ABSTRACT
We report data on the frequency of the paradoxical effect of echinocandins against Candida spp. (n = 602 incident isolates) using the EUCAST definitive document EDef 7.2 procedure. The paradoxical effect for one or more echinocandins was observed in 16% of the isolates. However, differences between species were found, and the paradoxical effect was more common in Candida tropicalis (P < 0.001). Caspofungin was the drug in which the paradoxical effect was most common, followed by anidulafungin and micafungin (P < 0.001).
KEYWORDS: paradoxical effect, echinocandins, Candida, EUCAST
TEXT
Treatment with echinocandins, i.e., caspofungin, micafungin, and anidulafungin, is recommended as the primary therapy for patients with candidemia (1, 2). Rates of resistance are low (3), but attenuation of activity at high concentrations, known as the paradoxical or Eagle effect, has been reported (4–7). Isolates are characterized by abnormal morphology when studied in the presence of high concentrations of echinocandins (8–10). Although it has been reported for all three echinocandins, differences in frequency by species have been pointed out, mostly by use of CLSI methodology (4, 11, 12). Antifungal lock therapy may prevent catheter removal in patients with candidemia. Since the procedure requires the catheter lumen to be filled with a solution containing a high concentration of echinocandins (13), the paradoxical effect may have a negative impact (14, 15).
(This study was presented in part at the 26th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Amsterdam, Netherlands, 9 to 12 April 2016 [electronic poster EP0008].)
We studied the frequency of the paradoxical effect of echinocandins against 602 echinocandin-susceptible Candida species incident isolates from the blood cultures of patients with candidemia who were admitted to Gregorio Marañón Hospital from January 2007 to March 2015. All the strains were molecularly identified (Table 1) (16), and antifungal susceptibility to micafungin (Astellas Pharma, Inc., Tokyo, Japan), anidulafungin (Pfizer Pharmaceutical Group, New York, NY), and caspofungin (Merck &Co., Inc., Rahway, New Jersey, NJ) was determined using the EUCAST definitive document EDef 7.2 procedure (17). The candin concentrations tested ranged from 0.015 to 8 μg/ml. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 isolates were used as quality control strains. The paradoxical effect was defined as an increase in optical density of 0.02 compared with the growth control in wells containing a candin concentration at least 2 drug dilutions higher than the MIC (Fig. 1). This definition was adapted from a previous study (9). We calculated the frequency of the paradoxical effect at each concentration tested, overall and by species, and the percentage of isolates in which the paradoxical effect was observed (Kruskal-Wallis test). This study was approved by the Ethics Committee of Hospital Gregorio Marañón (CEIC-A1; study no. 252/15).
TABLE 1.
Species distribution and percentage of isolates, overall and by species, in which the paradoxical effect of caspofungin, anidulafungin, micafungin, or any combination of the three was observed at any drug concentration
