ABSTRACT
Four trials were conducted to evaluate the impact of Escherichia coli probiotic strain ED1a administration to pigs on the gut carriage or survival in manure of extended-spectrum-β-lactamase-producing E. coli. Groups of pigs were orally inoculated with strain E. coli M63 carrying the blaCTX-M-1 gene (n = 84) or used as a control (n = 26). In the first two trials, 24 of 40 E. coli M63-inoculated pigs were given E. coli ED1a orally for 6 days starting 8 days after oral inoculation. In the third trial, 10 E. coli M63-inoculated pigs were given either E. coli ED1a or probiotic E. coli Nissle 1917 for 5 days. In the fourth trial, E. coli ED1a was given to a sow and its 12 piglets, and these 12 piglets plus 12 piglets that had not received E. coli ED1a were then inoculated with E. coli M63. Fecal shedding of cefotaxime-resistant Enterobacteriaceae (CTX-RE) was studied by culture, and blaCTX-M-1 genes were quantified by PCR. The persistence of CTX-RE in manure samples from inoculated pigs or manure samples inoculated in vitro with E. coli M63 with or without probiotics was studied. The results showed that E. coli M63 and ED1a were good gut colonizers. The reduction in the level of fecal excretion of CTX-RE in E. coli ED1a-treated pigs compared to that in nontreated pigs was usually less than 1 log10 CFU and was mainly observed during the probiotic administration period. The results obtained with E. coli Nissle 1917 did not differ significantly from those obtained with E. coli ED1a. CTX-RE survival did not differ significantly in manure samples with or without probiotic treatment. In conclusion, under our experimental conditions, E. coli ED1a and E. coli Nissle 1917 could not durably prevent CTX-RE colonization of the pig gut.
KEYWORDS: probiotic, cephalosporin resistance, pig, microbiota, antibiotic resistance, cephalosporin
INTRODUCTION
Resistance to extended-spectrum cephalosporins (ESC) is a human health concern. Enterobacteriaceae resistant to ESC are also isolated from clinical cases in veterinary medicine (1), and it is suspected that the reservoir of these ESC-resistant (ESCR) Escherichia coli isolates may be the microbiota of food-producing animals (2). Indeed, ESCR E. coli isolates are sometimes present at high levels in pigs (3, 4). Fecal material represents a potential source of carcass contamination at slaughter and, like Salmonella or Campylobacter, can be considered a foodborne biological hazard to public health. Moreover, farm workers are at risk of contamination (5), and the spread of manure containing ESCR bacteria could contaminate the environment (6).
In pigs, as in humans, the most frequently reported extended-spectrum β-lactamase (ESBL) is CTX-M (7), encoded by genes present on conjugative plasmids. The consumption of meat contaminated with Enterobacteriaceae isolates containing such plasmids may result in their establishment in the human microbiota or the transfer of their blaCTX-M gene to commensal or pathogenic human E. coli strains (8, 9). Depoorter et al. (10) recently published an assessment of human exposure to ESCR E. coli through the consumption of broiler meat in Belgium, concluding that the proportion of ESCR E. coli isolates on carcasses was one of the dominant factors in consumer exposure. We are not aware of such a study being published for pig meat, but it seems plausible that a similar finding could be obtained for swine production. Strategies to reduce the number of ESCR Enterobacteriaceae isolates in pig feces are therefore needed. The current study explores the possibility of competition between ESCR Enterobacteriaceae and a susceptible nonpathogenic E. coli strain known to be a good colonizer that could be administered as a probiotic. In the first two experiments, E. coli strain ED1a was chosen because previous studies had demonstrated that it is avirulent and competitive and that the numerous isolates obtained were susceptible to antimicrobials (11). The impact of the daily administration of E. coli ED1a to pigs previously experimentally inoculated with an ESCR E. coli strain on the fecal excretion of ESCR E. coli was studied. Next, the effects of probiotic strain E. coli Nissle 1917, which has been used as a probiotic in humans for decades (12), and E. coli strain ED1a were compared. Finally, the possibility of preventing the colonization of weaned specific-pathogen-free (SPF) piglets by an orally inoculated ESCR E. coli strain was explored by comparing the titers of ESCR isolates in piglets born to E. coli ED1a-treated sows and given ED1a from birth to weaning to those in nontreated piglets born to nontreated sows.
RESULTS
The titers of the E. coli M63 and the E. coli ED1a with E. coli Nissle 1917 inocula used for the different experiments are shown in Table 1. No ESCR Enterobacteriaceae were detected in any of the fecal samples before E. coli M63 inoculation or in uninoculated animals in any of the four trials.
TABLE 1.
Experimental design
| Trial | Group | E. coli M63 inoculum (no. of CFU/pig) on day 0 | Probiotic (no. of CFU/pig) | Days on which fecal samples were collectedd |
|---|---|---|---|---|
| 1 | 1-C | No | None | −1, 1, 3, 7, 10, 13, 17, 22, and 28 |
| 1-M63 | 7.8 × 109 | None | −1, 1, 3, 7, 10, 13, 17, 22, and 28 | |
| 1-M63-E | 7.8 × 109 | E. coli ED1a on days 8–13 (2.76 ×109–6.03 ×109a) | −1, 1, 3, 7, 10, 13, 17, 22, and 28 | |
| 2 | 2-C | No | None | 0, 3, 8, 10, 13, 16, 20, 23 27, and 31 |
| 2-M63 | 7.03 × 107 | None | 0, 3, 8, 10, 13, 16, 20, 23 27, and 31 | |
| 2-M63-EL | 7.03 × 107 | E. coli ED1a on days 8–13 (7.4 × 107b) | 0, 3, 8, 10, 13, 16, 20, 23 27, and 31 | |
| 2-M63-EH | 7.03 × 107 | E. coli ED1a on days 8–13 (6.5 × 108b) | 0, 3, 8, 10, 13, 16, 20, 23 27, and 31 | |
| 3 | 3-C | None | None | 0, 2, 3, 7, 9, 13, 16, and 20 |
| 3-M63-ED | 7.8 × 1010 | E. coli ED1a on days 8–12 (2 × 109–16 × 109a) | 0, 2, 3, 7, 9, 13, 16, and 20 | |
| 3-M63-EcN | 7.8 × 1010 | E. coli Nissle 1917 on days 8–12 (7 × 109–23 × 109a) | 0, 2, 3, 7, 9, 13, 16, and 20 | |
| 4 | 4-M63 | 2.5 × 107 | None | −29 (sows before farrowing); −17 and −10 (sow and piglets before weaning); 0, 4, 6, 11, 13, 18, 20, 25, and 32 (piglets after inoculation); and −43, −38, and −36 (sows before farrowing) |
| 2.5 × 107 | E. coli ED1a (1.35 × 109) for sows | −22, −17, and −10 (sows and piglets before weaning) | ||
| 4-ED-M63 | 2.5 × 107 | E. coli ED1a (1.35 × 108–5.4 × 108c) for piglets | 0, 4, 6, 11, 13, 18, 25, and 32 (piglets after inoculation) |
Several inocula were used for the different days.
A single inoculum was prepared and used for the different days.
The E. coli ED1a dose increased with piglet age (see the text for details).
For all trials, day 0 is the E. coli M63 inoculation day.
First trial.
During the first trial, signs of diarrhea were observed between day 17 and day 21 in seven animals from group 1-M63, and a rotavirus was detected by enzyme-linked immunosorbent assay at the local veterinary diagnostic laboratory (data not shown). Otherwise, no sign of diarrhea was detected during the 3 weeks following inoculation of E. coli M63. The pigs' rectal temperatures remained below 40°C for the 2 weeks following inoculation, except on day 2 (when one animal in group 1-M63 had a temperature of 41°C). No other clinical signs were observed. The statistical analysis of body weight gains showed no significant differences between groups except for group 1-M63, which had a lower weight gain during the third week, corresponding to the rotavirus infection period (data not shown). Throughout the study period, the mean weight gains of the animals in groups 1-C, 1-M63, and 1-M63-E were, respectively, 26.8, 25.6, and 23.6 kg (P > 0.05).
