Abstract
Klebsiella oxytoca is emerging as an important bacterial isolate causing hospital-acquired infection in adults and having multiple drug resistance to commonly used antibiotics. We analysed our data to observe the current pattern of drug resistance among K. oxytoca isolated from various samples during the period from January 2012 to March 2014 at a tertiary care hospital. A total of 17,335 samples were processed at the hospital laboratory during this period. Klebsiella was isolated from 654 of these clinical samples. Out of these Klebsiella species, 631 (96.48%) were K. pneumoniae and 23 (3.52%) were K. oxytoca. All the K. oxytoca isolates were sensitive to colistin and tigecyclin. However, these isolates showed 58% resistance to imipenem and meropenem. The resistance to gentamicin, amikacin and ceftriaxone was higher at 72%. Resistance to ciprofloxacin and aztreonam was less (58%). All the samples were from patients admitted to ICUs of a tertiary care centre. The data from our study show an increasing burden of infection caused by this bacterium, which is now gaining access to ICUs. It is recommended that Hospital Infection Control Committees must keep a close watch on the antibiotic pattern of K. oxytoca for better patient care.
Keywords: Klebsiella oxytoca, Antibiotic sensitivity, Emerging pathogen
Introduction
Klebsiella species are becoming an important pathogen of humans, and are being implicated in increasing morbidity amongst the patient population. Normally found in bowel of man and animals, water and soil, infections with these bacteria are leading to prolonged stays in hospitals. Multiple comorbidities and compromised immune status along with exposures to multiple antibiotics are main factors that increase the risks for infections and drug resistance. Colonization of the respiratory and the gastro-intestinal tract is common in hospitalised patients. Thus, Klebsiella species are often a cause of bronchopneumonia, urinary tract infection and septicaemia in admitted patients. This bacterium has the ability to cause outbreaks of nosocomial infection as it often exchanges plasmid-borne resistance with other bacteria, which are more common at tertiary and specialized centres. Klebsiella pneumoniae is the single, predominant species gaining more significance as it is developing multidrug resistance at tertiary care centres similar to Pseudomonas and Acinetobacter. Among the Klebsiella, K. oxytoca is now being isolated more frequently. This was earlier named Bacterium oxytocum by Flugge in 1886 and is an organism that forms indole, has a positive Voges–Proskauer reaction and liquefies gelatine along with other features of Klebsiella species. K. oxytoca is normally acquired from environmental sources. Some previous studies have concluded its pathogenic significance in sputum and blood culture in a few patients, while uncertain in others, more so in respiratory infections.1, 2
K. oxytoca has been isolated from different clinical samples mainly from the blood and respiratory secretions, and is gaining clinical significance in immunocompromised and debilitated patients admitted in Intensive Critical Care Units (ICUs). Further, different mechanisms are involved in development of multidrug resistance. These include production of extended-spectrum β-lactamases (ESBLs), AmpC lactamases, Klebsiella pneumonia carbapenemase (KPC) and aminoglycosides-modifying enzymes. Modi et al. have reported 89% ESBL-producing K. pneumoniae isolates on screening done in a neonatal care unit at a tertiary care centre.3 KPC are encoded by the gene blaKPC, which has the potential to get transferred from one species to other Gram-negative bacteria.
These KPC-producing bacteria have been found to be less sensitive to aminoglycosides and quinolones. Production of metallo-β-lactamases by these bacteria, on a highly mobile gene, is further limiting the use of all β-lactam agents. Thus, they are challenging modern treatment options being offered by tertiary care hospitals by becoming resistant to colistin by modulation of mgrB gene.4
Material and methods
The present study was carried out by analysing the data of all the clinical isolates of Klebsiella from January 2012 to March 2014 at a tertiary care hospital. Samples received from the wards were directly inoculated on Blood agar, MacConkey Agar, CLED and BacT/Alert culture bottles. Further identification was done as per routine laboratory protocols. Antimicrobial susceptibility testing was done in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines. Antibiotics disks included in this study, by Kirby–Bauer's disk diffusion method, were Ceftriaxone (30 μg), Ceftazidime (30 μg), Cefotaxime (30 μg), Aztreonam (30 μg), Gentamicin (10 μg), Amikacin (30 μg) Ciprofloxacin (5 μg), Imipenem (10 μg), Meropenem (10 μg), Tigecyclin (15 μg) and Colistin (10 μg) (Hi-Media Laboratories). These isolates were simultaneously processed by Vitek 2 System (Biomerieux), which gave sensitivity results of relevant antibiotics along with MIC values.
