Abstract
Medium-sized and medium-bridged rings are attractive structural motifs in natural products and therapeutic agents. Due to the unfavourable entropic and/or enthalpic factors with these ring systems, their efficient construction remains a formidable challenge. To address this problem, we herein disclose a radical-based approach for diversity-oriented synthesis of various benzannulated carbon- and heteroatom-containing 8–11(14)-membered ketone libraries. This strategy involves 1,4- or 1,5-aryl migration triggered by radical azidation, trifluoromethylation, phosphonylation, sulfonylation, or perfluoroalkylation of unactivated alkenes followed by intramolecular ring expansion. Demonstration of this method as a highly flexible tool for the construction of 37 synthetically challenging medium-sized and macrocyclic ring scaffolds including bridged rings with diverse functionalities and skeletons is highlighted. Some of these products showed potent inhibitory activity against the cancer cell or derivative of human embryonic kidney line in preliminary biological studies. The mechanism of this novel strategy is investigated by control experiments and DFT calculations.
Medium-sized ring systems are common in natural products, however their synthesis is challenging, largely due to entropic factors. Here the authors report a radical-based method for the synthesis of medium to large functionalized, carbon or heterocyclic scaffolds.
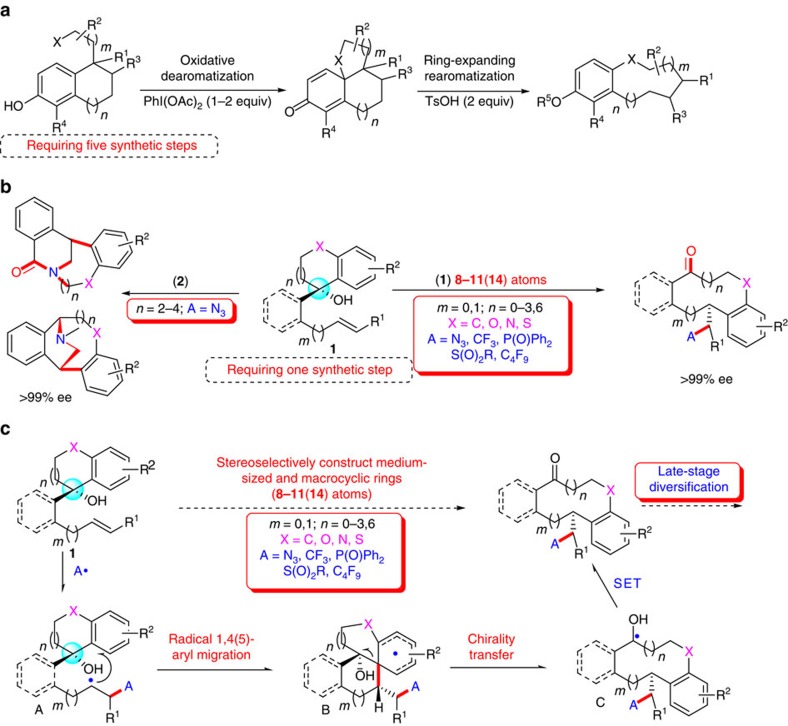
Collections and high-throughput screening of small-molecule libraries have become of paramount importance for discovering promising drug leads and biological probes. To meet this urgent need, the development of diversity-oriented synthesis approach for the rapid creation of complex molecules in the library has received increasing attention in both medicinal chemistry and chemical synthesis1,2,3. Although benzannulated medium- or medium-bridged rings constitute the basic skeletons of many important naturally occurring and biologically active molecules4,5,6,7,8,9, they are really rare among the top 200 drugs10. The main reason is probably the difficulty in developing general strategies for the diversity-oriented synthesis of such library of structures11,12,13. The achievement of high efficiency remains a formidable challenge with conventional cyclization-based methods, mainly due to unfavourable enthalpic and entropic reasons. To address this, an alternative, more flexible strategy is the use of ring expansion reactions of bicyclic or polycyclic substrates to the desired medium-sized rings14,15,16,17,18,19. For example, Harrowven has reported a ring expansion approach involving radical ipso-substitution triggered by sp2-radical intermediates generated from the dehalogenation of aryl or vinyl halides in the presence of toxic tin hydride reagents16. Recently, Tan and co-workers have developed an elegant biomimetic diversity-oriented synthesis of benzannulated medium-sized rings with rationally designed substrates via oxidative dearomatization followed by ring-expanding rearomatization (Fig. 1a)18. However, most reported methods rely heavily on the inherent nature of special substrates with preformed polycycles and/or highly strained small rings, which usually require tedious multistep preparation under harsh reaction conditions14,15,16,17,18,19. In addition, enantioselective ways to such chiral scaffolds have been rare because of the lack of efficient asymmetric approaches. Therefore, a general protocol for stereoselective and diversity-oriented synthesis of benzannulated medium- and related bridged-ring libraries, employing readily available starting materials and reagents under step/atom-economical and user-friendly synthetic conditions, is still highly desirable.
Figure 1. Radical strategy for asymmetric construction of medium-sized ketones and bridged amines.
(a) Previous work for construction of benzannulated medium-sized rings via ring expansion18. (b) Our radical-based strategy for construction of benzannulated medium- to large-sized ketones and bridged amines. (c) Proposed mechanism for the stereoselective construction of medium-sized rings.
Unactivated alkenes are one of the most abundant feedstocks and thus represent excellent building blocks for chemical synthesis. The selective addition of diverse radicals to unactivated alkenes represents an efficient way for the functionalization of such alkenes20,21. In addition, great advances have been achieved in the field of aryl migration following radical ipso substitution at an aromatic ring for constructing new organic compounds16,22,23,24,25,26,27,28,29,30,31. Despite these advances, no radical-based protocol has been available for the conversion of unactivated alkenes into medium- to large-sized and related bridged ring libraries via aryl migration, especially for access to such chiral skeletons. To address these challenges, we rationally designed alkenyl alcohols 1 bearing cyclic benzyl alcohol groups32,33, which are more readily accessible than those polycyclic substrates required in conventional methods14,15,16,17,18,19 (Fig. 1b). It is expected that an inherently high-energy sp3-carbon-centred alkyl radical A could be in situ generated from the addition of appropriate radicals to the unactivated alkenyl moiety of 1. Driven by the formation of a lower energy neutral ketyl radical C (refs 32, 33, 34), intermediate A would undergo an intramolecular radical remote 1,4- or 1,5-aryl migration/ring expansion sequence to provide desired medium-ring products (Fig. 1c). Furthermore, we envisioned that the use of easily available optically pure alcohols would lead to enantioenriched medium-sized rings through a remote chirality transfer strategy. Hence, this strategy can overcome the unfavourable entropic and/or enthalpic factors typically encountered by conventional cyclization-based approaches11,12,13. Nonetheless, the realization of such cascade sequence reactions remains an underdeveloped process. Several challenges are associated with the development of this reaction, such as (1) the unfavourable kinetic and/or thermodynamic factors to realize regioselective medium ring formation over other competitive 1,2-difunctionalizations of alkenes with oxygen-based nucleophiles35,36,37,38, (2) the compatibility between unactivated alkenyl substrates and various radical precursors with different reactivity properties, (3) the identification of mild reaction conditions to achieve high degree of enantiocontrol through remote chirality transfer strategy.
