ABSTRACT
We evaluated the MIC Strip Isavuconazole test against EUCAST E.Def 9.3 by using 40 wild-type and 39 CYP51A mutant Aspergillus fumigatus strains. The strip full inhibition endpoint (FIE) and 80% growth inhibition endpoint were determined by two independent readers, reader 1 (R1) and R2. The essential (within ±0, ±1, and ±2 twofold dilutions) and categorical agreements were best with the FIE (for R1/R2, 42%/41%, 75%/73%, and 90%/89% for essential agreement, and 91.1%/92.4% categorical agreement, with 6.3/8.9% very major errors and 0/1.3% major errors, respectively). The MIC Strip Isavuconazole test with the FIE appears to be useful.
KEYWORDS: antifungal susceptibility testing, MIC, isavuconazole, gradient strip, EUCAST, Aspergillus fumigatus, Cyp51A mutants, wild type
TEXT
Antifungal susceptibility testing of Aspergillus fumigatus has become increasingly important with the emergence of azole resistance (1–6). EUCAST has set clinical breakpoints for isavuconazole and Aspergillus (7). For A. fumigatus, the clinical breakpoint is 1 mg/liter, one step lower than the epidemiological cutoff value (ECOFF) (2 mg/liter) because the pharmacokinetic/pharmacodynamic breakpoint is 1 mg/liter and the MIC ranges for wild-type and resistant mutants overlap. Hence an MIC of 2 mg/liter may represent wild-type isolates as well as isolates with clinically relevant resistance mechanisms (1–3, 5, 8–15). In clinical practice, the adoption of a restrictive clinical breakpoint for interpretation of MICs generated by commercial tests may create a higher risk of misclassification unless the susceptibility test is very well standardized against the reference method and associated with low reader-to-reader and interlaboratory variations. An isavuconazole gradient strip (Etest; AB Biodisk, Solna, Sweden) was previously evaluated but is no longer available (16, 17). Thus, we evaluated the only commercially available isavuconazole susceptibility test, the MIC Strip Isavuconazole test (Liofilchem, Roseto degli Abruzzi, TE, Italy).
Forty wild-type and 39 CYP51A mutant A. fumigatus isolates with hot-spot alterations involving G54 (n = 10), M220 (n = 10), TR34/L98H (n = 9), and TR46/Y121F T289A (n = 10) were included. For the strip test (Liofilchem, Roseto degli Abruzzi, TE, Italy) a McFarland 0.5 conidial suspension and RPMI 1640 2% glucose agar (SSI Diagnostica, Hillerød, Denmark) were used. Strip MICs were read by two independent technicians (reader 1 [R1] and R2) blind to the CYP51A genotype at 24 and 48 h of incubation, with an 80% inhibition endpoint (80% IE) and a full inhibition endpoint (FIE). EUCAST testing was performed as previously recommended (7, 18). Four control strains were included (see Table S1 in the supplemental material) (7). The percent essential agreement between the tests was calculated. Isolates for which the MICs were above scale by both methods (EUCAST, >16 mg/liter; strip test, >32 mg/liter) were considered in agreement within ±0 twofold dilution. The categorical agreement between the methods was calculated as the percentage of isolates classified equally by both methods. Very major errors (VMEs) were defined as isolate categorization as resistant (R) by EUCAST but susceptible (S) by the strip test, and major errors (MEs) were defined as isolate categorization as S by the EUCAST method but R by the strip test.
Most isavuconazole strip MICs were above the recommended ranges for the two control Candida strains (Table S1). In contrast, the strip MICs for A. fumigatus ATCC 204305 and A. flavus CM1813 were within ±1 twofold dilution of the EUCAST MICs, suggesting better agreement for the Aspergillus strains and best when using the FIE for Aspergillus.
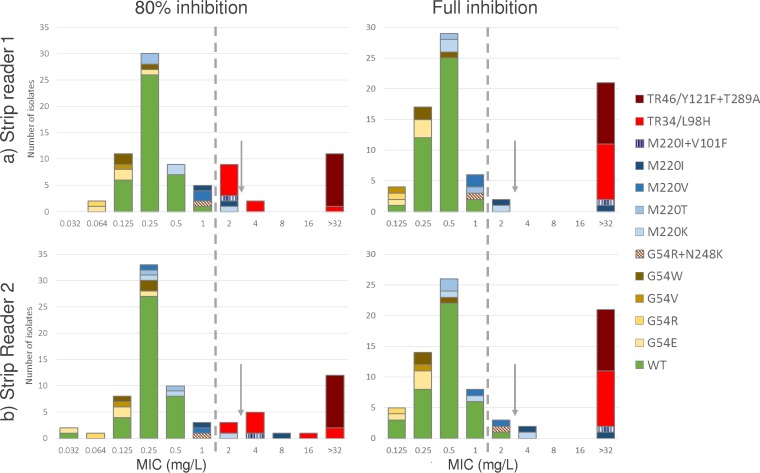
Nine isolates (11.4%) failed to grow sufficiently well to allow strip MIC reading on day 1, when, in general, zones were fuzzy and difficult to read. Day 2 MICs were lower with the 80% IE than with the FIE (Fig. 1). This was particularly evident for isolates harboring TR34/L98H alterations, for which the modal 80% IE MICs were 2 and 4 mg/liter, respectively but >32 mg/liter for both readers with the FIE. The essential agreement between the strip MICs from the two readers was highest, 97% at ±1 twofold dilution and 100% at ±2 twofold dilutions, when using the FIE (Table 1).