| Antifungal concentration (μg/ml) | % Isolates in which the paradoxical effect was observed fora: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All species (n = 602)b |
C. tropicalis (n = 50) |
Other Candida spp. (n = 24)c |
C. albicans (n = 291) |
C. parapsilosis (n = 164) |
C. glabrata (n = 62) |
|||||||||||||
| MYC | AND | CAS | MYC | AND | CAS | MYC | AND | CAS | MYC | AND | CAS | MYC | AND | CAS | MYC | AND | CAS | |
| 0.125 | 0 | 0.3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0.3 | NA | NA | NA | NA | 0 | 0 | NA |
| 0.25 | 0.2 | 0.7 | 0.2 | 0 | 4 | 0 | 0 | 0 | 4.2 | 0 | 0.7 | 0 | NA | NA | NA | 0 | 0 | 0 |
| 0.5 | 2.3 | 1.8 | 1.2 | 18 | 14 | 6 | 8.3 | 4.2 | 12.5 | 0.3 | 1 | 0 | NA | NA | NA | 0 | 0 | 0 |
| 1 | 3.2 | 2.2 | 1.8 | 26 | 16 | 8 | 12.5 | 8.3 | 16.7 | 1 | 1 | 0.3 | NA | NA | NA | 0 | 0 | 0 |
| 2 | 3.7 | 4.8 | 5.6 | 30 | 36 | 32 | 12.5 | 8.3 | 29.2 | 1 | 3.1 | 1 | 0 | NA | 0 | 0 | 0 | 0 |
| 4 | 4 | 7.6 | 7.6 | 38 | 44 | 50 | 12.5 | 12.5 | 29.2 | 1.4 | 5.8 | 2.4 | 0 | 1.2 | 2.4 | 1.6 | 3.2 | 0 |
| 8 | 4 | 8.1 | 11.6 | 38 | 40 | 54 | 8.3 | 16.7 | 25 | 0.3 | 8.2 | 2.4 | 0.6 | 0 | 4.3 | 1.6 | 3.2 | 0 |
| Overall | 4.8 | 10.5 | 13.3 | 38 | 48 | 62 | 16.7 | 16.7 | 29.2 | 0.3 | 10.7 | 9.6 | 0.6 | 1.2 | 5.5 | 1.6 | 3.2 | 0 |
Statistically significant differences (P < 0.05) are shown in bold. MYC, micafungin; AND, anidulafungin; CAS, caspofungin; NA, not applicable.
C. krusei isolates (n = 11) are not shown because none of them showed paradoxical effect for any echinocandin.
C. guilliermondii (n = 8), C. dubliniensis (n = 5), C. lusitaniae (n = 5), C. kefyr (n = 2), C. fermentati (n = 1), C. inconspicua (n = 1), C. pelliculosa (n = 1), Kodamaea ohmeri (n = 1).
FIG 1.
Example of an isolate showing paradoxical effect (PE) of caspofungin at MICs of 4 and 8 μg/ml. OD, optical density.
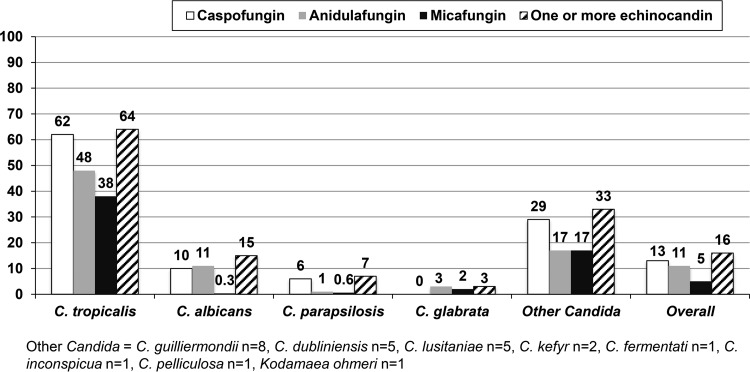
We used the EUCAST procedure and found a paradoxical effect for one or more echinocandins in 16% (n = 96) of the isolates; it was most frequent in caspofungin, followed by anidulafungin and micafungin (Fig. 2) (P < 0.001), as reported previously in studies using the method in CLSI document M27-A3 (4, 10, 18). Caspofungin had a paradoxical effect against Candida tropicalis, C. parapsilosis, and other Candida spp., whereas anidulafungin presented the effect more frequently than caspofungin and micafungin against Candida albicans and Candida glabrata. Micafungin was the agent for which the effect was least frequent. Overall, the three echinocandins are similar in terms of pharmacological properties and spectra of activity; however, micafungin tends to show lower MICs against C. glabrata. Furthermore, our observations support previous findings that micafungin causes the paradoxical effect in a lower proportion of C. glabrata isolates (11, 19).
FIG 2.
Percentage of isolates, overall and per species, in which the paradoxical effect of caspofungin, anidulafungin, micafungin, or any combination of the three was observed.