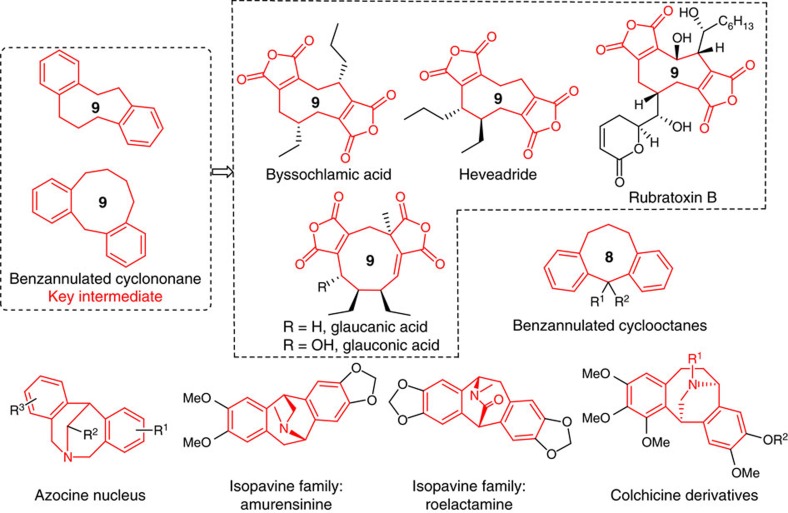
With this strategy, we report herein a practical strategy for diversity-oriented synthesis of benzannulated 8-11(14)-membered cyclic ketones along with concurrent installation of various functional groups from readily available starting materials. This strategy was realized through a concerted remote 1,4- or 1,5-aryl migration/ring expansion sequence triggered by radical azidation, trifluoromethylation, phosphonylation, sulfonylation or perfluoroalkylation of unactivated alkenes (Fig. 1b, path 1). Given the prevalence of medium-bridged amines and relative chiral compounds in medicinal chemistry39,40, we further demonstrate a distinct pathway for the convenient collection of useful medium-bridged amines with a high degree of skeletal complexity and functional diversity (Fig. 1b, path 2). In the context of a diversity-oriented synthesis of medium- and bridged-ring library, our strategy has displayed some exceptional advantages: (1) 1,4- and 1,5-aryl migration could be realized with the easily accessible substrates. (2) This method tolerates different opening-ring sizes of carbo- and heterocyclic alcohols from 5 to 11-membered ring, thus leading to a skeletally diverse medium- and large-ring collection. (3) A variety of radical sources, including azide, trifluoromethyl, phosphonyl, sulfonyl, perfluoroalkyl radical, are used as suitable radical precursors. (4) The functional groups in formed products serve as versatile handles for further diversification to afford diverse natural analogues in parallel. Therefore, this discovery will be a highly flexible tool for diverse collection of a variety of medium-sized rings and related bridged ring libraries with over 37 distinct scaffolds as well as the identification of some compounds with potent inhibitory activity against the cancer cell or derivative of human embryonic kidney lines. Noteworthy is that many formed benzannulated medium-sized or medium-bridged rings constitute the key synthetic intermediates and unnatural analogues of many important naturally occurring and biologically active molecules such as benzannulated cyclooctanes (antiarrhythmic, antidiarrheal, antiallergic and anti-inflammatory)4, nonadride family (byssochlamic acid, heveadride, rubratoxin B, glaucanic and glauconic acid with antifungal, antimicrobial and antitumor activities)5,6, isopavine family (neurological disorders inhibition)7,8 and colchicine derivatives (anticancer activity)9 (Fig. 2).
Figure 2. Representative natural products and biologically active molecules with benzannulated medium-sized rings and bridged amines.
Several core structures or their derivatives of natural products are synthesized in next section.
Results
Azidation-initiated medium/macro-sized ketones' synthesis
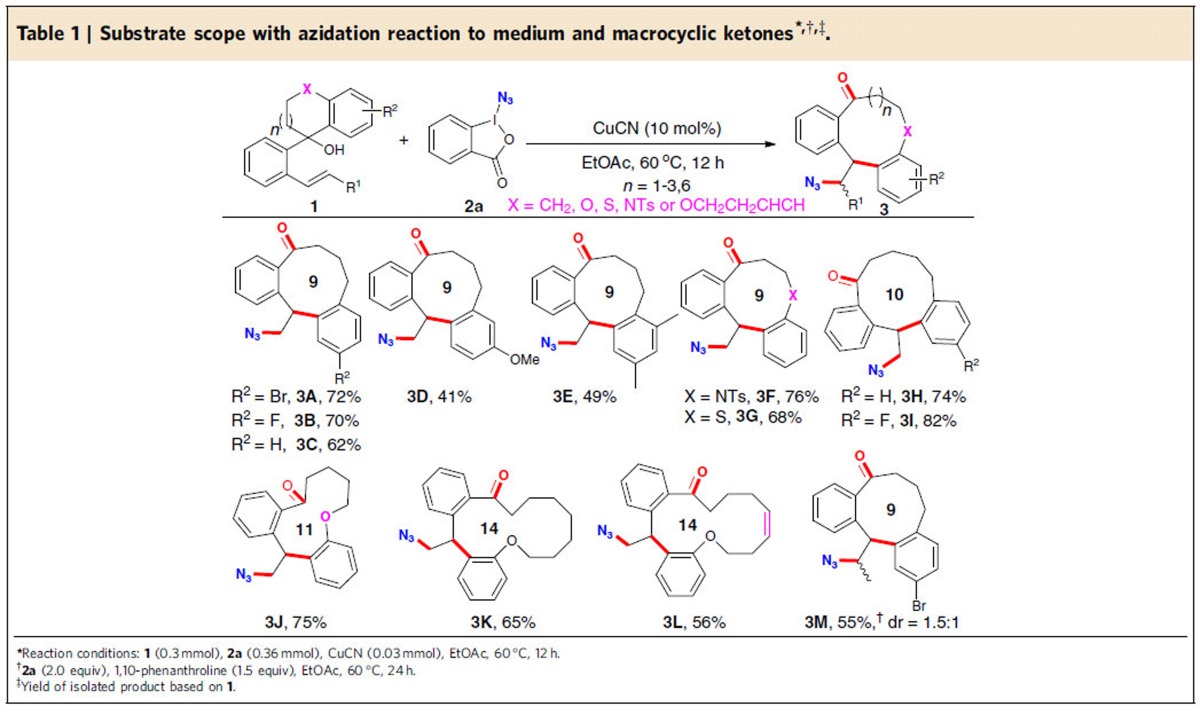
Modular synthetic access to these model substrates 1 would be readily achieved in good yields by straightforward and efficient one-step transformation from inexpensive starting materials (see details in Supplementary Information). Since the development of methods for the incorporation of an azide moiety into organic compounds has enormous significance in chemistry, biology and materials sciences41,42, we started our investigation to target the challenging nine-membered ketone formation along with the concurrent installation of an azide group based on the proposed pathway. As such, the reaction of substrate 1A with iodine (III) reagent azidoidinane (2a), first reported by Zhdankin and co-workers43, was selected for the optimization of reaction conditions (Supplementary Table 1). We were delighted to find that the desired nine-membered product 3A was indeed observed in the presence of CuI (10 mol%), albeit with 47% yield, suggesting that the remote radical 1,4-aryl migration is much more favourable than other reaction pathways in the current catalytic system. Upon optimizing the reaction conditions through variation of the copper catalysts, catalyst loadings, solvents and the molar ratio of the reactants, we identified the following protocol as optimal: reaction of 1A and 2a with the molar ratio of 1.0:1.2 in the presence of CuCN (10 mol%) in EtOAc at 60 °C for 12 h, 3A was obtained in 72% isolated yield (Table 1). With the optimized reaction conditions established, we first examined the substrate scope of alkenyl alcohols 1 featuring a six-membered ring and the results are summarized in Table 1. A range of substrates with 1,2,3,4-tetrahydronaphthalene moieties bearing mono-substituents, such as electron-withdrawing (-Br (1A), -F (1B)), electron-neutral (-H (1C)), and electron-donating (-OMe (1D)) groups, were found to be suitable to give the corresponding products 3A-3D in 41–72% yields. Moreover, the reaction of 1E with 2,5-dimethyl groups on the aryl ring proceeded smoothly to generate 3E (49% yield). Remarkably, the products 3A-3E are quite similar to the core structure of key intermediates towards the synthesis of glaucanic acid6. It is noteworthy that the alkenyl alcohols with heteroatom-tethering groups, such as sulfonamide- and sulfur-tethered substrates 1F and 1G, were also well tolerated to produce heterocyclic ketones 3F and 3G in 76% and 68% yields, respectively. To increase the diversity of such a library, the more challenging benzannulated 10- and 11-membered ketone products 3H-3J were also obtained in 74–82% yields from the corresponding substrates under the current reaction system. The high efficiency of the present protocol in preparing macrocyclic ring system was demonstrated in the isolation of 14-membered ketone 3K in 65% yield from 11-membered alkenyl alcohol 1K, which further extended the compound library. Notably, substrate 1L containing another reactive olefin also afforded the desired 14-membered alkenyl ketone 3L in 56% yield with the additional olefin being intact. Meanwhile, the alkenol 1M containing an internal alkene in the presence of 1.5 equiv of 1,10-phenanthroline afforded the desired product 3M as a 1.5:1 mixture of diastereomers in 55% yield.