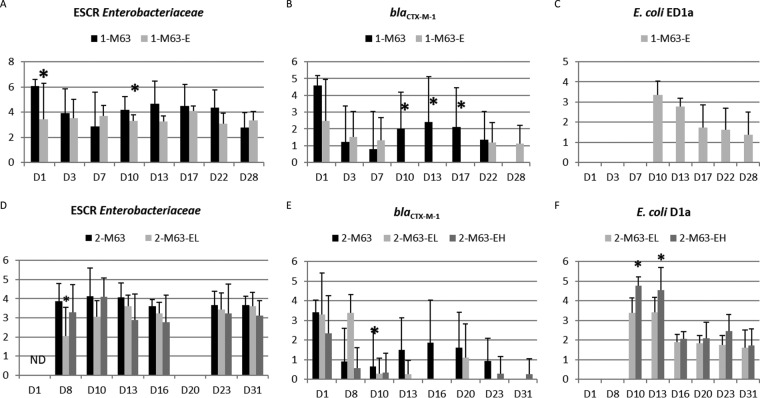
The mean titers obtained on cefotaxime-supplemented medium in the first trial are presented in Fig. 1A. Individual titers varied quite markedly, with the titers in some samples being under the detection limit, while other samples contained up to 1.25 × 108 CFU/g. Piglet no. 4314 from the 1-M63 group regularly excreted many more resistant bacteria than the other piglets and had titers 20 to 130 times higher than all the other piglets from day 3 to day 13. Comparisons between groups were therefore performed either with data for all the animals in the 1-M63 and 1-M63-E groups, with data for all the animals except this high shedder, or with data only for the 1-M63 group animals in a pen different from that with the high shedder because the high level of shedding by one animal probably resulted in a highly contaminated environment for pigs in the same pen. When the data for all the animals were considered, a significant difference (P = 0.01) was observed for the whole period, and analysis per day showed a significant difference between the titer on day 1 (before administration of E. coli ED1a) and that on day 10 (P = 0.048) and a tendency toward a significant difference on day 13 (P = 0.058), with higher ESCR Enterobacteriaceae titers being detected in the 1-M63 group. When the data for the high shedder were excluded, the difference between groups was less significant (P = 0.087 for the whole period, P = 0.08 for day 10, and P = 0.10 for day 13). When data for animals in the pen containing the high shedder were excluded, no significant difference was observed.
FIG 1.
Results of the first and second trials. Samples were cultured for ESCR Enterobacteriaceae, and qPCR for blaCTX-M-1 and E. coli ED1a was performed on the different sampling days. (A to C) First trial; (D to F) second trial. (A and D) Titers of ESCR Enterobacteriaceae (log10 number of CFU per gram, as indicated on the y axis); (B and E) titers of blaCTX-M-1 (log10 number of copies in 10 ng of DNA, as indicated on the y axis); (C and F) titers of E. coli ED1a (log10 number of copies in 10 ng of DNA, as indicated on the y axis). For each date, asterisks indicate significant differences between groups (P < 0.05). For both trials, E. coli M63 was inoculated on day 0 (D0), and E. coli ED1a was given from day 8 (D8) to day 13 (D13). ND, not done. Samples from animals not inoculated with E. coli M63 were negative for ESCR Enterobacteriaceae and blaCTX-M-1. Samples from animals not inoculated with E. coli ED1a were negative for E. coli ED1a.
Analysis of ESCR Enterobacteriaceae showed that most were identified to be E. coli by the PCR tests. A few isolates gave negative results but were later identified to be E. coli by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) at the local veterinary diagnostic laboratory (results not shown). The percentages of isolates resistant to rifampin varied (Table 2), being 100% during the first sampling days after inoculation but decreasing to 47% for the 1-M63 group on day 28. PCR showed that all 104 tested isolates obtained on cefotaxime-supplemented medium bore a blaCTX-M-1 gene. All 17 rifampin-resistant isolates tested belonged to the same phylogenetic group as E. coli M63, group B1. The 87 rifampin-susceptible isolates belonged to various groups, including B1, B2, A, C, E, and F (Table 2). The only isolate belonging to group B2 had a PFGE profile different from that of E. coli ED1a (data not shown) and gave a negative result with the E. coli ED1a-specific PCR. Two cefotaxime-resistant and rifampin-susceptible isolates belonging to phylogenetic group B1, one from group 1-M63 and one from group 1-M63-E, were analyzed by pulsed-field gel electrophoresis (PFGE) and were revealed to have profiles that not only differed from each other but also differed from the profile of the E. coli M63 strain inoculated.
TABLE 2.
Percentages of rifampin-susceptible isolates among the cefotaxime-resistant E. coli isolates obtained in the in vivo trials and their phylogenetic groups
| Trial and group | No. of rifampin-susceptible, cefotaxime-resistant isolates/total no. of cefotaxime-resistant isolates tested (%) | % rifampin-susceptible isolates on the following sampling day: |
No. of isolates in the following phylogenetic groupa: |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 6 | 7 | 8 | 9 | 10 | 11 | 13 | 16 | 17 | 18 | 20 | 22 | 23 | 25 | 28 | 31 | 32 | A | B1 | B2 | C | D | E | F | ||
| Trial 1 | ||||||||||||||||||||||||||||
| 1-M63 | 47/281 (17) | 0 | 0 | 0 | 10 | 26 | 5 | 28 | 53 | 3 | 28* | 1 | 1 | 15 | ||||||||||||||
| 1-M63-E | 40/287 (14) | 0 | 0 | 8 | 10 | 20 | 25 | 18 | 20 | 1 | 13 | 1 | 25* | |||||||||||||||
| Trial 2 | ||||||||||||||||||||||||||||
| 2-M63 | 64/224 (29)b | NDc | 41 | 46 | 28 | 23 | ND | 25 | 9 | 37 | 25* | 1 | ||||||||||||||||
| 2-M63-EL | 73/208 (35)b | ND | 71 | 34 | 33 | 35 | ND | 50 | 10 | 3 | 34* | 2 | 27 | 5 | ||||||||||||||
| 2-M63-EH | 89/198 (45)b | ND | 43 | 50 | 47 | 54 | ND | 40 | 50 | 84* | ||||||||||||||||||
| Trial 3 | ||||||||||||||||||||||||||||
| 3-M63-ED | 41/106 (39) | 20 | 32 | 23 | 71 | 65 | 24 | 8 | 9 | |||||||||||||||||||
| 3-M63-EN | 2/86 (2) | 0 | 0 | 17 | 6 | 0 | 2 | |||||||||||||||||||||
| Trial 4 | ||||||||||||||||||||||||||||
| 4-M63 | 32/176 (18) | 12 | 8 | 25 | 29 | 32 | 17 | 7 | 10 | 14* | 1 | 9 | 8* | |||||||||||||||
| 4-ED-M63 | 23/141 (16) | 0 | 0 | 0 | 5 | 9.5 | 58 | 69 | 22* | 1 | ||||||||||||||||||
For the first, second, and fourth trials, data with asterisks indicate that isolates of the phylogenetic group were obtained from the two separate pens in the room.
A few isolates were lost, and their phylogenetic group could not be determined or the isolates were not tested for their phylogenetic group.
ND, not determined.
The results of quantitative PCR (qPCR) for blaCTX-M-1 and E. coli ED1a are shown in Fig. 1B and C, respectively. The sensitivity of qPCR for the blaCTX-M-1 gene in fecal material was 104 copies per test (data not shown). All the samples collected prior to inoculation or from uninoculated piglets were negative. When data for all the pigs in the 1-M63 group were considered, statistical analysis showed that there were significantly more copies of blaCTX-M-1 in the 1-M63 group than in the 1-M63-E group on days 10, 13, and 17 (P < 0.05 each), and the total number of positive samples detected during and after the E. coli ED1a administration period was significantly different between the 1-M63 group (15/40 positive samples) and the 1-M63-E group (2/40 positive samples) (P < 0.001). When data for the high shedder were excluded, the number of copies of blaCTX-M-1 in the 1-M63 group tended to be higher on day 10 (P = 0.057), day 13 (P = 0.075), and day 17 (P = 0.057). When data for animals in the pen containing the high shedder were excluded, no significant difference was observed.
The qPCR developed to detect E. coli ED1a had a sensitivity of 31 cells per assay (data not shown). Negative results were obtained for all fecal samples obtained from piglets before administration of E. coli ED1a and from untreated pigs. The mean titers of E. coli ED1a obtained during administration were higher than those obtained after administration (P < 0.05), but on the last two sampling days, days 22 and 28, six and five of the eight piglets in the 1-M63-E group were still excreting E. coli ED1a, respectively.
Second trial.
During the second trial, moderate hyperthermia was recorded during the first week after inoculation of E. coli M63 in the three inoculated groups, and the mean weight gains differed significantly between groups (data not shown). However, the mean weight gains during the following weeks and for the global study period were not significantly different.
A culture of ESCR Enterobacteriaceae revealed that titers again varied between animals, with the mean titers per animal for the global period varying between 1.73 and 4.43 log10 CFU/g, but animals with high levels of shedding (>4.0 log10 CFU/g) were present in all groups. The mean titers per day did not differ significantly between groups except on day 8, before administration of E. coli ED1a (P = 0.008), when the mean titer for the 2-M63-EL group was lower than that for the other two groups (Fig. 1D).