The screening tests and confirmatory tests for ESBL producers were done as per CLSI recommended methodology. Isolates showing inhibition zone of ≤22 mm for Ceftazidime (30 μg) (MIC ≥16 μg/ml), ≤27 mm for Cefotaxime (30 μg)(MIC ≥4 μg/ml) and ≤27 mm for Aztreonam (30 μg) (MIC ≥16 μg/ml) were taken for ESBL confirmation. The confirmation test was done by CLSI phenotypic disk confirmatory tests using disks of ceftazidime (30 μg) and ceftazidime–clavulanic acid (30 μg/10 μg). A zone difference of ≥5 mm around ceftazidime and ceftazidime–clavulanic acid was taken as ESBL positive. K. pneumoniae ATCC 700603 and Escherichia coli ATCC 25922 were used as positive and negative controls, respectively. Ertapenem (10 μg) is being considered the best carbapenem for detection of KPC resistance. Isolates showing inhibition zone of ≤18 mm and MIC ≥4 μg/ml were being considered positive for KPC. A few isolates were processed with the Modified Hodge test (MHT).
Results
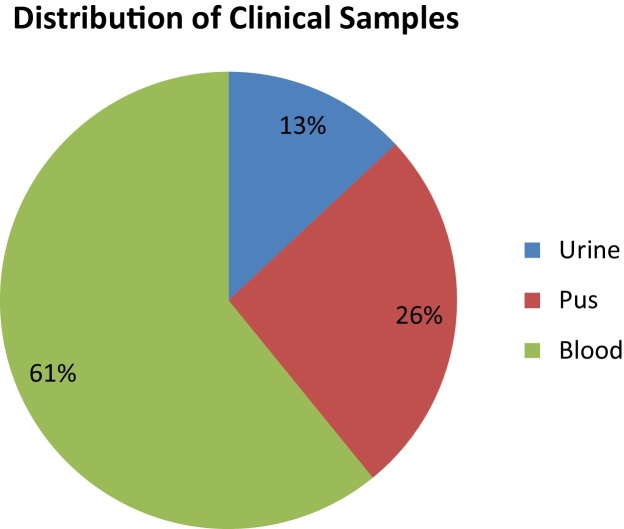
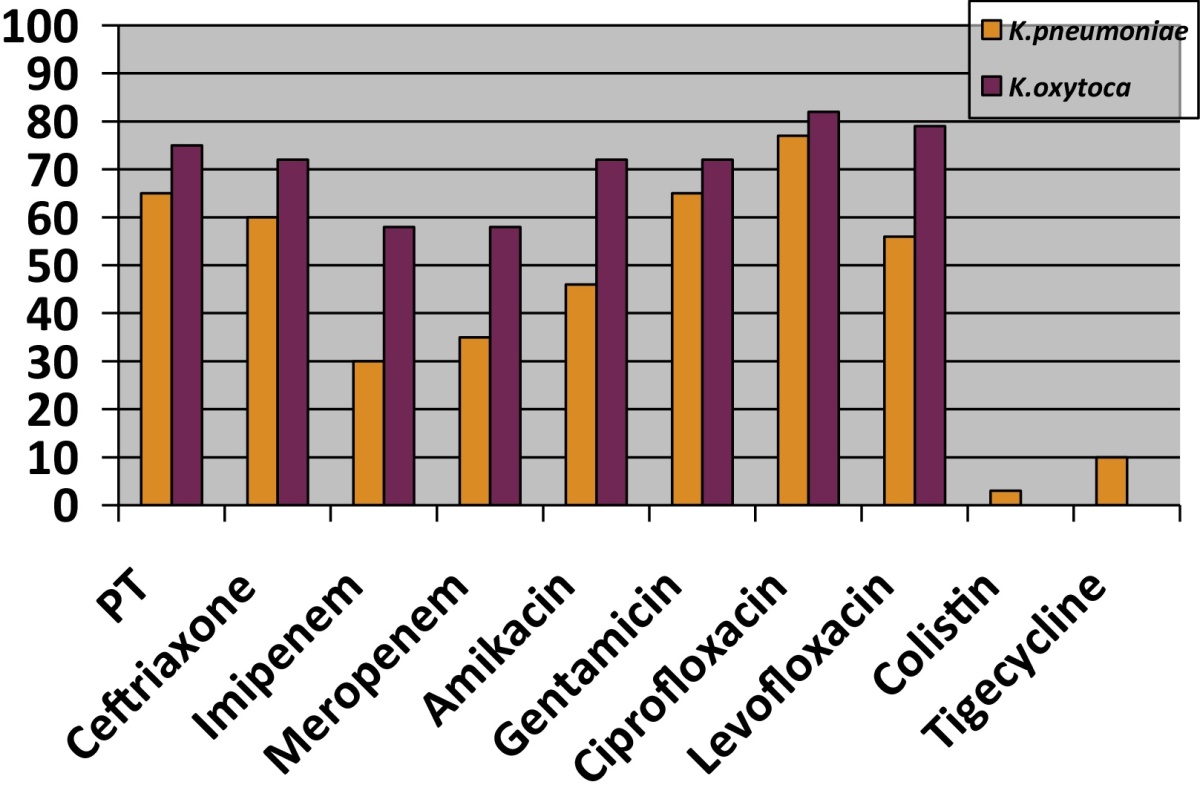
A total of 17,335 samples were processed at the hospital laboratory during the study period. Klebsiella was isolated from 654 of these clinical samples. Out of these Klebsiella species, 631 (96.48%) were K. pneumoniae and 23 (3.52%) were K. oxytoca. The distribution of these K. oxytoca isolates is given in Fig. 1. All these patients were admitted in the ICU, were critically ill and were already on broad-spectrum antibiotics. Minimum two samples were processed to confirm the bacterial isolates. Antibiotic sensitivity pattern was studied (Table 1), and it was found that all the K. oxytoca isolates were sensitive to colistin and tigecyclin. However, these isolates showed 58% resistance to imipenem and meropenem as compared to K. pneumoniae, which showed only 30% resistance. 72% resistance to Gentamicin, Amikacin, Ceftazidime and Ceftriaxone was observed. Sensitivity to ciprofloxacin and aztreonam was better (42%). Of these Klebsiella isolates, 58% were ESBL and KPC producers as compared to K. pneumoniae (30%).
Fig. 1.
Klebsiella oxytoca (n = 23).
Table 1.
Antibiotic resistance pattern of K. pneumoniae and K. oxytoca.
Discussion
Enterobacteriaceae are the leading causes of nosocomial infections, with Klebsiella being the second leading bacteria after E. coli. Majority of Klebsiella infections are caused by K. pneumoniae. There are mainly case reports and small case studies about K. oxytoca. In our study, we had 23 patients who had K. oxytoca infection. Earlier, it was only pseudomonas that showed high resistance to commonly used antibiotics, but now along with Acinetobacter, Klebsiella species