Table 1. Substrate scope with azidation reaction to medium and macrocyclic ketones *,†,‡.
Construction of trifluoromethylated medium-sized ketones
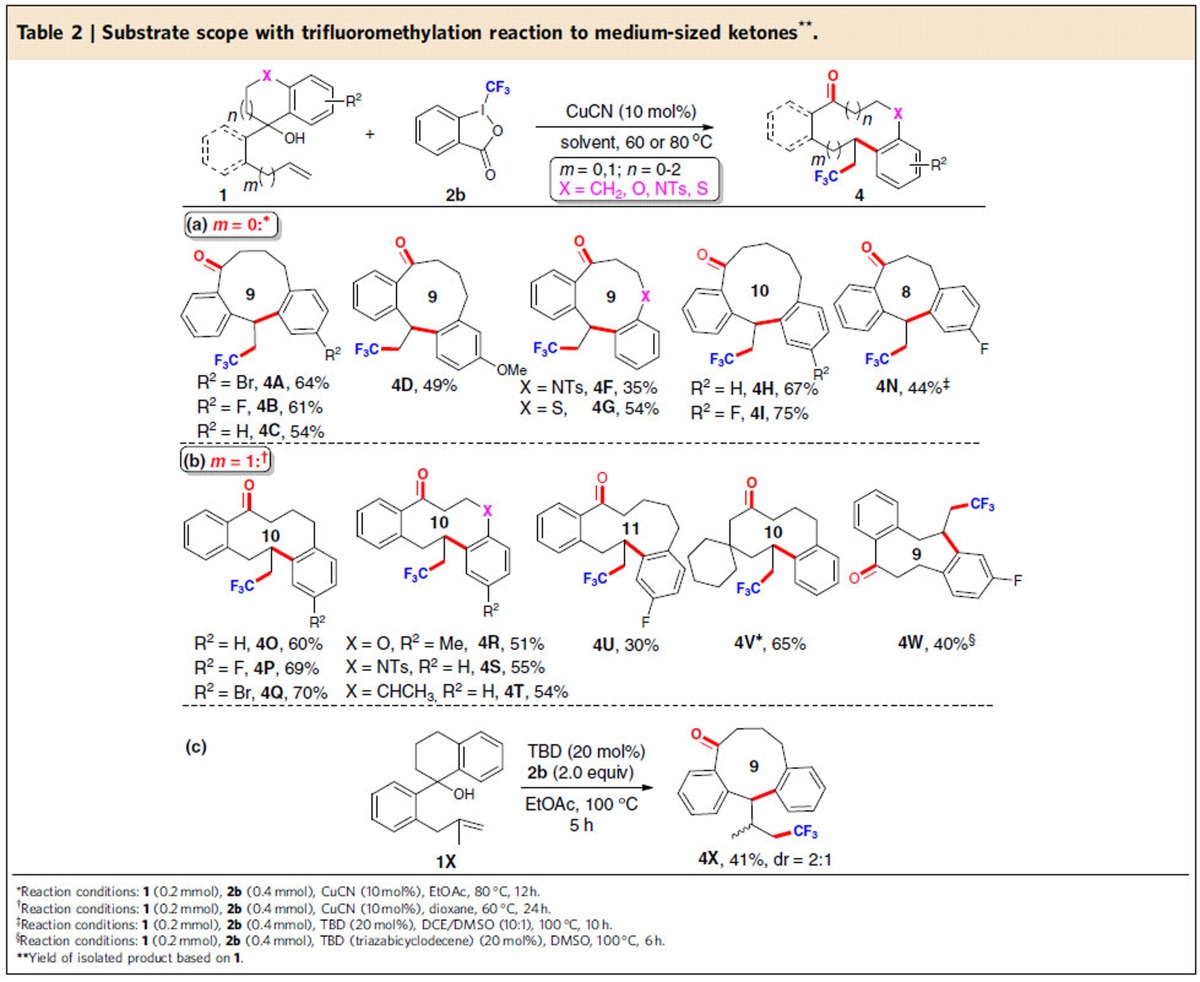
The increasing importance of trifluoromethyl organic molecules in agrochemicals, pharmaceuticals and molecular materials has spurred vigorous research efforts towards new versatile methodologies for the generation of the C-CF3 bond44,45. Impressive advances have been achieved in developing methods for direct introduction of the CF3 group into organic compounds by using radical trifluoromethylating reagents35,36. In this context, we focused our attention on realizing the incorporation of a CF3 group with concurrent construction of benzannulated medium-sized rings (Table 2). To our delight, after some optimization efforts (Supplementary Table 2), we found that the reaction of 1A with Togni's reagent46 2b (2.0 equiv) proceeded smoothly in the presence of CuCN (10 mol%), giving the desired product 4A in 64% yield with high chemoselectivity (Table 2a). A variety of alkenyl alcohols 1, bearing either electron-withdrawing or electron-donating groups on the phenyl ring, reacted smoothly with 2b to give the expected products 4B-4D in 49–61% yields. Notably, the alkenyl alcohols with heteroatom-tethering groups (1F and 1G) or seven-membered cycles (1H and 1I) were also suitable substrates to deliver nine- and ten-membered hetero- or carbocyclic ketones 4F-4I (35–75% yields). To verify the application of the current method in the synthesis of the core skeleton of the natural occurring benzannulated cyclooctanes4 (Fig. 2), we turned our attention to target benzannulated eight-membered ketone 4N and tested substrate 1N with a five-membered ring under the standard conditions. To our disappointment, such substrate preferentially underwent competitive 1,2-oxytrifluoromethylation37,38 to give product 4N′ in 65% yield with good diastereoselectivity (14:1 d.r.) (Supplementary Table 3). This observation clearly indicated that tuning the opening-ring size can have a profound influence on the control of the reaction outcome, probably owing to the presence of different favourable conformations and transition states during the reaction. To modulate the reactivity, we resorted to our recently developed organic base-catalysed radical trifluoromethylation of alkenes33, and obtained the desired eight-membered product 4N in 44% yield in the presence of triazabicyclodecene (TBD) (20 mol%) in a mixed solvent system of 1,2-dichloroethane (DCE)-dimethyl sulfoxide (DMSO) (10:1) (Supplementary Table 3).
Table 2. Substrate scope with trifluoromethylation reaction to medium-sized ketones**.