Globally, 404 out of the 631 ESCR isolates tested (64%) were resistant to rifampin. One week after inoculation, many isolates were found to be rifampin susceptible, with the proportion of rifampin-susceptible isolates ranging from 41% to 71% (Table 2). All but two cefotaxime-resistant E. coli isolates had the blaCTX-M gene. No attempt was made to determine the resistance mechanisms of these two ESCR E. coli isolates. Three piglets (piglets no. 4572, 4595, and 4611) in the 2-M63-EH group, which had high percentages of rifampin-susceptible isolates (69/75, 91%), excreted large amounts of ESCR Enterobacteriaceae, approximately 10 times more than all the other piglets, from day 10 to day 23 (data not shown). The phylogenetic groups of the cefotaxime-resistant, rifampin-susceptible isolates are shown in Table 2. No isolate belonged to the B2 group, which is the group to which E. coli ED1a belongs. All the isolates tested in the 2-M63-EH group belonged to the B1 group, whereas isolates belonging to groups A, B1, C, D, E, and F were identified in the 2-M63 and 2-M63-EL groups.
An intergroup comparison of the number of copies of blaCTX-M-1 showed no statistically significant differences between groups except on day 10 for the 2-M63 group compared to the 2-M63-EL group (1.49 ± 1.63 versus 0.25 ± 0.71 log10 copies of blaCTX-M-1, respectively [P = 0.01]) (Fig. 1E). The total number of samples positive for the blaCTX-M-1 gene during and after administration of E. coli ED1a tended to be higher in the 2-M63 group (in which 12 of 56 pigs were positive) than in the 2-M63-EH group (in which 4 of 55 pigs were positive) (P = 0.056). E. coli ED1a DNA was not detected by PCR before inoculation or in uninoculated groups. The mean titers obtained for E. coli ED1a in the 2-M63-EH group were significantly higher than the mean titers in the 2-M63-EL group (Wilcoxon test for the period from day 10 to day 31, P < 0.05) (Fig. 1F). In the two groups, six of the seven piglets tested were still shedding E. coli ED1a on the last sampling day.
Third trial.
No clinical signs or lesions were recorded during the third trial.
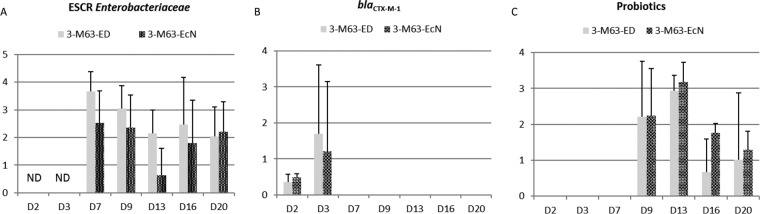
No ESCR Enterobacteriaceae or blaCTX-M-1 genes were detected before inoculation or in uninoculated piglets. The mean individual titers of ESCR Enterobacteriaceae for animals receiving either E. coli ED1a or E. coli Nissle 1917 were determined only on days 7, 9, 13, 16, and 20 (Fig. 2A), and no significant difference between groups could be detected (P > 0.05). The percentage of isolates resistant to rifampin was as low as 29% for group 3-M63-ED on day 16 (Table 2). On the other hand, only 2 out of a total of 86 isolates (2.3%) in the 3-M63-EcN group were rifampin susceptible for the whole assay; this rate is significantly lower than the rate for the 3-M63-ED group (41/106, 38.6%) (P < 0.001). This proportion of cefotaxime-resistant, rifampin-susceptible isolates was also significantly different from that obtained for all the other E. coli M63-inoculated groups in the first and second trials, whether or not they were treated with probiotics (P < 0.01). All cefotaxime-resistant isolates had the blaCTX-M-1 gene. Rifampin-susceptible isolates belonged to the B1, C, or F phylogenetic group but not to the B2 group, to which E. coli ED1a or E. coli Nissle 1917 belong. PFGE analysis showed that isolates from phylogenetic group B1 were different from the inoculated E. coli M63 strain.
FIG 2.
Results of the third trial. Samples were cultured for ESCR Enterobacteriaceae, and qPCR for blaCTX-M-1 and probiotics (E. coli ED1a and E. coli Nissle 1917) was performed on the different sampling days. (A) Titers of ESCR Enterobacteriaceae (log10 number of CFU per gram, as indicated on the y axis); (B) titers of blaCTX-M-1 (log10 number of copies in 10 ng of DNA, as indicated on the y axis); (C) titers of probiotics E. coli ED1a and E. coli Nissle 1917 (log10 number of copies in 10 ng of DNA, as indicated on the y axis). E. coli M63 was inoculated on day 0, and E. coli ED1a or E. coli Nissle 1917 was given from day 8 to day 12. Samples from animals not inoculated with E. coli M63 were negative for ESCR Enterobacteriaceae and blaCTX-M-1. Samples from animals not inoculated with E. coli ED1a and E. coli Nissle 1917 were negative for E. coli ED1a and E. coli Nissle 1917, respectively.
From day 7, even before administration of the probiotics, the blaCTX-M-1 gene was rarely detected in the feces of inoculated pigs (Fig. 2B).
E. coli ED1a was not detected in the uninoculated pigs but was present in four and five of the five tested pigs on day 9 and day 13, respectively. On the last sampling day (day 20), four out of five pigs were still positive by PCR (Fig. 2C). The PCR assay for E. coli Nissle 1917 had a sensitivity of approximately 8 E. coli Nissle 1917 cells per assay (data not shown). E. coli Nissle 1917 was not detected in the uninoculated pigs. The mean titers obtained during the E. coli Nissle 1917 administration period or the day after (day 9 and day 13, respectively) were significantly higher than those obtained afterwards (day 16 and day 20) (P < 0.01). All fecal samples collected from the 3-M63-EcN group from day 9 to the last sampling day (day 20) were positive for E. coli Nissle 1917.
Fourth trial.
E. coli ED1a was administered to the sows during the 15 days before farrowing, and no symptoms were noted. Unfortunately, one of the two treated sows and her piglets died during farrowing because the sow's pelvis was too narrow. Thus, the 4-ED-M63 group comprised 12 piglets from a single sow, whereas the 4-M63 group comprised 12 piglets from three different sows. The piglets' temperature most often remained below 40°C and always remained below 41.0°C. The mean birth weight of the piglets in the 4-M63 group was 1.17 kg, whereas that of the piglets in the 4-ED-M63 group was 1.325 kg (P = 0.01), but the mean body weights on the two groups' weaning days were not significantly different (7.31 kg for the 4-M63 group versus 6.66 kg for the 4-ED-M63 group; P > 0.05). The mean body weight gain from birth to 2 months of age was 0.365 kg/day for the 4-M63 group and 0.293 kg/day for the 4-ED-M63 group (P < 0.001). No lesions were observed.
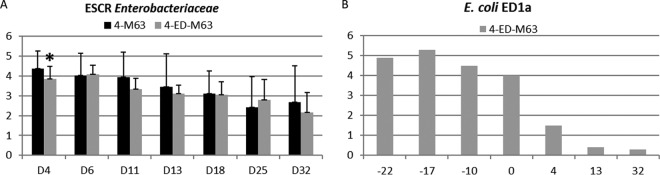
The titers of ESCR Enterobacteriaceae are presented in Fig. 3A. Statistical analysis showed that the titers for treated group 4-ED-M63 were significantly lower than the titers for nontreated group 4-M63 on the first sampling day after inoculation only (on day 4, 3.87 ± 0.87 log10 CFU/g for the 4-ED-M63 group compared to 4.38 ± 0.63 log10 CFU/g for the 4-M63 group). No ESCR Enterobacteriaceae could be detected in 2 out of 84 fecal samples collected from nontreated group 4-M63, whereas 10 out of 84 samples collected from the 4-ED-M63 group were negative by culture (P = 0.03). These negative samples were detected on days 12 to 33 in the 4-ED-M63 group and on days 26 and 33 in the 4-M63 group. Interestingly, all 10 negative samples in the 4-ED-M63 group were collected from piglets in the same pen.
FIG 3.
Results of the fourth trial. Samples were cultured for ESCR Enterobacteriaceae, and qPCR for E. coli ED1a in fecal samples from piglets was performed on the different sampling days. (A) Titers of ESCR Enterobacteriaceae (log10 number of CFU per gram, as indicated on the y axis); (B) titers of E. coli ED1a (log10 number of copies in 10 ng of DNA, as indicated on the y axis). E. coli M63 was inoculated on day 0, and E. coli ED1a was given to the sow from before farrowing (days −42 to −28 before E. coli M63 inoculation) and to the piglets from birth to weaning day (days −27 to day −1 before E. coli M63 inoculation). Samples from animals not inoculated with E. coli M63 were negative for ESCR Enterobacteriaceae. Samples from animals not inoculated with E. coli ED1a were negative for E. coli ED1a.
