 | 1.38 | 1.02–1.77 |
| D21 | 0.26 | 0.16–0.36 | 0.25 | 0.18–9.33 | 0.92 | 0.56–1.26 |
| D28 | 0.20 | 0.11–0.30 | 0.25 | 0.18–9.33 | 0.72 | 0.39–1.05 |
Data represent median and 90% confidence interval values for trough concentrations prior to dose (C24) and 2 h after dose (C2) on day 7 (D7) and day 14 (D14) as well as washout concentrations on day 21 (D21) and day 28 (D28).
The mechanism of action of auranofin in rheumatoid arthritis has been poorly understood. In contrast, the major target of auranofin in parasitic infections is known to be the oxidant protective system. This was first demonstrated in the crystal model of the Schistosoma mansoni thioredoxin-glutathione reductase, where the gold from auranofin substituted for the active-site selenium (13). The E. histolytica thioredoxin reductase (EhTrxR) and the Giardia TrxR are more similar to the low-molecular-weight TrxR of Escherichia coli, which does not contain selenium (6). We hypothesized that the monovalent gold from auranofin would bind the redox-active dithiol group in the active site of thioredoxin; however, this was not the case in crystal structural studies of EhTrxR and EhTrx, so the exact mechanism of action remains to be determined (21).
The results from the phase I trial described here support the idea of the safety of auranofin in short-term, anti-infective therapy. The first clinical trial for parasitic infections (amebiasis and giardiasis) is under way. Higher-dose therapy and longer-duration therapy also appear safe in CLL trials (NCT01419691). This is becoming increasingly important as the potential indications for auranofin continue to expand. Besides the potential of auranofin as a broadly active antiparasitic and chemotherapeutic agent, a recent publication also demonstrated its efficacy against drug-resistant Gram-positive bacteria, including Mycobacteria tuberculosis (22). Thus, auranofin has the potential to be an important repurposed drug.
MATERIALS AND METHODS
Trial design.
A phase I, single-center, open label, multiple-dose study of the PK of gold in auranofin was held at Quintiles Phase One Services (Quintiles, Overland Park, KS). A standard dose of auranofin (Ridaura) for the treatment of rheumatoid arthritis, 6 mg (29% gold), was administered orally once daily for 7 days. Fifteen healthy male or female subjects of non-childbearing potential, aged 18 to 45 years (inclusive), were admitted to the study center on day −1 and received the first dose of study drug on day 1. The study drug was administered in the study center on days 1, 2, and 7 and was self-administered at home on days 3 to 6. The pharmacokinetics of gold in plasma was evaluated up to day 126 (approximately 5 half-lives) and in feces up to day 42. Treatment-emergent adverse events (TEAE) were monitored from day 1 until day 14 and serious adverse events (SAEs) until day 126 based on clinical laboratory evaluations, vital signs, and physical examinations.
Pharmacokinetic analysis.
Single-dose (day 1) and multiple-dose (day 7) noncompartmental PK analysis was performed on the plasma gold concentrations using the computer program Phoenix WinNonlin version 6.3 (Pharsight Corporation, St. Louis, MO, USA). PK parameters determined included maximum plasma concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration-time curve from time zero to time of last measureable concentration (AUC0–last), area under the plasma concentration-time curve from time zero to 24 h postdose (AUC0–24) (day 7 only), day 7/day 1 accumulation ratio for AUC(0–24) (RAUC), and accumulation ratio for Cmax (RCmax). Additional multiple-dose PK parameters included the terminal rate constant, λZ, and the terminal t1/2. The AUC values were calculated using the linear trapezoid method, with postdose gold concentrations below the limit of quantitation (BLQ) treated as zero. The terminal slope λz was determined using a log-linear regression analysis, with gold concentrations greater than the limit of quantitation and terminal half-life (t1/2) values calculated as 0.693/λz. Fecal gold concentrations were determined predose and at day 1 (+1 day), day 7 (± 1 day), day 14 (±1 day), day 28 (±3 days), and day 42 (±3 days). Subjects with quantifiable plasma gold concentrations prior to the first dose were excluded from the noncompartmental PK analysis and summary statistics of gold plasma concentration data.