We found the effect to be species specific; C. tropicalis had the highest proportion of strains (64%) in which one or more echinocandins produced a paradoxical effect, followed by C. albicans (14.8%) (Fig. 2). The high percentage of C. tropicalis isolates showing paradoxical effect was previously reported (18). The high percentage of other Candida species isolates in which the effect was observed has been mainly attributed to Candida dubliniensis (4/5) and Candida guilliermondii (4/8) (11).
Chamilos et al. (18) showed that the paradoxical effect of caspofungin was found in a high proportion (90%) of C. parapsilosis isolates when using the CLSI method; however, we only detected the effect in 6.7% of C. parapsilosis isolates with the EUCAST procedure. This discrepancy may be due to differences between the EUCAST and CLSI methods, the low number of isolates analyzed by Chamilos et al., or the chosen definitions of the paradoxical effect.
The paradoxical effect was observed at low concentrations of ≥0.125 μg/ml (anidulafungin) and ≥0.25 μg/ml (caspofungin and micafungin); previous studies using the CLSI method reported the presence of the paradoxical effect at high concentrations (2 to 64 μg/ml) (4, 11, 18). Use of the EUCAST procedure would probably explain the presence of the effect at lower concentrations; however, we found that the higher the echinocandin concentration, the higher the proportion of isolates in which the effect was observed (Table 1). Furthermore, the phenomenon was not only concentration dependent but also drug dependent, as shown by the fact that caspofungin and anidulafungin caused the paradoxical effect in a higher percentage of isolates than caused by micafungin, to the extent that the drug concentration rose (P < 0.05) (Table 1). However, in vitro antifungal activity of caspofungin obtained by the EUCAST or CLSI procedure should be interpreted carefully because of the interlaboratory variations reported when using this drug (20). This observation was not reported with use of the colorimetric marketed system YeastOne assay (21).
The concentrations at which the paradoxical effect was observed in vitro were easily reached in plasma (5); the addition of serum to the trays reversed the effect (10), probably as a consequence of albumin binding, suggesting that the paradoxical effect may only have a clinical impact on anatomical sites at which the free fraction of echinocandins reached is high. Few data support the clinical impact of the paradoxical effect, and high-dose echinocandin regimens have been shown to be as effective as regular dose regimens (14, 15). Antifungal lock therapy requires concentrations that are at least 1,000-fold greater than the MIC over a prolonged period in order to provide a suitable environment for the paradoxical effect (13). Future studies should evaluate the clinical impact of promoting the paradoxical effect when using echinocandins in lock therapy (5, 7).
The mechanism of action of the paradoxical effect is not fully understood, but it probably involves the integrity of the cell wall (5, 7, 10). Since these changes enable the cell to adapt to an environment with high echinocandin concentrations, the paradoxical effect is more a mechanism of tolerance than of resistance, albeit with a cost in fitness and virulence (9).
We only tested echinocandin concentrations of up to 8 μg/ml. Previous studies using the CLSI method showed the presence of the paradoxical effect at concentrations ranging from 2 to 64 μg/ml (4, 8, 11, 18). However, a key strength of our study is the detection of the paradoxical effect coupled with the MIC reading. This finding may prove particularly relevant if the phenomenon is shown to have a clinical impact.
We conclude that the paradoxical effect is easily detected using the EUCAST procedure, and it is seen mainly with caspofungin and in C. tropicalis.
ACKNOWLEDGMENTS
We thank Thomas O'Boyle for editing the article.
We have no conflicts of interest to report.
This study was supported by grant ref. CM-SANTANDER (GR3/2014; group 920200), grant no. PI14/00740 from Fondo de Investigación Sanitaria (FIS; Instituto de Salud Carlos III, Madrid, Spain; Plan Nacional de I+D+I 2013-2016), and the European Regional Development Fund (FEDER; “A way of making Europe”).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
P.E. (CPI15/00115), J.G. (CPII15/00006), and L.J.M.-Z. (FI12/00265) are supported by FIS.