One hundred seventy-seven isolates from the 4-M63 group were studied. Most (176/177) were E. coli and had the blaCTX-M gene. Thirty-two E. coli isolates (18.2%) were rifampin susceptible and belonged to phylogenetic groups B1 (14 isolates), F (8 isolates), C (9 isolates), and B2 (1 isolate, which was negative when tested with the ED1a-specific PCR test). One isolate obtained on day 32 formed small pink colonies on MacConkey agar and was positive for the group 1 blaCTX-M gene; it was identified as Pseudomonas oryzihabitans by MALDI-TOF MS (data not shown). A total of 141 E. coli isolates from the 4-ED-M63 group were studied. All had the blaCTX-M gene. Twenty-three of them (16.3%) were rifampin susceptible; 22 of these 23 isolates belonged to phylogenetic group A, and the remaining isolate belonged to group B1. An isolate obtained on day 6 formed white colonies on MacConkey agar, contained the group 1 blaCTX-M gene, and was identified as Acinetobacter guillouiae by MALDI-TOF MS (data not shown). The two non-E. coli blaCTX-M-positive isolates were not characterized further.
Samples collected from sows not treated with ED1a 29 days before and 1 day after E. coli M63 inoculation and from piglets in group 4-M63 just before and 32 days after E. coli M63 inoculation were negative according to the ED1a-specific qPCR. Samples collected from the treated sow were negative before administration of E. coli ED1a, and then all five samples collected from the 7th day of administration of E. coli ED1a until the day after weaning were positive, with the mean titer being 3.36 log10 copies of ED1a per 10 ng of DNA (Fig. 3B). All 12 piglets were positive 22, 17, and 10 days before inoculation of E. coli M63 and on the inoculation day, but only 7/12, 2/12, and 2/12 were still positive 4, 13, and 32 days after E. coli M63 inoculation, respectively.
Analysis of manure samples obtained from inoculated pigs.
For the first and second trials, neither ESCR E. coli isolates nor the blaCTX-M-1 gene could be detected in the manure collected from the uninoculated groups; no E. coli ED1a DNA was detected in manure samples from pigs not given E. coli ED1a.
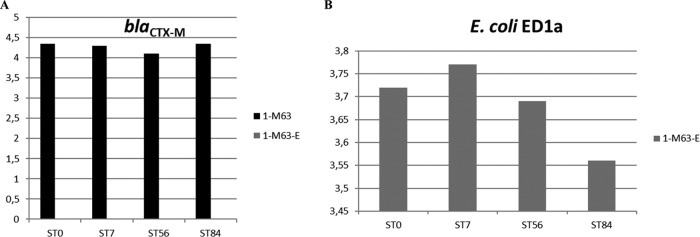
For the first trial, culture of the manure collected on day 7 on supplemented medium showed that the initial titers for manure samples from E. coli M63-inoculated pigs were high (mean, 5.5 log10 CFU per ml of manure). A slight increase was observed after 1 week of storage at 5 ± 3°C, but then the titers slowly decreased and a mean decrease of 0.6 log10 CFU/ml was noted after 84 days. Similarly, the number of copies of E. coli, blaCTX-M, and E. coli ED1a determined by qPCR remained stable during the 3-month storage period at 5 ± 3°C. For E. coli, the mean log10 numbers of copies per 10 ng of DNA for all samples on the first and last day of storage were 4.6 and 4.5, respectively, and the respective values for E. coli ED1a were 2.7 and 2.65 log10 copy numbers per 10 ng of DNA. Because of the poor sensitivity of the test for blaCTX-M-1, many samples were negative. All 13 samples obtained from the 1-M63-E group after administration of E. coli ED1a were negative for blaCTX-M-1, whereas 7 out of 13 samples obtained from the 1-M63 group were positive (P = 0.005). Figures 4A and B show the evolution of the number of copies of blaCTX-M and E. coli ED1a in the manure samples collected on day 14.
FIG 4.
Results of the first trial. A qPCR for blaCTX-M and E. coli ED1a in manure samples collected on day 14 and stored at 5 ± 3°C was performed. (A) Titer of blaCTX-M-1 (log10 number of copies in 10 ng of DNA, as indicated on the y axis); (B) titer of E. coli ED1a (log10 number of copies in 10 ng of DNA, as indicated on the y axis). E. coli M63 was inoculated on day 0, and E. coli ED1a was given from day 8 to day 13. Manure samples were collected on day 14 and analyzed after 0 (ST0), 7 (ST7), 56 (ST56), and 84 (ST84) days of storage at 5 ± 3°C. No isolates of the ESCR Enterobacteriaceae were cultured. Samples from animals not inoculated with E. coli M63 were negative for blaCTX-M-1. Samples from animals not inoculated with E. coli ED1a were negative for E. coli ED1a.
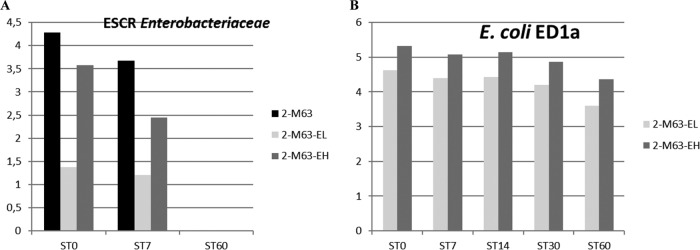
For the second experiment, manure samples were stored at room temperature (mean temperature, 20.7°C; minimum temperature, 17.4°C; maximum temperature, 25.2°C). The initial titers of ESCR Enterobacteriaceae in manure collected on days 8, 13, and 27 for the 2-M63, 2-M63-EL, and 2-M63-EH groups were between 3.2 and 4.5 log10 CFU/ml, except for the sample from the 2-M63-EL group collected on D13—at the end of the E. coli ED1a administration period—which was lower (1.4 log10 CFU/ml). No ESCR Enterobacteriaceae could be cultivated from the samples after 2 months of storage at room temperature (Fig. 5A). PCR tests indicated that 152/155 isolates of ESCR Enterobacteriaceae analyzed belonged to the species E. coli. For each group, the distribution of the phylogenetic groups observed for the manure isolates tested was similar to that observed for the fecal isolates (data not shown). After 3 months at room temperature, the mean log10 copy numbers of E. coli and E. coli ED1a in 10 ng of DNA for the treated groups decreased from 4.9 to 3.7 and from 3.9 to 1.85, respectively. Figure 5B shows the evolution of the copy numbers of E. coli ED1a in the manure samples collected on day 13. The blaCTX-M-1 gene was detected significantly more often in samples from the 2-M63 group (11/41) than in samples from the 2-M63-EL and 2-M63-EH groups (2/41 for each group) (P = 0.006).
FIG 5.
Results of the second trial. Samples were cultured for ESCR Enterobacteriaceae, and a qPCR for the detection of E. coli ED1a in manure samples collected on day 13 and stored at room temperature was performed. (A) Titer of ESCR Enterobacteriaceae (log10 number of CFU per gram, as indicated on the y axis); (B) titer of E. coli ED1a (log10 number of copies in 10 ng of DNA, as indicated on the y axis). E. coli M63 was inoculated on day 0, and E. coli ED1a was given from day 8 to day 13. Manure samples were collected on day 13 and analyzed after 0 (ST0), 7 (ST7), 14 (ST14), 30 (ST30), or 60 (ST60) days of storage at room temperature. All samples from animals not inoculated with E. coli M63 were negative for ESCR Enterobacteriaceae, and all samples from animals not inoculated with E. coli ED1a were negative for E. coli ED1a. Most samples were negative for the blaCTX-M gene.
Analysis of the in vitro-inoculated manure samples.
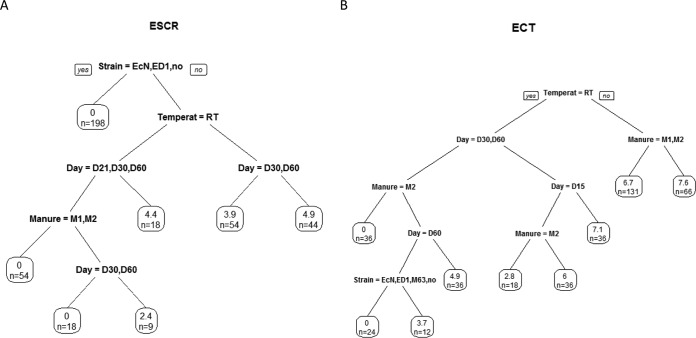
The titers of the E. coli M63, ED1a, and Nissle 1917 inocula for assays on in vitro-inoculated manure samples were, respectively, 2.65 ×107 CFU/ml, 4.7 ×107 CFU/ml, and 5.7 ×107 CFU/ml. The titers of E. coli and ESCR Enterobacteriaceae were determined and grouped by the segmentation method (Fig. 6). The results clearly showed the critical impact of temperature. The other factors included time and manure effects. The addition of a probiotic had no impact on the evolution of the ESCR isolate population or the E. coli population.