Since subjects had not reached the steady state by day 7, a compartmental population pharmacokinetic analysis was also performed using complete pharmacokinetic profiles (day 1 through day 126) to generate PK parameter estimates for apparent clearance and Vss. The computer program NONMEM (version 7.2; ICON, Dublin, Ireland) was used for this analysis. A three-compartment model, with first-order absorption (ADVAN 12) and the first order conditional estimation with interaction (FOCEI) subroutine with proportional residual error, was used to characterize the pharmacokinetic data. PK samples with concentrations below the quantitative limit of the assay were excluded from the analysis. Within-subject, interoccasion variability (IOV) for bioavailability (F) was included for the doses within the period of intensive PK sampling (day 1 and day 7). The apparent volume of distribution at the steady state (Vss/F) was calculated as the sum of all of the apparent individual compartment volumes (V1/F + V2/F + V3/F). Given the small number (n = 15) of subjects and the relatively homogenous nature of the health volunteer study population, no exploratory covariate analysis of population PK parameters was attempted. Empirical Bayesian post hoc estimates of individual subject PK parameters were generated. A 1,000-sample bootstrap analysis was performed using the final model to determine the 95% CI for PK parameter estimates using Wings for NONMEM (v7.2).
Subjects with quantifiable concentrations in the predose drug samples collected on day 1 required modification of the observed postdose concentration before inclusion in the population PK analysis. These predose values represented >5% of the Cmax, and the postdose washout concentrations were greater than the corresponding prestudy drug concentrations. Since gold is found in the environment and can be ingested from other sources, the finding of day 1 predose gold concentration values that were greater than BLQ was assumed to be due to ingestion of gold from environmental sources. For the analysis, it was assumed that the level of environmental gold “contamination” remained constant throughout the study duration for these subjects. Thus, the postdrug gold concentrations in these subjects were adjusted using their day 1 predose gold concentrations (observed prestudy concentrations) before inclusion in the population analysis. Therefore, the population pharmacokinetic analysis was performed on all 15 subjects.
The population pharmacokinetic model was used to perform Monte Carlo (MC) simulations with 1,000 virtual subjects to predict gold exposure with various auranofin dosing regimens. Median and 5th and 95th percentiles of the predicted concentrations were determined.
Analytical methodology.
Plasma and fecal concentrations of gold (Au) were determined by means of inductively coupled plasma-mass spectrometry (ICP-MS). The limits of quantification for gold in plasma and feces were 0.020 μg/ml and 0.100 μg/g, respectively.
ACKNOWLEDGMENTS
We thank M. John Rogers for his dedication to research on neglected tropical diseases and for his personal support for these studies to find new drugs for parasitic infections. We also thank Walt Jones and Blaire Osborn, Greg Deye, and Lee Hall of DMID, who made the phase I trial possible.
We have no conflict of interest.
This work was supported by the National Institutes of Health (Study DMID 12-0101 under Phase 1 Unit Contract Number HHSN272200800024C to S.L.R., UO1 A1077822 to S.L.R. and J.H.M., and UO1 AI110435 to S.L.R.).
REFERENCES
- 1.Wassmann C, Hellberg A, Tannich E, Bruchhaus I. 1999. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem 274:1–8. 10.1074/jbc.274.37.26051. [DOI] [PubMed] [Google Scholar]
- 2.Upcroft P, Upcroft JA. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 14:150–164. 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAuley JB, Herwaldt BL, Stokes SL, Becher JA, Roberts JM, Michelson MK, Juranek DD. 1992. Diloxanide furoate for treating asymptomatic cyst passers: 14 years' experience in the United States. Clin Infect Dis 15:464–468. 10.1093/clind/15.3.464. [DOI] [PubMed] [Google Scholar]
- 4.Rossignol JF, Ayoub A, Ayers MS. 2001. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double blind, placebo-controlled study of nitazoxanide. J Infect Dis 184:381–384. 10.1086/322038. [DOI] [PubMed] [Google Scholar]
- 5.Wright JM, Dunn LA, Upcroft P, Upcroft JA. 2003. Efficacy of antigiardial drugs. Expert Opin Drug Saf 2:529–541. doi: 10.1517/14740338.2.6.529. [DOI] [PubMed] [Google Scholar]