are rapidly developing multidrug resistance. Klebsiella are opportunistic pathogens that cause severe diseases in hospital setting. This organism causes pneumonia, urinary tract infection, soft tissue infection and septicaemia, which often leads to septic shock.2 K. oxytoca has been isolated more frequently from neonatal ICUs in the past, but now it is being isolated even from various samples of adult patients admitted at critical care centres. It is showing multidrug resistance and is showing higher drug resistance as compared to K. pneumoniae. In our study, K. oxytoca showed higher resistance (75%) to piperacillin/tazobactam as compared to K. pneumoniae (65%). Mechanism of piperacillin/tazobactam resistance sometimes differs between K. pneumoniae and K. oxytoca. Identified ESBLs amongst K. pneumoniae isolates were CTX-M-2, -3, -14 and -15 and SHV-12, while OXY-2 was conferred to K. oxytoca. These give different sensitivity patterns. CTX phenotype pattern has better sensitivity to piperacillin/tazobactam, while OXY-2 ESBLs reduce sensitivity to piperacillin/tazobactam.5 ESBL production by K. pneumoniae has been found variable and is being reported up to 96%.3
In our study, 58% of K. oxytoca isolates were KPC producers, as compared to K. pneumoniae (30%). Similar results were observed in another study were reported meropenem resistance was up to 58%.6 Hoenigl et al. have described a nosocomial outbreak of KPC-producing K. oxytoca, highlighting the clinical importance of infection with this microorganism.7 K. oxytoca infected individuals may remain asymptomatic; however, it is still considered to be an opportunistic pathogen. The clinical significance of this is now recognized by its association with nosocomial infections in cohorts of hospitalized patients, including children and neonates.8, 9, 10 KPC-producing K. pneumoniae are responsible for higher mortality as compared to non-KPC producers.11 We observed three isolates of K. pneumoniae that were resistant to colistin and 10 isolates showed resistance to tigecyclin, while none of K. oxytoca showed any resistance to these two antibiotics. In vitro susceptibility to polymyxins among clinical KPC-producing isolates ranges from 90 to 100%.12
Aminoglycosides are an important treatment option for KPC-producing organisms. It has been found that the production of carbapenemases is the most important molecular mechanism of such resistance, both epidemiologically and clinically. The data from our study show an increasing burden of infection caused by this bacterium, which is now gaining access to ICCUs, which needs to be corroborated by studies across more centres. Of clinical significance is that the organism appears to become resistant to a range of antibiotics; however, our study found that resistance had not yet set in to colistin and tigecyclin. However, pan-resistant bacteria have been reported that are resistant to tigecycline, polymixin and aminoglycosides besides carbapenems.