FIG 6.
Culture of ESCR Enterobacteriaceae and total E. coli (ECT) in in vitro-inoculated manure samples: segmentation of total E. coli and ESCR strains. (A) Titers of ESCR Enterobacteriaceae; (B) titers of all E. coli strains. Each leaf indicates the mean titer for the subgroup and the number of samples tested. The titers of ESCR Enterobacteriaceae were determined on days 2, 7, 21, 30, and 60. The titers of all E. coli strains were determined on days 2, 7, 15, 30, and 60. Temperat, temperature; RT, room temperature; EcN, E. coli Nissle 1917; ED, E. coli ED1a.
DISCUSSION
The presence of ESBL-producing Enterobacteriaceae in the fecal flora of food-producing animals is worrisome. The risk factors for such carriage are antimicrobial treatments (2) which may select for and increase the persistence of ESCR Enterobacteriaceae (13). A longitudinal study on three farms showed that the fecal counts of cefotaxime (CTX)-resistant coliforms decreased significantly from approximately 107 CFU/g in piglets to 103 CFU/g in finishers (14), but variations between animals existed and samples collected from pigs at the slaughterhouse contained a mean of 105 CFU/g (range, 100 to 4.7 × 105 CFU/g ESBL-producing E. coli) (15). Methods to decrease the carriage and excretion of ESCR Enterobacteriaceae are therefore needed to reduce exposure for farm workers and consumers, in addition to environmental contamination by manure. Bearing this in mind, we decided to evaluate the impact of the oral administration of E. coli strains on the excretion of ESCR Enterobacteriaceae in feces and their survival in manure through an experimental model of ESCR E. coli infection (16). The E. coli M63 strain used in this model seemed to be very well suited to the pig microbiota and persisted in all groups up to the end of the trials.
The results also showed that, as previously observed for E. coli Nissle 1917 (17) and for the first time for E. coli ED1a, both strains could readily colonize the pig gut, at least when the probiotic was given to weaned pigs. Most of these animals were still excreting the probiotic strain on the last sampling days, more than 2 weeks after the last probiotic administration. The fecal titers of E. coli ED1a after the administration period were not modified by the titers of the ED1a inocula. However, when E. coli ED1a was given to the sow and then to the suckling piglet, the strain was no longer detected in most piglets 2 weeks after the last administration. A possible explanation for this shorter persistence of the probiotic in piglets could be the modifications in their intestinal microbiota around the weaning period with the transition from milk to solid food. No adverse effects of ED1a administration were observed; indeed, in the fourth trial, the mean body weight of the newborn piglets born to the ED1a-treated sow was significantly higher than that of the other newborn piglets, but—besides a potential positive effect of the ED1a probiotic—this could have been the result of the age of the sows and their genetic characteristics. However, the growth of the treated piglets was not as good as that of the nontreated piglets, though, again, this may have been related to the piglets' origins.
The results from the first trial showed significantly lower ESCR E. coli titers and significantly fewer numbers of copies of the blaCTX-M-1 gene in the feces of E. coli ED1a-treated pigs than nontreated ones and significantly fewer blaCTX-M-1-positive samples among the fecal samples from E. coli ED1a-treated pigs than nontreated ones. These findings were mainly obtained during the E. coli ED1a administration period. However, because of the presence of a high shedder in the nontreated group, there was no clear-cut interpretation of the results, so a second trial was undertaken. In this second trial, there was no difference in the culture results for ESCR Enterobacteriaceae from fecal samples between the groups, and significant differences in the results of the molecular analysis between fecal samples from the 2-M63-EL group and those from the 2-M63 group were obtained only on day 10. On the basis of the results of ESCR E. coli cultures and the blaCTX-M-1 titers obtained from the third trial, we could not evidence a greater impact of either E. coli Nissle 1917 or E. coli ED1a administration. In the fourth trial, the ED1a probiotic strain was administered to the sow and piglets in order to optimize its colonization of the piglets' microbiota, with the idea being that E. coli ED1a would be among the first colonizers of their digestive tract because it was present in both their environment and their food. However, the persistence of E. coli ED1a after the weaning period appeared to be shorter when the probiotic strain was given before weaning; the potential protective effect of ED1a administration against ESCR Enterobacteriaceae could be evidenced on the first sampling after M63 inoculation and on the basis of the total number of ESCR Enterobacteriaceae-negative samples. However, these ESCR Enterobacteriaceae-negative samples were obtained from day 13, when probiotic strain ED1a was no longer detected in most fecal samples.
The different trials showed that the blaCTX-M-1 gene was frequently transferred from the inoculated E. coli M63 strain to other E. coli strains in the microbiota in all groups, with the exception of the group receiving the E. coli Nissle 1917 probiotic. Importantly, we could not evidence any transfer of the blaCTX-M-1 gene to the probiotic strains, which is quite reassuring, as it is important that such strongly colonizing strains remain free of this ESBL gene.
The molecular analysis of the manure samples from the first two in vivo trials detected significantly fewer blaCTX-M-1-positive samples from pigs given E. coli ED1a. Under our experimental conditions, manure samples consisted of a mixture of the droppings and urine from all the pigs in a pen collected over a whole week. It is therefore likely that the use of these manure samples amplifies small differences in the quantities of resistant isolates or resistance genes in individual fecal samples. Moreover, the repeated sampling of the manure over time may also have tended to magnify the initial differences between fecal samples in the groups. In order to determine whether the probiotics could have an impact on ESCR Enterobacteriaceae during manure storage, assays were conducted with in vitro-inoculated manure samples. The results indicated that the probiotics had no effect on the persistence of ESCR Enterobacteriaceae during storage either at room temperature or at 5 ± 3°C.
In human and veterinary medicine, a few papers have reported on experimental trials or ecological studies to control the carriage of resistant bacteria. Abecasis et al. (18), for example, have claimed that selective digestive decontamination by the use of parenteral cefotaxime and enteral polymyxin E-tobramycin in children was effective in controlling ESBL-producing aerobic Gram-negative bacilli and could avoid outbreaks of carriage or infection, yet other teams have sometimes reported contrary results, and the selective decontamination method raises many concerns in hospitals because of the use of antimicrobials of last resort, such as colistin (19, 20). Furthermore, this method is obviously unacceptable for animals. Another trial method used to decrease the carriage of ESCR bacteria involved the administration of an autogenous vaccine against E. coli bearing a blaCTX-M-14 plasmid gene to cattle, but no significant difference in the shedding of E. coli blaCTX-M-14 was found between vaccinated calves and calves receiving placebo throughout the study period (21). In 2007, an Australian team published the results of a randomized controlled trial designed to determine whether the consumption of Lactobacillus rhamnosus GG (LGG) in the form of commercially available yoghurt could improve the clearance of vancomycin-resistant enterococci (VRE). Their results showed the probiotic's efficacy in clearing VRE from fecal specimens (22). However, more recently, Lactobacillus rhamnosus Lcr35 has been shown to have little or no effect in eradicating or preventing VRE intestinal colonization in mice or human patients (23). The effect of a probiotic product containing different species (Bifidobacterium bifidum, B. lactis, Enterococcus faecium, Lactobacillus acidophilus L. paracasei, L. plantarum, L. rhamnosus, and L. salivarius) against ampicillin-resistant enterococcal intestinal colonization was studied in wards with a documented high prevalence of such bacteria, but the probiotics could not prevent colonization by the resistant enterococci (24). Other published assays concern resistant E. coli: a double-blind, placebo-controlled trial was conducted to evaluate whether a probiotic product with E. coli strain Nissle 1917 (Mutaflor) could compete with the multidrug-resistant E. coli isolates in patients in long-term-care facilities, but after 5 weeks of treatment, the authors did not observe a significant difference in the carriage of multiresistant E. coli between the probiotic and placebo groups (25). These disappointing observations concerning the ability of probiotics to competitively exclude resistant bacteria are somewhat counterbalanced by the findings described in a paper by Nuotio et al. (26). These authors hypothesized that the difference between the prevalence of ESCR E. coli in Finnish and Swedish broilers could be linked to the very frequent use in Finland of Broilact (Orion, Finland), a preparation of freeze-dried bacteria selected from the intestine of a normal adult fowl and usually given to day-old chicks for the competitive exclusion of Salmonella. In the experiment described by the authors, chicks were given the probiotic on their day of hatching and challenged on the day after. ESCR bacteria from the ceca were enumerated on the 5th day postchallenge. The study showed a substantial reduction in the amount of ESCR bacteria in birds receiving the probiotic. Moreover, Hofacre et al. (27) similarly reported that the use of another commercially available competitive exclusion product significantly reduced colonization of the small intestine, large intestine, and cecum by a multidrug-resistant E. coli strain pathogenic for poultry—E. coli O78:K80—1 and 2 weeks postchallenge. In the trials of Hofacre et al. (27), the probiotic was given to one-day-old animals 1 day before administration of the resistant challenge strain, which was probably a more favorable situation for colonization by the probiotic and resulted in a greater impact in reducing the excretion of the resistant strains administered afterwards.