To expand the synthetic utility of this methodology for access to other novel benzannulated medium-sized skeletons, we explored the use of allylbenzene derivatives as the substrates in place of styrene derivatives (Table 2b). After some experimentation (Supplementary Table 4), such a process for the formation of benzannulated ten-membered ketones could be successfully realized directly from readily available alkenyl alcohols. For example, substrates 1 bearing various functional groups (1O-1Q) on the aryl ring, heteroatom-tethering groups (such as oxygen- and sulfonamide-tethered substrates 1R and 1S), and methyl-substituted 1,2,3,4-tetrahydronaphthalene group (1T), reacted efficiently to afford the corresponding products 4O-4T in 51–70% yields. The structure of the product 4O was also established by X-ray crystallographic analysis (Supplementary Fig. 1). Particularly noteworthy is that the 11-memerbed ketone 4U could also be constructed with the current process, albeit in moderate yield with substrate 1U bearing a seven-membered ring. It is noteworthy that the current method could be extended to the aliphatic substrate 1V. Under the standard reaction conditions, product 4V was obtained in 65% yield, indicating that the reaction was not significantly affected by switching the benzylic alcohol (1O) to an aliphatic substrate (1V). The resemblance of 4W to the core structure of a key intermediate towards the synthesis of byssochlamic acid analogues5 prompted us to test substrate 1W. It was found that 4W was generated smoothly as well, further elaborating the powerful utility of the current method in natural product synthesis. An interesting result was that the reaction of gem-disubstituted alkenyl alcohol 1X generated the unexpected product 4X in 41% yield as a 2:1 mixture of diastereomers, probably via a 1,2-hydrogen atom transfer of intermediate A followed by a 1,4-aryl migration process (Table 2c).
Synthetic application towards diverse radicals
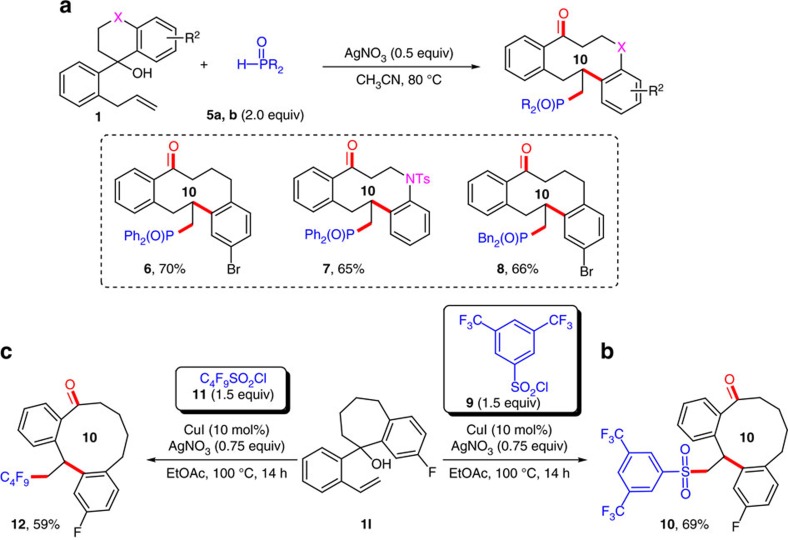
The high efficiency of the above diversity-oriented synthesis approach encouraged us to expand the scope to other radical precursors. As expected, the reaction of substrate 1Q with Ph2P(O)H (5a, 2.0 equiv) in the presence of AgNO3 (0.5 equiv) delivered phosphonyl-containing ten-membered ketone 6 (Fig. 3a and Supplementary Table 5). Furthermore, the phosphonyl-containing ten-membered ketone 7 with a sulfonamide-tethering group was obtained in 65% yield. Moreover, dibenzylphosphine oxide 5b also participated in this reaction to give product 8 in 66% yield, suggesting the feasibility of other types of phosphonyl radicals in this reaction. In addition to phosphonyl radical, in situ generated sulfonyl radical was also a suitable reaction partner: the reaction of substrate 1I with 1.5 equiv of 3,5-bis(trifluoromethyl)benzenesulfonyl chloride 9 in the presence of CuI (10 mol%) and AgNO3 (0.75 equiv) afforded the corresponding ketone 10 in 69% yield (Fig. 3b). The final success of C4F9 radical installation to generate 12 with perfluorobutanesulfonyl chloride (11, 1.5 equiv) via extrusion of sulfur dioxide under the otherwise identical reaction conditions was in great support of the wide application of the present methodology with different radicals in enriching the library of medium- and large-sized compounds (Fig. 3c).
Figure 3. Addition of diverse radicals to alkenes for the construction of medium-sized ketones.
(a) Radical alkene phosphonylation-initiated reaction. (b) Radical alkene sulfonylation-initiated reaction. (c) Radical alkene perfluoroalkylation-initiated reaction.
Chirality transfer strategy and mechanistic investigations
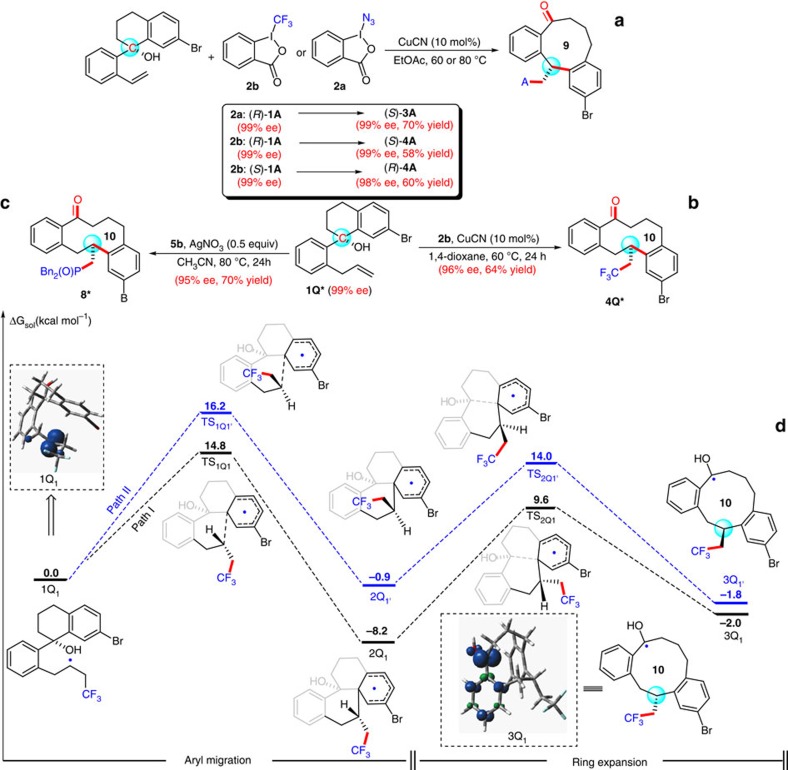
On the basis of the concerted nature of the intramolecular radical aryl migration/ring expansion process as proposed in Fig. 1c, we expected that the stereochemical information of the tertiary alcohol would be completely transferred to the remote new-formed carbon chiral centre in a highly stereoselective way47,48 to construct optically pure medium-sized ketone. However, this chirality transfer strategy might be difficult due to the presence of several highly reactive radical intermediates, as well as the requirement of discrimination between the two enantiotopic faces in the intermediate B. To our delight, we found that the desired product (S)-3A was obtained in 70% yield with 99% ee via an almost complete chirality transfer strategy upon treatment of (R)-1A (99% ee) with 2a under the otherwise identical conditions (Fig. 4a). Remarkably, almost complete chirality transfer could also be achieved for substrates (R)-1A and (S)-1A upon treatment with Togni's reagent 2b under the standard conditions to give (S)-4A and (R)-4A with 99% ee (58% yield) and 98% ee (60% yield), respectively, thus indicating the present transformation is highly stereoselective (Fig. 4a). The absolute configurations of the substrate (R)-1A and the product (R)-4A were established by X-ray crystallographic analysis (Supplementary Fig. 1), and those of other materials and products were assigned by analogy (Fig. 4a). To further evaluate the practicality of this methodology, we expanded the substrate scope with the enantiomerically pure allylbenzene derivative 1Q*. Treatment of substrate 1Q* with 2b or 5b under the otherwise identical conditions also underwent almost complete chirality transfer to give the corresponding enantioenriched benzannulated ten-membered trifluoromethyl- and phosphorus-containing ketones 4Q* and 8* in 64% and 70% yields with 96% and 95% ee, respectively (Fig. 4b,c).