FIG 1.
Isavuconazole strip MICs for wild-type and CYP51A mutant A. fumigatus isolates determined at 80% inhibition (left side) and full inhibition endpoints (right side) and by two independent readers, R1 (top) and R2 (bottom).
TABLE 1.
Essential agreement between R1 and R2 of MIC Strip Isavuconazole test and between strip and EUCAST MICs
| CYP51A profile | n | Strip MIC agreement (%)a between R1 and R2 |
Agreement (%)a between Strip MIC and EUCAST MIC |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 vs R2, FIE |
R1 vs R2, 80% IE |
R1 FIE vs EUCAST |
R2 FIE vs EUCAST |
R1 80% IE vs EUCAST |
R2 80% IE vs EUCAST |
||||||||||||||
| ±0b | ±1 | ±2 | ±0 | ±1 | ±2 | ±0 | ±1 | ±2 | ±0 | ±1 | ±2 | ±0 | ±1 | ±2 | ±0 | ±1 | ±2 | ||
| Wild type | 40 | 55 | 95 | 100 | 83 | 98 | 98 | 38 | 80 | 98 | 28 | 78 | 95 | 18 | 63 | 88 | 15 | 63 | 88 |
| G54 alterations | |||||||||||||||||||
| G54E | 4 | 100 | 100 | 100 | 75 | 100 | 100 | 75 | 100 | 100 | 75 | 100 | 100 | 0 | 100 | 100 | 0 | 75 | 100 |
| G54R | 1 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| G54V | 1 | 0 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 0 | 100 | 100 |
| G54W | 3 | 100 | 100 | 100 | 67 | 100 | 100 | 33 | 100 | 100 | 33 | 100 | 100 | 67 | 100 | 100 | 100 | 100 | 100 |
| G54R N248K | 1 | 0 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 0 | 100 | 100 |
| M220 alterations | |||||||||||||||||||
| M220K | 3 | 33 | 100 | 100 | 67 | 100 | 100 | 0 | 100 | 100 | 67 | 100 | 100 | 0 | 100 | 100 | 0 | 67 | 100 |
| M220T | 2 | 50 | 100 | 100 | 50 | 100 | 100 | 100 | 100 | 100 | 50 | 100 | 100 | 0 | 50 | 100 | 50 | 50 | 100 |
| M220V | 2 | 50 | 100 | 100 | 50 | 50 | 100 | 0 | 50 | 100 | 50 | 50 | 100 | 0 | 50 | 100 | 0 | 50 | 50 |
| M220I | 2 | 50 | 100 | 100 | 50 | 50 | 100 | 50 | 50 | 50 | 0 | 50 | 50 | 0 | 50 | 100 | 50 | 100 | 100 |
| M220I V101F | 1 | 100 | 100 | 100 | 0 | 100 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 100 |
| Mutants with TRc | |||||||||||||||||||
| TR34/L98H | 9 | 100 | 100 | 100 | 33 | 78 | 89 | 11 | 11 | 33 | 11 | 11 | 33 | 11 | 22 | 100 | 33 | 67 | 100 |
| TR46/Y121F T289A | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| All isolates | 79 | 68 | 97 | 100 | 75 | 94 | 97 | 42 | 75 | 90 | 41 | 73 | 89 | 25 | 66 | 91 | 30 | 70 | 91 |
Percent essential agreement within ±0, ±1, and ±2 twofold dilutions, respectively. MICs were read after 2 days of incubation. Strip MICs were read by using the FIE or 80% IE endpoint.
Number of twofold dilutions.
TR, tandem repeat in the CYP51A promoter region.