Our first protocol was based on the inoculation of E. coli M63 followed by administration of the probiotic. The E. coli M63 strain was such an efficient gut colonizer and plasmid donor that the administration of probiotics 1 week after the E. coli M63 inoculation was probably too challenging for the probiotic strains, which could not compete with the ESCR M63 strain and the in vivo transconjugants. In light of the interesting results cited previously (26, 27), we then tried another protocol wherein the probiotic was administered prior to the inoculation of E. coli M63, because such a situation could represent an option in field situations where young animals free of ESCR Enterobacteriaceae are moved into a contaminated environment. Our results showed a moderate impact of E. coli ED1a administration, which was limited to the very first days after the probiotic administration period.
Conclusion.
Four different trials were conducted to evaluate the capacity of probiotics, E. coli ED1a or E. coli Nissle 1917, to reduce the fecal shedding of ESCR Enterobacteriaceae from inoculated pigs. The ESCR strain E. coli M63 was inoculated either before or after probiotic administration. This resistant strain had an excellent gut-colonizing capacity, and its blaCTX-M-1 gene was readily transferred to different E. coli isolates in the pig gut. Under our experimental conditions, the protective effect of E. coli ED1a was a small reduction in ESCR Enterobacteriaceae shedding that was limited over time. Further assays with modified protocols are necessary to evaluate whether continuous administration of the probiotic prior to and after ESCR E. coli inoculation could result in more promising results.
MATERIALS AND METHODS
Strains.
E. coli ED1a is a commensal human strain belonging to phylogroup B2, subgroup VIII (sequence type [ST] c452), with an O81 serotype (28). This clone has been reported to be human specific. It is a very good gut colonizer and is very rarely isolated under pathological conditions. It is avirulent in a mouse model of septicemia (11). E. coli Nissle 1917 is also a human commensal strain of phylogroup B2, but it belongs to subgroup II (STc73) and is O6:K5:H1 (12). This strain forms the basis of the probiotic preparation Mutaflor, which is used to treat various intestinal disorders and which is known to be a successful colonizer of the human gut (12). Of note, this strain is highly virulent in the model of septicemia cited above, as it kills 100% of inoculated mice (unpublished data). The E. coli ED1a and E. coli Nissle 1917 inocula used for the first and third trials and for the 2-M63-EL group (see below) were prepared from bacteria cultured at 37 ± 1°C on Mueller-Hinton (MH) agar plates. A suspension was prepared for the 2-M63-EH group (see below) by centrifuging cultures in MH broth.
As previously described (16), the ESCR E. coli strain to be inoculated in the pigs was constructed in our laboratory in order to increase the likelihood of colonization. Briefly, a pan-susceptible E. coli isolate from the feces of piglets from the specific-pathogen-free (SPF) experimental swine herd belonging to ANSES, Ploufragan, France, was first isolated on MacConkey medium. This isolate, named UB12/059-3, was then made resistant to rifampin and used as a receptor for in vitro conjugation with an ESCR E. coli isolate obtained from a healthy pig at a slaughterhouse and previously shown to contain a blaCTX-M-1 gene. The resulting transconjugant, E. coli M63, belongs to the B1 phylogenetic group and contains resistance genes blaCTX-M-1, blaCMY-59, aadA5, sul2, and dfrA17 (16). It is resistant to cefotaxime, rifampin, streptomycin, and trimethoprim-sulfamethoxazole. The inocula consisted of suspensions of the E. coli M63 cultures obtained on cefotaxime-supplemented MH agar plates.
Animals and experimental design.
The experiments were performed in accordance with French animal welfare regulations, and the protocol was approved by the ANSES/ENVA/UPEC ethical committee (ComEth authorizations 12-032 and 15-050 [no. APAFIS: 2015061813246553]). The different protocols are summarized in Table 1. The four experiments were conducted in the ANSES, Ploufragan, France, animal facilities. Strict biosecurity measures were implemented in order to avoid contamination of the pigs, including the use of an air filtration system and airlocks for each unit, unit-specific clothes for the researchers, and compulsory showering by the researchers after they visited the pigs. The trials were conducted with SPF Large White piglets.
For the first two trials, the 7-week-old piglets were randomized before the experiment. The animals did not receive any antibiotic treatment prior to the trial and were given the same nonsupplemented feed. Each room contained eight piglets placed in two pens of four animals each. The first trial included three different groups, with each group being housed in a separate room, as follows: an uninoculated control group (group 1-C), an E. coli M63-inoculated group (group 1-M63), and an E. coli M63-inoculated group to which ED1a was administered (group 1-M63-E). The second trial included four groups: an uninoculated control group (group 2-C), an E. coli M63-inoculated group (group 2-M63), an E. coli M63-inoculated group to which a low ED1a dose was administered (group 2-M63-EL), and an E. coli M63-inoculated group to which a high ED1a dose was administered (group 2-M63-EH).
For both trials, E. coli M63 was inoculated on the first day (day 0). Each piglet was orally given a 10-ml suspension prepared from E. coli M63 cultivated on MH agar containing cefotaxime (2 mg/liter). The titer of this suspension was determined by spreading 10-fold dilutions on MH medium plates. Piglets in the 1-C and 2-C groups were similarly inoculated with a sterile medium. On day 8 to day 13, piglets from groups 1-M63-E, 2-M63-EL, and 2-M63-EH were given a 10-ml dose of a titrated suspension of E. coli ED1a daily.
The weights of the individual animals were recorded once a week. During the week, daily clinical examinations consisted of evaluations for general clinical signs and recording of rectal temperatures. Individual fecal samples were collected from all animals on day 0 before inoculation and on days 1, 3, 7, 10, 13, 17, 22, and 28 (the first trial) and days 3, 8, 10, 13, 16, 20, 23, 27, and 31 (the second trial). The fecal samples were stored at −70°C until analysis. The pigs were euthanized at the end of the experiment on days 35 to 37 postinoculation, and the animals were searched for lesions.
In the third trial, the impact of two E. coli strains—either E. coli ED1a or E. coli Nissle 1917—on the excretion of E. coli M63 was compared. Three groups of 8-week-old SPF pigs were housed five to a pen in three separate animal rooms; one group (group 3-C) was not inoculated, while the other two groups were orally inoculated on day 0 with 10 ml of a suspension prepared from E. coli M63. From day 8 to day 12, the pigs were orally given either E. coli ED1a (group 3-M63-ED) or E. coli Nissle 1917 (group 3-M63-EcN). Fecal samples were collected on days 2, 3, 7, 9, 13, 16, and 20.
In the fourth trial, a group of three pregnant SPF sows was housed in one animal room. After farrowing, the piglets were left with their mother for 4 weeks before weaning. Then, 12 piglets (group 4-M63, comprising six males and six females) were moved to another animal room. Another group of two pregnant SPF sows was housed in a different animal room. From 12 days before the expected day of farrowing and up to the weaning day, the sows were daily given a 10-ml suspension of E. coli ED1a, which was deposited in a small quantity of each animal's food before the full meal was offered, to ensure that the sows ate all the E. coli ED1a suspension. Unfortunately, one of the treated sows died during farrowing and no piglets could be obtained from this sow. During the suckling period, 12 piglets from the surviving treated sow were daily given the E. coli ED1a suspension by oral gavage from the first day of life up to the weaning day at increasing doses (1 ml the 1st week, 2 ml the 2nd week, 3 ml the 3rd week, and 4 ml the 4th week). After weaning, at 4 weeks of age, 12 of these piglets (group 4-ED-M63, comprising six males and six females) were moved to another room. On the day after weaning (day 0), all 24 piglets from either the treated or the nontreated sows received a 5-ml inoculum of a suspension prepared from E. coli M63. Clinical signs and rectal temperatures were recorded, and the piglets were weighed once a week. Fecal samples were regularly collected from the sows before farrowing and up to the weaning of their piglets and from the piglets up to the age of 2 months (Table 1). Two uninoculated piglets were kept as controls in another room. All the piglets were sacrificed at 2 months of age, and the lesions were recorded.
Study of in vivo- and in vitro-inoculated manure samples.