Figure 4. Reactions for enantioenriched medium-sized ketones through chirality transfer strategy and DFT calculation.
(a) Access to enantioenriched azido- and trifluoromethyl substituted medium-sized ketone from enantiopure 1A. (b,c) Access to enantioenriched trifluoromethyl- and phosphonyl substituted medium-sized ketone from enantiomerically pure 1Q*. (d) DFT calculation for mechanistic investigation. The calculated potential energy surfaces for aryl migration and ring expansion processes at M11/BS level of theory in 1,4-dioxane with the full optimization using smd model (BS refers to the used basis sets. For C, H, O, F atoms, the 6−31+G** basis set was used and for Br atoms, the Aug-cc-PVTZ basis set was used).
To gain some more insights into the reaction mechanism, we tested some model reactions in the presence of radical scavengers such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) (for azidation, trifluoromethylation or phosphonylation reaction), 2,6-di-tert-butyl-4-methylphenol (BHT) (for azidation reaction) or 1,4-dinitrobenzene (for trifluoromethylation or phosphonylation reaction) under the standard conditions, and found that the reactions were significantly inhibited by these reagents (Supplementary Fig. 2), suggesting that a radical process is involved in these reactions. To probe the origin of the observed stereoselectivity, we further investigated the reaction mechanism computationally using M11 method. The generally assumed alkyl sp3-carbon-centred radical species 1Q1 was chosen as the starting point to locate two reaction pathways responsible for the stereoselective reactions. The calculated results revealed that this reaction occurs stepwise, involving the formation of the bicyclic rings 2Q1/2Q1′ and the ring expansion to result in the formation of intermediates 3Q1/3Q1′ (Fig. 4d). Some notable points from these calculations are as follows: (i) The addition of sp3-carbon-centred radical species to the aryl group (from 1Q1 to 2Q1/2Q1′) is exothermic. In addition, the reversed process (2Q1/2Q1′ to 1Q1) has higher reaction barriers than the subsequent ring expansion (2Q1/2Q1′ to 3Q1/3Q1′). As such, reaction barriers of the aryl migration step could be used to mirror the observed stereoselectivity. (ii) The energy of transition state TS1Q1, leading to the major (S)-product 3Q1, is lower than that of transition state TS1Q1′ forming the minor (R)-product 3Q1′ by 1.4 kcal/mol when (R)-1Q* is employed as the reactant. This difference in energy appears to result from steric repulsion between the trifluoroethyl group and the six-membered ring and agrees well with experiment findings (for 4Q* from (R)-1Q in a highly stereoselective way as shown in Fig. 4b). (iii) As going from 1Q1 to 3Q1, the sequential cascade reaction is exothermic by 2.0 kcal/mol along with the formation of the more stable neutral ketyl radical species. To understand the factors that contribute to the greater stability of radical species 3Q1 than that of 1Q1, we performed the natural bond orbital (NBO) analysis of spin density distribution of the above two species (Fig. 4d). The spin density of 1Q1 is exclusively localized on the carbon atom of the substrate, while that of 3Q1 is partially distributed on the carbon atom with significant radical character on the O atom and the phenyl group. This reveals that the presence of a OH group as a π-donating moiety can significantly stabilize the resultant radical species 3Q1, providing an important driving force that makes the formation of ketyl radical species 3Q1 thermodynamically favourable and increases the power of the overall process substantially to generate medium-sized ring systems. DFT calculations on an alternative mechanism involving a Cu species have also been performed49,50, showing that the reaction of the alkyl radical 1Q with CuII species is highly energetically unfavourable, as revealed by a 6.1 kcal higher reaction barrier of the forming Cu intermediate than the radical aryl migration process of alkyl radical intermediate 1Q1 (Supplementary Fig. 3). Therefore, all these experimental and calculated results, together with the observed highly efficient radical chirality transfer during the ring expansion, are in support of our initial proposal as shown in Fig. 1c, in which the selective addition of a variety of in situ generated radicals to unactivated alkenes could trigger ring expansion via remote 1,4- or 1,5-aryl migration processes.
Diverse synthetic applications
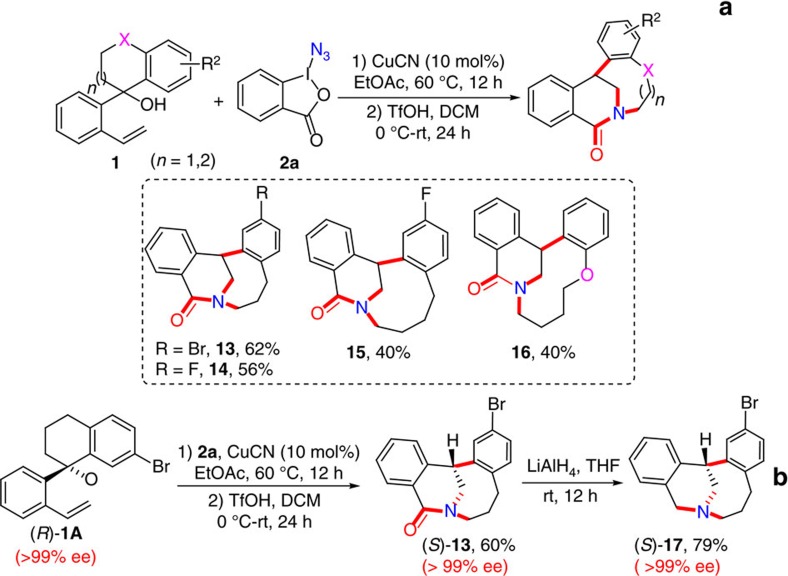
An important aspect of this current methodology is that both medium-sized carbo- and heterocyclic ketones and C-X (X=N3, CF3, P(O)R2, SO2Ar, C4F9) are efficiently constructed. Consequently, the resultant functionalized compounds can serve as a convenient handle to access other valuable medium-sized and related compounds. Medium-bridged lactams, exhibiting unique reactivity dissimilar to that of traditional amides due to the limited nN→π*C=O conjugation, have been used as important building blocks in accessing pharmaceutical and natural relevant molecules39. Therefore, a number of methodologies have been developed for the synthesis of these useful scaffolds39. However, convergent one-step or one-pot methods to prepare such complex skeletons are extremely rare especially with high levels of enantiocontrol, and often require the use of inconvenient polycyclic substrates via tedious multistep synthesis with poor selectivity. To demonstrate one important synthetic utility of this methodology, we reasoned that intramolecular Schmidt-Aubé reaction51,52 of the resultant azido-substituted medium-sized ketones 3 in the presence of an appropriate Brønsted acid might afford medium-bridged lactam derivatives. Our original screening of reaction conditions proved that the treatment of 3A with TfOH easily afforded the desired bridged lactam 13 in 85% yield, with complete regioselectivity (Supplementary Table 6). To further simplify the reaction protocol, we investigated the possibility of performing the two distinct reaction sequence in a one-pot fashion. To our delight, after optimization of different reaction parameters (Supplementary Table 7), a simple one-pot procedure was realized: alkenyl alcohols 1 were firstly converted into azido-substituted medium-sized ketones 3 in the presence of CuCN (10 mol%) with EtOAc as solvent. Then, after replacement of the solvent EtOAc with DCM, 3 were subjected to the intramolecular Schmidt-Aubé reaction with TfOH to afford the medium-bridged lactams 13–16 with distinct backbones in 40–62% yields (Fig. 5a). Moreover, the enantioenriched (S)-13 was easily obtained in 60% yield with >99% ee from (R)-1A (>99% ee) following the one-pot procedure (Fig. 5b). It is encouraging to note that the present process is a rather general protocol for the one-pot synthesis of useful complex medium-bridged lactams in high efficiency, which is clearly complementary to previous conventional synthetic methods39. Most importantly, our procedure is also applicable to the synthesis of a novel type of chiral bridged tertiary amines at the bridgehead position, which have a great potential for applications as effective asymmetric organocatalysts or ligands53 and could be further converted into other alkaloid analogues of potential medicinal importance39. For example, the reduction of the resultant (S)-13 with LiAlH4 efficiently delivered the medium-bridgehead tertiary amine (S)-17 in 79% yield without a decrease in the ee value (Fig. 5b).