REFERENCES
- 1.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:1–4. doi: 10.1093/cid/civ93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlin DS. 2015. Echinocandin resistance in Candida. Clin Infect Dis 61(Suppl 6):S612–S617. doi: 10.1093/cid/civ791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens DA, Espiritu M, Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanstraelen K, Lagrou K, Maertens J, Wauters J, Willems L, Spriet I. 2013. The Eagle-like effect of echinocandins: what's in a name? Expert Rev Anti Infect Ther 11:1179–1191. doi: 10.1586/14787210.2013.841543. [DOI] [PubMed] [Google Scholar]
- 6.Stevens DA. 2009. Frequency of paradoxical effect with caspofungin in Candida albicans. Eur J Clin Microbiol Infect Dis 28:717. doi: 10.1007/s10096-008-0688-y. [DOI] [PubMed] [Google Scholar]
- 7.Steinbach WJ, Lamoth F, Juvvadi PR. 2015. Potential microbiological effects of higher dosing of echinocandins. Clin Infect Dis 61(Suppl 6):S669–S677. doi: 10.1093/cid/civ725. [DOI] [PubMed] [Google Scholar]
- 8.Bizerra FC, Melo AS, Katchburian E, Freymuller E, Straus AH, Takahashi HK, Colombo AL. 2011. Changes in cell wall synthesis and ultrastructure during paradoxical growth effect of caspofungin on four different Candida species. Antimicrob Agents Chemother 55:302–310. doi: 10.1128/AAC.00633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rueda C, Cuenca-Estrella M, Zaragoza O. 2014. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother 58:1071–1083. doi: 10.1128/AAC.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields RK, Nguyen MH, Du C, Press E, Cheng S, Clancy CJ. 2011. Paradoxical effect of caspofungin against Candida bloodstream isolates is mediated by multiple pathways but eliminated in human serum. Antimicrob Agents Chemother 55:2641–2647. doi: 10.1128/AAC.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis 27:127–131. doi: 10.1007/s10096-007-0411-4. [DOI] [PubMed] [Google Scholar]
- 12.Miceli MH, Bernardo SM, Lee SA. 2009. In vitro analysis of the occurrence of a paradoxical effect with different echinocandins and Candida albicans biofilms. Int J Antimicrob Agents 34:500–502. doi: 10.1016/j.ijantimicag.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Walraven CJ, Lee SA. 2013. Antifungal lock therapy. Antimicrob Agents Chemother 57:1–8. doi: 10.1128/AAC.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, Herbrecht R, Ruiz-Palacios G, Young JA, Baddley JW, Strohmaier KM, Tucker KA, Taylor AF, Kartsonis NA. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 48:1676–1684. doi: 10.1086/598933. [DOI] [PubMed] [Google Scholar]
- 15.Safdar A, Rodriguez G, Rolston KV, O'Brien S, Khouri IF, Shpall EJ, Keating MJ, Kantarjian HM, Champlin RE, Raad II, Kontoyiannis DP. 2007. High-dose caspofungin combination antifungal therapy in patients with hematologic malignancies and hematopoietic stem cell transplantation. Bone Marrow Transplant 39:157–164. doi: 10.1038/sj.bmt.1705559. [DOI] [PubMed] [Google Scholar]
- 16.White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 322 In Michael A, Innis DHG, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 17.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 18.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob Agents Chemother 51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, Hope W. 2012. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother 56:2435–2442. doi: 10.1128/AAC.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eschenauer GA, Nguyen MH, Shoham S, Vazquez JA, Morris AJ, Pasculle WA, Kubin CJ, Klinker KP, Carver PL, Hanson KE, Chen S, Lam SW, Potoski BA, Clarke LG, Shields RK, Clancy CJ. 2014. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob Agents Chemother 58:1897–1906. doi: 10.1128/AAC.02163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]