During the first and second experiments, large plastic boxes were placed under the pens to collect the mixture of feces and urine emitted by the animals over several days. These mixtures were collected on day 7 (the first trial) and on days 8, 13, and 27 (the second trial). The feces and urine were vigorously mixed, and the resulting liquid manure was then stored in the laboratory in glass bottles either at 5 ± 3°C (the first trial), or at room temperature (the second trial). The room temperature was recorded weekly. Subsamples of manure were taken from these bottles after various periods of time ranging from 1 week to 3 months, and the survival of ESCR E. coli in manure was studied.
Moreover, three different samples of manure (samples M1, M2, and M3) were obtained from SPF pigs. Each manure sample was divided into 10 parts and placed in 10 flasks, with 5 flasks being stored at room temperature and 5 being stored at 5 ± 3°C. Each flask contained 100 ml of manure and was either uninoculated or inoculated with 1 ml of a 0.5 McFarland suspension of E. coli M63 combined with E. coli Nissle 1917 and ED1a, M63 with ED1a, or M63 with Nissle 1917. The titers of the ESCR Enterobacteriaceae in manure samples were determined in the same way after 2, 7, 21, 30, and 60 days, and the titers of E. coli were determined after 2, 7, 15, 30, and 60 days.
Bacteriological analysis of fecal and manure samples.
The titers of ESCR Enterobacteriaceae in the individual fecal samples were determined by spreading 100 μl of 10-fold dilutions on MacConkey agar plates containing 2 mg/liter cefotaxime in triplicate. After incubation at 37°C, the ESCR colonies on supplemented MacConkey agar plates were enumerated, and the titer was calculated for each pig per day. The detection limit was 100 CFU/g of feces. As far as possible, five (trials 1 and 2) or two (trial 4) isolates per pig per day were further analyzed. Their resistance to cefotaxime was confirmed by restreaking the isolates on MacConkey agar plates containing 2 mg/liter cefotaxime, and their susceptibility to rifampin was studied by inoculation on MacConkey agar plates containing 250 mg/liter rifampin. They were identified with an E. coli-specific PCR (29, 30), and the presence of the blaCTX-M-1 gene was sought by PCR (31). According to their origin, the isolates were also tested by PCRs specific for E. coli ED1a or E. coli Nissle 1917, as described below. After digestion with SmaI, the pulsed-field gel electrophoresis (PFGE) profiles of a few isolates were compared with the PFGE profiles of the inoculated ESCR strain and the administered probiotics (32).
For the first trial, the titers of ESCR Enterobacteriaceae in the manure samples obtained on day 7 were determined either immediately or after 7, 56, or 84 days of storage at 5 ± 3°C. For the second trial, the titers in the manure samples collected on day 8, 13, or 27 were determined either immediately or after 1 week or 2 months of storage at room temperature. The goal was to evaluate the persistence of culturable ESCR Enterobacteriaceae in manure.
Molecular analysis.
DNA extracts were prepared from 0.2 g of each fecal or manure sample using a protocol adapted from that of Yu and Morrison (33), including bead beating with high concentrations of sodium dodecyl sulfate (SDS), salt, and EDTA, followed by analysis with a Qiagen DNA stool kit (Qiagen). DNA solutions were stored at −20°C until analysis. Each DNA extract was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific) and then adjusted to a concentration of 10 ng/μl.
The numbers of E. coli, E. coli ED1a, E. coli Nissle 1917, and group 1 blaCTX-M copies in feces and manure were quantified according to the conditions described in Table 3 (30, 34). The specific primers and the probe for E. coli ED1a were designed with Beacon Designer software (Sigma-Aldrich) to amplify a 118-bp fragment of the E. coli ED1a ICE gene (NCBI accession no. CU928162). PCRs were performed in a final reaction mixture volume of 10 μl containing 1 μl of diluted DNA samples, primers and probes, 1× IQ Supermix (Bio-Rad), and 0.25× an exogenous internal positive control (Applied Biosystems, Saint-Aubin, France) to check for PCR inhibitors. For the blaCTX-M gene, samples containing 10 ng of DNA were first tested. In the event of negative results, nondiluted samples were tested. For each sample, results were expressed as the log10 number of copies of DNA per 10 ng of DNA.
TABLE 3.
Primers, probes, and conditions of PCR tests
| Target | Primer or probe | Sequencea (5′ → 3′) | Primer or probe concn (μM) | Cycle | Reference or source |
|---|---|---|---|---|---|
| E. coli | EcF | CATGCCGCGTGTATGAAGAA | 0.3 | 95°C for 10 min, followed by 40 cycles at 95°C for 30 s and 60°C for 60 s | 30 |
| EcR | 5′CGGGTAACGTCAATGAGCAAA | 0.3 | |||
| EcP | FAM-TCCTCCCCGCTGAA-BHQ1 | 0.1 | |||
| E. coli ED1a | ED1aF | GAACCGAATGGGATTTACAGGAAG | 0.1 | 95°C for 5 min, followed by 40 cycles at 95°C for 30 s and 60°C for 60 s | This study |
| ED1aR | AAGATAGCTGCTGCTAATGCTTCAAG | 0.1 | |||
| ED1aP | FAM-TACCACTGCCGTTGGGACCAGCAC-BHQ1 | 0.2 | |||
| E. coli Nissle 1917 | PM2-5011F | GCGCCAAACACTGGAATATGT | 0.3 | 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 60 s | C. Miossec (Da Volterra, Paris, France), personal communication |
| PM2-5081R | CATCCTCGGTCAGCAAAACC | 0.3 | |||
| Pmut2-5036S | FAM-CAAAGCGGACGTTTGCCGTTC-BHQ1 | 0.2 | |||
| blaCTX-M-1 | CTXGp1F | GGAATCTGACGCTGGGTAAA | 0.2 | 95°C for 5 min, followed by 25 cycles at 95°C for 20 s, 56°C for 35 s, and 72°C for 35 s | 34 |
| CTXGp1R | GGTTGAGGCTGGGTGAAGTA | 0.2 | |||
| CTXGp1P | FAM-ACTATGGCACCACCAACGAT-BHQ1 | 0.05 |
FAM, 6-carboxyfluorescein; BHQ1, black hole quencher 1.
Statistical analysis.
Differences between the weight gains of animals from the different rooms or groups were analyzed by a Kruskal-Wallis test. The individual titers of ESCR Enterobacteriaceae and gene copy numbers were log10 transformed. The first step entailed a search for significant differences between groups using a Kruskal-Wallis test. The second one entailed investigation of significant differences between each pair of groups through adjusted Mann-Whitney-Wilcoxon tests. The distribution of the number of positive tests was compared by a χ2 test with a Fisher correction when needed (small theoretical values in the contingency table). The results were considered significant when P was <0.05.
For in vitro-inoculated manure samples, in order to understand the influence of the four experimental design parameters—temperature, day, manure, and inoculated strain—on the E. coli titers on the one hand and on the ESCR Enterobacteriaceae titers on the other, decision trees were processed. As parametric hypotheses are clearly not verified, decision trees are used as a nonparametric regression (35). The goal of decision trees is to build a model that explains and predicts the value of a target variable (i.e., E. coli or ESCR strain titers) by creating simple decision rules inferred from data features (i.e., temperature, day, manure, and strain). The first stage of the procedure consists of building the full tree by sequentially finding the parameters that best split the data into two subgroups differentiating the target variable means either until the subgroups reach a minimum size or until no further improvement can be made. The criterion to be minimized is the model's mean squared error. The second stage consists of using cross validation to trim the full tree in order to avoid overfitting. The final model is that subtree with the lowest estimate of risk. The models are fitted using the rpart function of R software (http://cran.r-project.org/web/packages/rpart/index.html).
ACKNOWLEDGMENTS
We are grateful to C. Miossec (Da Volterra, Paris) for sharing the PCR protocol for E. coli Nissle 1917 and to N. Morin, A. Guérin, and Q. Allard (ANSES) for their technical assistance.