Isavuconazole MICs for isolates with wild-type CYP51A or single alterations at the G54 codon were all below the EUCAST ECOFF for the strip test with the FIE, as well as for EUCAST (Table 2). Likewise, the MICs for isolates harboring M220I alterations or TR34/L98H or TR46/Y121F T289A were all above the clinical breakpoint for both methods when the FIE was used for the strip test. However, the MICs for TR34/L98H isolates were higher when determined by the strip test (MIC range, >32 mg/liter) than when determined by EUCAST (MIC50 of 8 mg/liter; range, 4 to >16 mg/liter) (Table 2). The overall essential agreement between strip MICs and EUCAST MICs within ±0, ±1, and ±2 twofold dilutions was best when using the FIE (R1/R2: 42/41, 75/73, and 90/89%) than when using the 80% IE (R1/R2: 25/30, 66/70, and 91/91%). At least 95% essential agreement between the strip test and EUCAST within ±2 twofold dilutions was seen for all CYP51A genotypes except those harboring the TR34/L98H mechanism or the M220I alteration. Similarly, the categorical agreement was better for the FIE reading of the strip test (91.1 to 92.4% with 6.3 to 8.9% VMEs and 0 to 1.3% MEs) than for the 80% IE (89.9% with 10.1% VMEs and 0% MEs for both readers). VMEs included four isolates with the wild-type CYP51A genotype and one to four isolates harboring M220V, M220I, or G54R N248K alterations, respectively.
TABLE 2.
Isavuconazole susceptibility of wild-type and CYP51A mutant A. fumigatus isolates determined by strip testa and EUCAST E.Def 9.3
| CYP51A profilea | No. of isolates | EUCAST E.Def 9.3 |
Gradient strip R1 |
Gradient strip R2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50b | MIC range | % >ECOFFc | % R | MIC50b | MIC range | % >ECOFF | % R | MIC50b | MIC range | % >ECOFF | % R | ||
| Wild type | 40 | 0.5 | 0.25–2 | 0 | 10 | 0.5 | 0.125–1 | 0 | 0 | 0.5 | 0.125–2 | 0 | 3 |
| G54 alterations | |||||||||||||
| G54E | 4 | 0.125–0.25 | 0 | 0 | 0.125–0.25 | 0 | 0 | 0.125–0.25 | 0 | 0 | |||
| G54R | 1 | 0.5 | 0 | 0 | 0.125 | 0 | 0 | 0.125 | 0 | 0 | |||
| G54V | 1 | 0.25 | 0 | 0 | 0.125 | 0 | 0 | 0.25 | 0 | 0 | |||
| G54W | 3 | 0.125–0.25 | 0 | 0 | 0.25–0.5 | 0 | 0 | 0.25–0.5 | 0 | 0 | |||
| G54R N248K | 1 | 2 | 0 | 100 | 1 | 0 | 0 | 2 | 0 | 100 | |||
| M220alterations | |||||||||||||
| M220K | 3 | 1–4 | 33 | 33 | 0.5–2 | 0 | 33 | 0.5–1 | 33 | 33 | |||
| M220T | 2 | 0.5–1 | 0 | 0 | 0.5–1 | 0 | 0 | 0.5 | 0 | 0 | |||
| M220V | 2 | 2–4 | 50 | 100 | 1 | 0 | 0 | 1–2 | 0 | 50 | |||
| M220I | 2 | 2–8 | 50 | 100 | 2 to >32 | 50 | 100 | 4 to >32 | 100 | 100 | |||
| M220I V101F | 1 | 16 | 100 | 100 | >32 | 100 | 100 | >32 | 100 | 100 | |||
| Mutants with TRd | |||||||||||||
| TR34/L98H | 9 | 4 to >16 | 100 | 100 | >32 | 100 | 100 | >32 | 100 | 100 | |||
| TR46/Y121F T289A | 10 | >16 | >16 | 100 | 100 | >32 | >32 | 100 | 100 | >32 | >32 | 100 | 100 |
| All isolates | 79 | 1 | 0.125 to >16 | 29 | 38 | 0.5 | 0.125 to >32 | 27 | 29 | 0.5 | 0.125 to >32 | 29 | 33 |
Plates were read by two independent readers using the FIE endpoint after 2 days of incubation.
MIC50s (mg/liter) are presented only for genotypes represented by ≥10 isolates.
Percentage of isolates with MICs above the EUCAST isavuconazole ECOFF (2 mg/liter) and clinical (1 mg/liter) breakpoints.
TR, tandem repeat in the CYP51A promoter region.