Figure 5. Versatile transformations (I).
(a) One-pot Schmidt-Aubé reaction for the construction of medium-bridged lactams. (b) Access to enantioenriched medium-bridged tertiary amines.
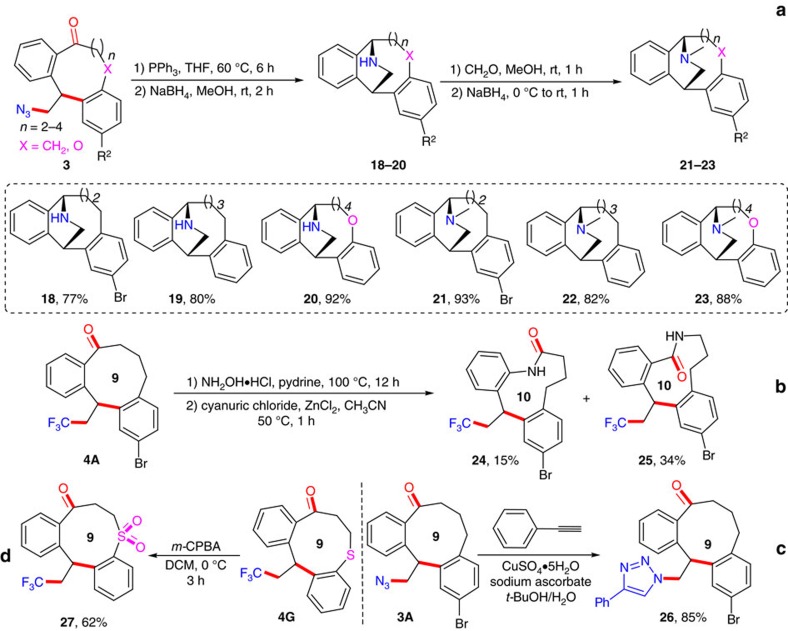
The easy access to azido-substituted medium-sized ketones prompts us to exploit the synthetic application of the current methodology to the rapid accumulation of analogues of isopavine alkaloids, which possess important pharmacological activities (Fig. 2). As envisioned, the PPh3-promoted intramolecular Staudinger/aza-Wittig reaction of 3, followed by subsequent NaBH4 reduction of in situ generated imines provided amines 18–20 smoothly in 77–92% yields (Fig. 6a). Finally, the amines were directly converted to isopavine analogues 21–23 in 82–93% yields by reductive amination with formaldehyde in the presence of NaBH4 (Fig. 6a). In addition, the resultant ketone group in the obtained medium-ring product inspires us to extend the ring expansion strategy towards lactams via Beckmann rearrangement. An example was the efficient conversion of CF3-containing nine-membered ketone 4A to the ten-membered lactams 24 and 25 in 15% and 34% overall yields via Beckmann rearrangement of the corresponding E- or Z-oximes, respectively (Fig. 6b); such synthetically challenging medium-ring lactams are widespread subunits in biologically active natural products and therapeutic agents54. Meanwhile, 3A successfully underwent the click reaction to give the corresponding triazole 26 in 85% yield (Fig. 6c). Furthermore, manipulation of functional groups at other sites of the ring proved also feasible. For example, sulfur-tethered ketone 4G could be selectively oxidized to deliver sulfonyl-substituted nine-membered ketone 27 in 62% yield (Fig. 6d).
Figure 6. Versatile transformations (II).
(a) Rapid accumulation of a small library of isopavine analogues. (b) Preparation of medium-sized lactams via Beckmann rearrangement. (c,d) Click chemistry and preparation of sulfone.
Cheminformatic analysis
We have used a cheminformatic approach involving principal component analysis to evaluate the diversity of our compound library compared with natural products, drug-like compounds and drugs18 (see Supplementary Figs 4–10 and Supplementary Tables 8 and 9). The results reveal comparably diverse but distinct chemical spaces occupied by our synthetic molecules and similar benzannulated medium-ring natural products. In addition, compared with the naturally occurring counterpart, our compound library displays less overlaps with the chemical spaces determined by drug-like compounds and drugs. Both of these two features indicate a wide and less-explored chemical space spanned by our compound library, thus demonstrating its great potential for future drug discovery.
Preliminary biological studies
The structure resemblance to a variety of biologically active compounds also encourages us to evaluate the biological activity of our products. Our preliminary biological studies revealed that compounds 3I, 13 and 15 exhibited good cytotoxicities against both H1299 (human non-small cell lung carcinoma, with IC50 values of 10.8 and 19.5 μM for 3I and 15, respectively) and 293T (derivative of human embryonic kidney transformed by adenovirus, with IC50 values of 18.5 and 10.9 μM for 13 and 15, respectively) cell lines (Supplementary Table 10).
Discussion
In summary, we have developed a strategically novel, general and powerful approach for diversity-oriented synthesis of skeletally- and functionally diverse benzannulated medium- and macro-ring scaffolds through 1,4(5)-aryl migration/ring expansion triggered by addition of the diverse radicals to alkenes. Furthermore, the newly developed protocol provides a facile and straightforward access to useful medium-bridged amine derivatives, such as the analogues of the azocine nucleus and the isopavine family. This protocol features a diverse product scope including a variety of carbo-, oxygen-, nitrogen- or sulfur-containing 8 to 14-membered cyclic ketones and medium-bridged amines with over 37 distinct skeletally complex scaffolds as well as the identification of some compounds with potent activity in anticancer and proliferation inhibition, wide functional group compatibility, high enantioselectivity (up to 99% ee) by a chirality transfer strategy, and readily available substrates and reagents. The mechanism of this novel strategy was also investigated by control experiments and DFT calculations. Considering the broad utility for diversity-oriented synthesis of various medium rings and bridged rings of this new protocol, we further anticipate that this efficient strategy will motivate the design of other related processes for the more efficient synthesis of complex natural products and other bioactive molecules.
Methods
General procedure for azidation reactions
Under argon, a 25 ml Schlenk tube equipped with a magnetic stir bar were charged with 1 (0.3 mmol, 1.0 equiv), 2a (104 mg, 0.36 mmol, 1.2 equiv), CuCN (2.7 mg, 0.03 mmol, 0.1 equiv) and EtOAc (super dry, 3.0 ml). The sealed tube was then stirred at 60 °C for 12 h. After completion (monitored by thin-layer chromatography (TLC)), EtOAc (30 ml) was added. The organic phase was washed with saturated NaHCO3 solution (2 × 5 ml), dried over anhydrous Na2SO4, filtered and concentrated to afford the crude product, which was purified by flash column chromatography to afford the corresponding product 3.