- 6.Debnath A, Parsonage D, Andrade RM, He C, Cobo E, Hirata K, Chen S, Garcia-Rivera G, Orozco E, Martinez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, McKerrow JH, Reed SL. 2012. A high throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med 18:956–960. 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tejman-Yarden N, Miyamoto Y, Leitsch D, Santini J, Denath A, Gut J, McKerrow J, Reed SL, Eckman L. 2013. A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia. Antimicrob Agents Chemother 57:2029–2035. 10.1128/AAC.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debnath A, Ndao M, Reed SL. 2013. Reprofiled drug targets ancient protozoans. Gut Microbes 4:66–67. 10.4161/gmic.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulman CA, Bidlow CM, Lustigman S, Cho-Ngwa F, Williams D, Rascón AA Jr, Tricoche N, Samje M, Bell A, Suzuki B, Lim KC, Supakorndej N, Supakorndej P, Wolfe AR, Knudsen GM, Chen S, Wilson C, Ang KH, Arkin M, Gut J, Franklin C, Marcellino C, McKerrow JH, Debnath A, Sakanari JA. 2015. Repurposing auranofin as a lead candidate for treatment of lymphatic filariasis and onchocerciasis. PLoS Negl Trop Dis 9:e0003534 10.1371/journal.pntd.0003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade RM, Chapparo JD, Capparelli E, Reed L. 2014. Auranofin is highly efficacious against Toxoplasma gondii in vitro and in an in vivo experimental model of acute toxoplasmosis. PLoS Negl Trop Dis 8:e2973 10.1371/journal.pntd.0002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobanov AV, Gromer S, Salinas G, Gladyshev VN. 2006. Selenium metabolism in Trypanosoma; characterization of selenoproteomes and identification of a Kinetoplastida-specific selenoprotein. Nucleic Acids Res 34:4012–4024. 10.1093/nar/gkl541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilari A, Baiocco P, Messori L, Fiorillo A, Boffi A, Gramiccia M, Muccio TD, Coilotti G. 2012. A gold-containing drug against parasitic polyamine metabolism: the X-ray structure of trypanothione reductase from Leishmania infantum in complex with auranofin reveals a dual mechanism of enzyme inhibition. Amino Acids 42:803–811. 10.1007/s00726-011-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelucci F, Sayed AA, Williams D, Boumis G, Brunori M, Dimastrogiovanni D, Miele AE, Pauly F, Bellelli A. 2009. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin. J Biol Chem 284:289777–289785. 10.1074/jbc.M109.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arnér ES, Williams DL. 2007. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med 4:e206 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furst DE. 1983. Mechanism of action pharmacology, clinical efficacy and side effects of auranofin. Pharmacotherapy 3:284–298. 10.1002/j.1875-9114.1983.tb03277.x. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb NL. 1986. Pharmacology of auranofin: overview and update. Scand J Rheumatol Suppl 63:19–28. http://www.ncbi.nlm.nih.gov/pubmed/3110942. [PubMed] [Google Scholar]
- 17.Kean WF, Hart L, Buchanan WW. 1997. Auranofin. Br J Rheumatol 36:560–572. 10.1093/rheumatology/36.5.560. [DOI] [PubMed] [Google Scholar]
- 18.Rose C, Parker A, Jefferson B, Cartmell E. 2015. The characterization of feces and urine: review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol 45:1827–1879. 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss TE. 1983. Auranofin: dose-related risk to benefit. Am J Med 75:128–132. https://doi.org/10.1016/0002-9343(83)90485-0. [DOI] [PubMed] [Google Scholar]
- 20.Medical Economics Co. 1996. Non-human toxicity excerpts, p 2513. Physicians' desk reference, 50th ed. https://pubchem.ncbi.nlm.nih.gov/compound/6333901#section=Non-Human-Toxicity-Excerpts Accessed 30 March 2016.
- 21.Parsonage D, Sheng F, Hirata K, Debnath A, McKerrow JH, Reed SL, Abagyan R, Poole LB, Podust LM. 2016. X-ray structures of thioredoxin and thioredoxin reductase from Entamoeba histolytica and prevailing hypothesis of the mechanism of auranofin action. J Struct Biol 194:180–190. 10.1016/j.jsb.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harbut HB, Vilcheze C, Luo X, Hensler ME, Guo H, Yang B, Chatterjee AK, Nizet V, Jacobs WR, Schultz PG, Wang F. 2015. Auranofin exerts broad-spectrum bactericidal activity by targeting thiol-redox homeostasis. Proc Nat Acad Sci 112:4453–4458. 10.1073/pnas.1504022112. [DOI] [PMC free article] [PubMed] [Google Scholar]