The MIC Strip Isavuconazole test manufacturer recommends an 80% IE reading, but in this study, higher interreader essential agreement, better separation between wild-type and resistant strains, and greater essential and categorical agreement compared to EUCAST results were achieved with the FIE. Thus, the FIE criterion was found to be superior although the MICs for the recommended Candida control strains were out of range (7). When using the FIE, the essential agreements with EUCAST within ±1 and ±2 twofold dilutions were 73 to 75% and 89 to 90% and thus better than previously found for the isavuconazole Etest versus the CLSI method, even though challenged here with a strain collection including a significant number of non-wild-type isolates (16). The categorical agreement was >91% when interpreting the MICs according to EUCAST breakpoints, and notably, among the 6 to 9% VMEs, half were isolates with a wild-type CYP51A target gene that either may be harboring other resistance mechanisms or may be isolates that are truly susceptible but misclassified as R by the EUCAST reference method because of the conservative EUCAST susceptibility breakpoint (7). Finally, the separation between wild-type and TR34/L98H and TR46/Y121F T289A mutant isolates was greater for the MIC strip test, rendering it a potentially promising routine lab tool for detecting R environmental mutants, provided the FIE is used (2, 5, 19–21).
The CYP51A amino acid alterations have been associated with a codon-specific susceptibility pattern (4, 13). Here, both the strip and EUCAST isavuconazole MICs indeed straddled the clinical breakpoint for isolates harboring M220 alterations and for the G54R N24K double mutant, which will inevitably lead to the random classification of such isolates as S or R in routine testing. Hence, as long as clinical outcome data are unavailable for such mutants, other measures such itraconazole MIC testing or CYP51A sequencing should be undertaken to detect these genotypes.
This study has limitations. We investigated strip test reader-to-reader agreement but no other factors associated with variation, such as variation across different lots and brands of RPMI agar plates, inoculum preparation, etc. Therefore, the promising performance reported here needs confirmation in a multicenter study.
Supplementary Material
ACKNOWLEDGMENTS
Isavuconazole MIC strips and the pure substance were kindly provided at no cost by Basilea. We thank Birgit Brandt and Désiré Mageme Nahimana for excellent technical assistance.
Maiken Cavling Arendrup has received research grants or speaker honoraria from Astellas, Basilea, Gilead, MSD, Novartis, Pfizer, and T2Biosystems. She is the current chairman of the EUCAST-AFST and has previously served on advisory boards for MSD (until 2014) and Pfizer (until 2012). Paul Verweij has received research grants from Astellas, Basilea, F2G, Gilead Sciences, Merck, and Pfizer; has been a consultant to Basilea, F2G, Gilead Sciences, Merck, and Pfizer; and has received speaker's fees from Basilea, Gilead Sciences, and Merck. Henrik Vedel Nielsen has no conflicts to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01659-16.
REFERENCES
- 1.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother 65:1–5. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 2.Astvad KM, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, Leatherbarrow H, Mellado E, Arendrup MC. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol 49:2243–2251. doi: 10.1128/JCM.00213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Alessandro C, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1-4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Howard SJ, Lass-Flörl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother 57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
- 10.Wu C-J, Wang H-C, Lee J-C, Lo H-J, Dai C-T, Chou P-H, Ko W-C, Chen Y-C. 2015. Azole-resistant Aspergillus fumigatus isolates carrying TR34 /L98H mutations in Taiwan. Mycoses 58:544–549. doi: 10.1111/myc.12354. [DOI] [PubMed] [Google Scholar]
- 11.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, Sanders C, Fan H, Fothergill AW, Sutton DA. 2016. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol 54:168–171. doi: 10.1128/JCM.02478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen RH, Hagen F, Astvad KM, Tyron A, Meis JF, Arendrup MC. 2016. Azole resistant Aspergillus fumigatus in Denmark: a laboratory based study on resistance mechanisms and genotypes. Clin Microbiol Infect 22:570.e1-9. doi: 10.1016/j.cmi.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Stensvold CR, Jørgensen LN, Arendrup MC. 2012. Azole-resistant invasive aspergillosis: relationship to agriculture. Curr Fungal Infect Rep 6:178–191. doi: 10.1007/s12281-012-0097-7. [DOI] [Google Scholar]
- 14.Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Møller N, Khan H, Melchers WJ, Verweij PE. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One 5:e10080. doi: 10.1371/journal.pone.0010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snelders E, Huis In 't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinea J, Peláez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 52:1396–1400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson GR, Fothergill AW, Wiederhold NP, Vallor AC, Wickes BL, Patterson TF. 2008. Evaluation of Etest method for determining isavuconazole MICs for Cryptococcus gattii and Cryptococcus neoformans. Antimicrob Agents Chemother 52:2959–2961. doi: 10.1128/AAC.00646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendrup MC, Howard S, Lass-Flörl C, Mouton JW, Meletiadis J, Cuenca-Estrella M. 2014. EUCAST testing of isavuconazole susceptibility in Aspergillus: comparison of results for inoculum standardization using conidium counting versus optical density. Antimicrob Agents Chemother 58:6432–6436. doi: 10.1128/AAC.03779-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJA, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 21.Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole-resistant aspergillosis. N Engl J Med 356:1481–1483. doi: 10.1056/NEJMc061720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.