General procedure for trifluoromethylation reactions (a)
Under argon, a 25 ml Schlenk tube equipped with a magnetic stir bar were charged with 1 (0.2 mmol, 1.0 equiv), 2b (126 mg, 0.4 mmol, 2.0 equiv), CuCN (1.8 mg, 0.02 mmol, 0.1 equiv) and EtOAc (2.0 ml). The sealed tube was then stirred at 80 °C for 12 h. After completion (monitored by TLC), EtOAc (30 ml) was added and the reaction mixture was washed with saturated NaHCO3 (2 × 5 ml) solution. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to afford the crude product, which was purified by flash column chromatography to afford the product 4.
General procedure for trifluoromethylation reactions (b)
Under argon, a 25 ml Schlenk tube equipped with a magnetic stir bar was charged with 1 (0.2 mmol, 1.0 equiv), 2b (126 mg, 0.4 mmol, 2.0 equiv), CuCN (1.8 mg, 0.02 mmol, 0.1 equiv) and dioxane (4.0 ml). The sealed tube was then stirred at 60 °C for 24 h. After completion (monitored by TLC), EtOAc (30 ml) was added and the reaction mixture was washed with saturated NaHCO3 solution (2 × 5 ml). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to afford the crude product, which was purified by flash column chromatography to afford the product 4.
General procedure for phosphonylation reactions
To a flame-dried Schlenk tube equipped with a magnetic stir bar were added 1 (0.2 mmol, 1.0 equiv), 5a/5b (0.4 mmol, 2.0 equiv) and AgNO3 (18 mg, 0.10 mmol, 0.5 equiv). The tube was evacuated and backfilled with argon for three times, and then CH3CN (2.0 ml) was added. The tube was stirred at 80 °C for 24 h and then H2O (5 ml) was added. The product was extracted with EtOAc (3 × 10 ml). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated to afford the crude product, which was purified by flash column chromatography to afford the product 6–8.
For NMR and HPLC spectra, see Supplementary Figs 11–214.
Data availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1451652 (4O), CCDC 1451653 ((R)-1A) and CCDC 1451654 ((R)-4A). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Any further relevant data are available from the authors on reasonable request.
Additional information
How to cite this article: Li, L. et al. Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings. Nat. Commun. 7, 13852 doi: 10.1038/ncomms13852 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary tables, supplementary notes, supplementary methods and supplementary references.
Calculated total energies and geometrical coordinates for structures.
Acknowledgments
Financial support from the National Natural Science Foundation of China (Nos. 215722096, 21302088), the National Key Basic Research Program of China (973 Program 2013CB834802), Shenzhen overseas high-level talents innovation plan of technical innovation project (KQCX20150331101823702), Shenzhen special funds for the development of biomedicine, Internet, new energy and new material industries (JCYJ20150430160022517) and South University of Science and Technology of China is greatly appreciated.
Footnotes
Author contributions L.L., Z.-L.L. and F.-L.W. performed experiments. Z.G. performed DFT calculations. Y.-F.C. and N.W. helped with characterizing all new compounds. X.-W.D. performed cheminformatic analysis. C.F., J.L. and C.H. performed biological studies. B.T. revised the paper. X.-Y.L. conceived and directed the project and wrote the paper.
References
- O' Connor C. J., Beckmann H. S. G. & Spring D. R. Diversity-oriented synthesis: producing chemical tools for dissecting biology. Chem. Soc. Rev. 41, 4444–4456 (2012). [DOI] [PubMed] [Google Scholar]
- Tan D. S. Diversity-oriented synthesis: exploring the intersections between chemistry and biology. Nat. Chem. Biol. 1, 74–84 (2005). [DOI] [PubMed] [Google Scholar]
- Galloway W. R. J. D., Isidro-Llobet A. & Spring D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80 (2010). [DOI] [PubMed] [Google Scholar]
- Wong J. K., Piwinski J. J. & Green M. J. Bis-benzo, cyclohepta piperidylidene, piperidine and piperazine compounds, compositions and method of use. US patent 5416087 (1995).
- Stork G., Tabak J. M. & Blount J. F. Total synthesis of (+-)-byssochlamic acid. J. Am. Chem. Soc. 94, 4735–4737 (1972). [Google Scholar]
- Chen X., Zheng Y. & Shen Y. Natural products with maleic anhydride structure: nonadrides, tautomycin, chaetomellic anhydride, and other compounds. Chem. Rev. 107, 1777–1830 (2007). [DOI] [PubMed] [Google Scholar]
- Tambar U. K., Ebner D. C. & Stoltz B. M. A convergent and enantioselective synthesis of (+)-amurensinine via selective C-H and C-C bond insertion reactions. J. Am. Chem. Soc. 128, 11752–11753 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanessian S., Parthasarathy S. & Mauduit M. The power of visual imagery in drug design. Isopavines as a new class of morphinomimetics and their human opioid receptor binding activity. J. Med. Chem. 46, 34–48 (2003). [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C., Valiulin R. A., Pokorski J. K., Chang V. & Chen J. S. Bio-inspired synthesis and biological evaluation of a colchicine-related compound library. Bioorg. Med. Chem. Lett. 22, 3776–3780 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath N. A., Brichacek M. & Njardarson J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010). [Google Scholar]
- Shiina I. Total synthesis of natural 8- and 9-membered lactones: recent advancements in medium-sized ring formation. Chem. Rev. 107, 239–273 (2007). [DOI] [PubMed] [Google Scholar]
- Sharma A., Appukkuttan P. & Van der Eycken E. Microwave-assisted synthesis of medium-sized heterocycles. Chem. Commun. 48, 1623–1637 (2012). [DOI] [PubMed] [Google Scholar]
- Parenty A., Moreau X., Niel G. & Campagne J.-M. Update 1 of: Macrolactonizations in the total synthesis of natural products. Chem. Rev 113, PR1–PR40 (2013). [DOI] [PubMed] [Google Scholar]
- Hesse M. Ring Enlargement in Organic Chemistry VCH (1991). [Google Scholar]
- Roxburgh C. J. Syntheses of medium sized rings by ring expansion reactions. Tetrahedron 49, 10749–10784 (1993). [Google Scholar]
- Harrowven D. C., Helias N. L., Moseley J. D., Blumire N. J. & Flanagan S. R. Medium ring synthesis by radical ipso-substitution. Chem. Commun. 21, 2658–2659 (2003). [DOI] [PubMed] [Google Scholar]
- Zhao W.-X., Wang Z.-B. & Sun J.-W. Synthesis of eight-membered lactones: intermolecular [6+2] cyclization of amphoteric molecules with siloxy alkynes. Angew. Chem. Int. Ed. 51, 6209–6213 (2012). [DOI] [PubMed] [Google Scholar]
- Bauer R. A., Wenderski T. A. & Tan D. S. Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion. Nat. Chem. Biol. 9, 21–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Fan X., Yu J. & Zhu C. Silver-catalyzed ring-opening strategy for the synthesis of β- and γ-fluorinated ketones. J. Am. Chem. Soc. 137, 3490–3493 (2015). [DOI] [PubMed] [Google Scholar]
- Baralle A. et al. in Encyclopedia of Radicals in Chemistry, Biology and Materials (eds Chatgilialoglu, C. & Studer, A.) Ch. 27 (John Wiley & Sons, Ltd., 2012).
- Hata S. & Sibi M. P. in Science of Synthesis—Stereoselective Reactions of C-C double bonds (ed. de Vries, J.G.) 873 (Georg Thieme Verlag, 2011).