This work was supported by grant 2012-0388 from France-Agrimer.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.ANSES. 2013. Réseau d'épidémiosurveillance de l'antibiorésistance des bactéries pathogènes animales: Bilan 2012. ANSES, Ploufragan, France: http://www.resapath.anses.fr/resapath_uploadfiles/files/Documents/2012%20RESAPATH%20Rapport%20Annuel%20Fr.pdf. [Google Scholar]
- 2.Panel on Biological Hazards. 2011. Scientific opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J 9:1. doi: 10.2903/j.efsa.2011.2322. [DOI] [Google Scholar]
- 3.Hu YY, Cai JC, Zhou HW, Chi D, Zhang XF, Chen WL, Zhang R, Chen GX. 2013. Molecular typing of CTX-M-producing Escherichia coli isolates from environmental water, swine feces, specimens from healthy humans, and human patients. Appl Environ Microbiol 79:5988–16. doi: 10.1128/AEM.01740-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos S, Silva N, Dias D, Sousa M, Capelo-Martinez JL, Brito F, Canica M, Igrejas G, Poeta P. 2013. Clonal diversity of ESBL-producing Escherichia coli in pigs at slaughter level in Portugal. Foodborne Pathog Dis 10:74–79. doi: 10.1089/fpd.2012.1173. [DOI] [PubMed] [Google Scholar]
- 5.Moodley A, Guardabassi L. 2009. Transmission of IncN plasmids carrying blaCTX-M-1 between commensal Escherichia coli in pigs and farm workers. Antimicrob Agents Chemother 53:1709–1711. doi: 10.1128/AAC.01014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues C, Machado E, Peixe L, Novais A. 2013. IncI1/ST3 and IncN/ST1 plasmids drive the spread of blaTEM-52 and blaCTX-M-1/-32 in diverse Escherichia coli clones from different piggeries. J Antimicrob Chemother 68:2245–2248. doi: 10.1093/jac/dkt187. [DOI] [PubMed] [Google Scholar]
- 7.Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. 2012. Extended-spectrum beta-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect 18:646–655. doi: 10.1111/j.1469-0691.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 8.Smet A, Rasschaert G, Martel A, Persoons D, Dewulf J, Butaye P, Catry B, Haesebrouck F, Herman L, Heyndrickx M. 2011. In situ ESBL conjugation from avian to human Escherichia coli during cefotaxime administration. J Appl Microbiol 110:541–549. doi: 10.1111/j.1365-2672.2010.04907.x. [DOI] [PubMed] [Google Scholar]
- 9.Faure S, Perrin-Guyomard A, Delmas JM, Chatre P, Laurentie M. 2010. Transfer of plasmid-mediated CTX-M-9 from Salmonella enterica serotype Virchow to Enterobacteriaceae in human flora-associated rats treated with cefixime. Antimicrob Agents Chemother 54:164–169. doi: 10.1128/AAC.00310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depoorter P, Persoons D, Uyttendaele M, Butaye P, De Zutter L, Dierick K, Herman L, Imberechts H, Van Huffel X, Dewulf J. 2012. Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium Int J Food Microbiol 159:30–38. doi: 10.1016/j.ijfoodmicro.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Clermont O, Lescat M, O'Brien CL, Gordon DM, Tenaillon O, Denamur E. 2008. Evidence for a human-specific Escherichia coli clone. Environ Microbiol 10:1000–1006. doi: 10.1111/j.1462-2920.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 12.Hancock V, Dahl M, Klemm P. 2010. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J Med Microbiol 59:392–399. doi: 10.1099/jmm.0.008672-0. [DOI] [PubMed] [Google Scholar]
- 13.Cavaco LM, Abatih E, Aarestrup FM, Guardabassi L. 2008. Selection and persistence of CTX-M-producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrob Agents Chemother 52:3612–3616. doi: 10.1128/AAC.00354-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen KH, Damborg P, Andreasen M, Nielsen SS, Guardabassi L. 2013. Carriage and fecal counts of cefotaxime M-producing Escherichia coli in pigs: a longitudinal study. Appl Environ Microbiol 79:794–798. doi: 10.1128/AEM.02399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton RA, Randall LP, Snary EL, Cockrem H, Lotz S, Wearing H, Duncan D, Rabie A, McLaren I, Watson E, La Ragione RM, Coldham NG. 2011. Fecal carriage and shedding density of CTX-M extended-spectrum {beta}-lactamase-producing Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl Environ Microbiol 77:3715–3719. doi: 10.1128/AEM.02831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleury MA, Mourand G, Jouy E, Touzain F, Le Devendec L, De Boisseson C, Eono F, Cariolet R, Guérin A, Le Goff O, Blanquet-Diot S, Alric M, Kempf I. 2015. Impact of ceftiofur injection on gut microbiota and Escherichia coli resistance in pigs. Antimicrob Agents Chemother 59:5171–5180. doi: 10.1128/AAC.00177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barth S, Duncker S, Hempe J, Breves G, Baljer G, Bauerfeind R. 2009. Escherichia coli Nissle 1917 for probiotic use in piglets: evidence for intestinal colonization. J Appl Microbiol 107:1697–1710. doi: 10.1111/j.1365-2672.2009.04361.x. [DOI] [PubMed] [Google Scholar]
- 18.Abecasis F, Sarginson RE, Kerr S, Taylor N, van Saene HK. 2011. Is selective digestive decontamination useful in controlling aerobic gram-negative bacilli producing extended spectrum beta-lactamases? Microb Drug Resist 17:17–23. doi: 10.1089/mdr.2010.0060. [DOI] [PubMed] [Google Scholar]
- 19.Oostdijk EA, de Smet AM, Blok HE, Thieme Groen ES, van Asselt GJ, Benus RF, Bernards SA, Frenay IH, Jansz AR, de Jongh BM, Kaan JA, Leverstein-van Hall MA, Mascini EM, Pauw W, Sturm PD, Thijsen SF, Kluytmans JA, Bonten MJ. 2010. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med 181:452–457. doi: 10.1164/rccm.200908-1210OC. [DOI] [PubMed] [Google Scholar]
- 20.Petros AJ, Silvestri L, Taylor N, Abecasis F, Damjanovic V, de la Cal MA, Zandstra D, van Saene HK. 2014. Comment on: selective decontamination of the oropharynx and the digestive tract, and antimicrobial resistance: a 4 year ecological study in 38 intensive care units in the Netherlands. J Antimicrob Chemother 69:860. doi: 10.1093/jac/dkt485. [DOI] [PubMed] [Google Scholar]
- 21.Reeves HE, Lotz SB, Kennedy E, Randall LP, Coldham NG, La Ragione RM. 2013. Evaluation of an autogenous vaccine in cattle against Escherichia coli bearing the CTX-M-14 plasmid. Res Vet Sci 94:419–424. doi: 10.1016/j.rvsc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Manley KJ, Fraenkel MB, Mayall BC, Power DA. 2007. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. Med J Aust 186:454–457. [DOI] [PubMed] [Google Scholar]
- 23.Vidal M, Forestier C, Charbonnel N, Henard S, Rabaud C, Lesens O. 2010. Probiotics and intestinal colonization by vancomycin-resistant enterococci in mice and humans. J Clin Microbiol 48:2595–2598. doi: 10.1128/JCM.00473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Regt MJ, Willems RJ, Hene RJ, Siersema PD, Verhaar HJ, Hopmans TE, Bonten MJ. 2010. Effects of probiotics on acquisition and spread of multiresistant enterococci. Antimicrob Agents Chemother 54:2801–2805. doi: 10.1128/AAC.01765-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannock GW, Tiong IS, Priest P, Munro K, Taylor C, Richardson A, Schultz M. 2011. Testing probiotic strain Escherichia coli Nissle 1917 (Mutaflor) for its ability to reduce carriage of multidrug-resistant E. coli by elderly residents in long-term care facilities. J Med Microbiol 60:366–370. doi: 10.1099/jmm.0.025874-0. [DOI] [PubMed] [Google Scholar]
- 26.Nuotio L, Schneitz C, Nilsson O. 2013. Effect of competitive exclusion in reducing the occurrence of Escherichia coli producing extended-spectrum beta-lactamases in the ceca of broiler chicks. Poult Sci 92:250–254. doi: 10.3382/ps.2012-02575. [DOI] [PubMed] [Google Scholar]
- 27.Hofacre CL, Johnson AC, Kelly BJ, Froyman R. 2002. Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens. Avian Dis 46:198–202. doi: 10.1637/0005-2086(2002)046[0198:EOACCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Clermont O, Gordon D, Denamur E. 2015. Guide to the various phylogenetic classification schemes for Escherichia coli and the correspondence among schemes. Microbiology 161:980–988. doi: 10.1099/mic.0.000063. [DOI] [PubMed] [Google Scholar]
- 29.Bej AK, DiCesare JL, Haff L, Atlas RM. 1991. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol 57:1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furet JP, Firmesse O, Gourmelon M, Bridonneau C, Tap J, Mondot S, Dore J, Corthier G. 2009. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol Ecol 68:351–362. doi: 10.1111/j.1574-6941.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 31.Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J Antimicrob Chemother 57:154–155. doi: 10.1093/jac/dki412. [DOI] [PubMed] [Google Scholar]
- 32.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 33.Yu Z, Morrison M. 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812. [DOI] [PubMed] [Google Scholar]
- 34.Ellem J, Partridge SR, Iredell JR. 2011. Efficient direct extended-spectrum β-lactamase detection by multiplex real-time PCR: accurate assignment of phenotype by use of a limited set of genetic markers. J Clin Microbiol 49:3074–3077. doi: 10.1128/JCM.02647-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breiman L, Friedman JH, Olshen RA, Stone CJ. 1984. Classification and regression trees (Wadsworth statistics/probability). Chapman & Hall/CRC, Boca Raton, FL. [Google Scholar]