- Studer A. & Bossart M. Radical aryl migration reactions. Tetrahedron 57, 9649–9667 (2001). [Google Scholar]
- Chen Z.-M., Zhang X.-M. & Tu Y.-Q. Radical aryl migration reactions and synthetic applications. Chem. Soc. Rev. 44, 5220–5245 (2015). [DOI] [PubMed] [Google Scholar]
- Gao P. et al. Copper-catalyzed one-pot trifluoromethylation/aryl migration/carbonyl formation with homopropargylic alcohols. Angew. Chem. Int. Ed. 53, 7629–7633 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou T.-G., Luo F.-X., Yang M.-Y. & Shi Z.-J. Silver-catalyzed long-distance aryl migration from carbon center to nitrogen center. J. Am. Chem. Soc. 137, 14586–14589 (2015). [DOI] [PubMed] [Google Scholar]
- Kong W., Casimiro M., Merino E. & Nevado C. Copper-catalyzed one-pot trifluoromethylation/aryl migration/desulfonylation and C(sp2)–N bond formation of conjugated tosyl amides. J. Am. Chem. Soc. 135, 14480–14483 (2013). [DOI] [PubMed] [Google Scholar]
- Kong W., Casimiro M., Fuentes N., Merino E. & Nevado C. Metal-free aryltrifluoromethylation of activated alkenes. Angew. Chem. Int. Ed. 52, 13086–13090 (2013). [DOI] [PubMed] [Google Scholar]
- Kong W., Merino E. & Nevado C. Arylphosphonylation and arylazidation of activated alkenes. Angew. Chem. Int. Ed. 53, 5078–5082 (2014). [DOI] [PubMed] [Google Scholar]
- Zeng Y., Ni C. & Hu J. Recent advances in the one-step synthesis of distally fluorinated ketones. Chem. Eur. J. 22, 3210–3223 (2016). [DOI] [PubMed] [Google Scholar]
- Chen Z.-M. et al. Copper-catalyzed tandem trifluoromethylation/semipinacol rearrangement of allylic alcohols. Angew. Chem. Int. Ed. 52, 9781–9785 (2013). [DOI] [PubMed] [Google Scholar]
- Bunescu A., Wang Q. & Zhu J. Copper-catalyzed cyanomethylation of allylic alcohols with concomitant 1,2-aryl migration: efficient synthesis of functionalized ketones containing an α-quaternary center. Angew. Chem. Int. Ed. 54, 3132–3135 (2015). [DOI] [PubMed] [Google Scholar]
- Yu P. et al. Enantioselective C-H bond functionalization triggered by radical trifluoromethylation of unactivated alkene. Angew. Chem. Int. Ed. 53, 11890–11894 (2014). [DOI] [PubMed] [Google Scholar]
- Yu P., Zheng S.-C., Yang N.-Y., Tan B. & Liu X.-Y. Phosphine-catalyzed remote β-C-H functionalization of amines triggered by trifluoromethylation of alkenes: one-pot synthesis of bistrifluoromethylated enamides and oxazoles. Angew. Chem. Int. Ed. 54, 4041–4045 (2015). [DOI] [PubMed] [Google Scholar]
- Wu Z., Ren R. & Zhu C. Combination of a cyano migration strategy and alkene difunctionalization: the elusive selective azidocyanation of unactivated olefins. Angew. Chem. Int. Ed. 55, 10821–10824 (2016). [DOI] [PubMed] [Google Scholar]
- Charpentier J., Früh N. & Togni A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem. Rev. 115, 650–682 (2015). [DOI] [PubMed] [Google Scholar]
- Studer A. & Curran D. P. The electron is a catalyst. Nat. Chem. 6, 765–773 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu R. & Buchwald S. L. Copper-catalyzed oxytrifluoromethylation of unactivated alkenes. J. Am. Chem. Soc. 134, 12462–12465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.-Y. & Qing F.-L. Copper-catalyzed three-component oxytrifluoromethylation of alkenes with sodium trifluoromethanesulfinate and hydroxamic acid. Angew. Chem. Int. Ed. 52, 14177–14180 (2013). [DOI] [PubMed] [Google Scholar]
- Szostak M. & Aubé J. Chemistry of bridged lactams and related heterocycles. Chem. Rev. 113, 5701–5765 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H. M. & Dong G. Cooperative activation of cyclobutanones and olefins leads to bridged ring systems by a catalytic [4+2] coupling. Nat. Chem. 6, 739–744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung N. & Bräse S. Vinyl and alkynyl azides: well-known intermediates in the focus of modern synthetic methods. Angew. Chem. Int. Ed. 51, 12169–12171 (2013). [DOI] [PubMed] [Google Scholar]
- Song W., Kozhushkov S. I. & Ackermann L. Site-selective catalytic C(sp2)-H bond azidations. Angew. Chem. Int. Ed. 52, 6576–6578 (2013). [DOI] [PubMed] [Google Scholar]
- Zhdankin V. V. et al. Preparation, X-ray crystal structure, and chemistry of stable azidoiodinanes derivatives of benziodoxole. J. Am. Chem. Soc. 118, 5192–5197 (1996). [Google Scholar]
- Müller K., Faeh C. & Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007). [DOI] [PubMed] [Google Scholar]
- Furuya T., Kamlet A. S. & Ritter T. Metal-catalysed cross-coupling reactions. Nature 473, 470–477 (2011).21614074 [Google Scholar]
- Eisenberger P., Gischig S. & Togni A. Novel 10-I-3 hypervalent iodine-based compounds for electrophilic trifluoromethylation. Chem. Eur. J. 12, 2579–2586 (2006). [DOI] [PubMed] [Google Scholar]
- Amrein S., Bossart M., Vasella T. & Studer A. Stereoselective radical aryl migration from silicon to carbon. J. Org. Chem. 65, 4281–4288 (2000). [DOI] [PubMed] [Google Scholar]
- Tomohara K., Yoshimura T., Hyakutake R., Yang P. & Kawabata T. Asymmetric α-arylation of amino acid derivatives by clayden rearrangement of ester enolates via memory of chirality. J. Am. Chem. Soc. 135, 13294–13297 (2013). [DOI] [PubMed] [Google Scholar]
- Jones G. O., Liu P., Houk K. N. & Buchwald S. L. Computational explorations of mechanisms and ligand-directed selectivities of copper-catalyzed Ullmann-type reactions. J. Am. Chem. Soc. 132, 6205–6213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.-Z., Jiang Y.-Y., Fu Y. & Liu L. Alternative mechanistic explanation for ligand-dependent selectivities in copper-catalyzed N- and O-arylation reactions. J. Am. Chem. Soc. 132, 18078–18091 (2010). [DOI] [PubMed] [Google Scholar]
- Tani K. & Stoltz B. M. Synthesis and structural analysis of 2-quinuclidonium tetrafluoroborate. Nature 441, 731–734 (2006). [DOI] [PubMed] [Google Scholar]
- Milligan G. L., Mossman C. J. & Aubé J. Intramolecular schmidt reactions of alkyl azides with ketones: scope and stereochemical studies. J. Am. Chem. Soc. 117, 10449–10459 (1995). [Google Scholar]
- Wei Y. & Shi M. Recent advances in organocatalytic asymmetric morita–baylis–hillman/aza-morita–baylis–hillman reactions. Chem. Rev. 113, 6659–6690 (2013). [DOI] [PubMed] [Google Scholar]
- Yet L. Metal-mediated synthesis of medium-sized rings. Chem. Rev. 100, 2963–3008 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary tables, supplementary notes, supplementary methods and supplementary references.
Calculated total energies and geometrical coordinates for structures.
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1451652 (4O), CCDC 1451653 ((R)-1A) and CCDC 1451654 ((R)-4A). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Any further relevant data are available from the authors on reasonable request.