Active surveillance of the Gram-negative pathogenic bacteria K. oxytoca and Klebsiella pneumoniae that produce ESBL and carbapenemases enzymes is an essential function of hospital laboratories. Further, K. oxytoca has shown reduced sensitivity to the widely used antiseptic, chlorhexidine.13 This will undoubtedly increase the further burden of such infections in the future. Hospital Infection Control Committees must keep a close watch on K. oxytoca amongst other microorganisms of concern.
Conflicts of interest
The authors have none to declare.
References
- 1.Power J.T., Calder M.A. Pathogenic significance of K. oxytoca in acute respiratory tract infection. Thorax. 1983;38(3):205–208. doi: 10.1136/thx.38.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagiwara S., Murata M., Aoki M., Kaneko M., Oshima K. Septic shock caused by K. oxytoca: an autopsy case and a survival case with driving Extracorporeal Membrane Oxygenation. Hippokratia. 2013;17(2):171–173. [PMC free article] [PubMed] [Google Scholar]
- 3.Modi D.J., Patel B.V., Patel M.H. A study of extended spectrum beta lactamases (ESBL) and AmpC beta lactamase producing K. pneumoniae in neonatal intensive care unit at tertiary care hospital, Ahmedabad. Natl J Community Med. 2012;3:523–528. [Google Scholar]
- 4.Jayol A., Poirel L., Villegas M.V., Nordmann P. Modulation of mgrB gene expression as a source of colistin resistance in K. oxytoca. Int J Antimicrob Agents. 2015;46(July (1)):108–110. doi: 10.1016/j.ijantimicag.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Sato T., Hara T., Horyama T. Mechanism of resistance and bacterial susceptibility in extended spectrum beta lactamases phenotype Klebsiella pneumoniae and Klebsiella oxytoca isolated between 2000 and 2010 in Japan. J Med Microbiol. 2015;64:538–543. doi: 10.1099/jmm.0.000057. [DOI] [PubMed] [Google Scholar]
- 6.Gajul S.V., Mohite S.T., Mangalgi S.S. Klebsiella pneumoniae in septicemic neonates with special reference to extended spectrum β-lactamase, AmpC, metallo β-lactamase production and multiple drug resistance in tertiary care hospital. J Lab Physicians. 2015;7:32–37. doi: 10.4103/0974-2727.151689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoenigl M., Valentin T., Zarfel G. Nosocomial outbreak of Klebsiella pneumoniae carbapenemase-producing Klebsiella oxytoca in Austria. Antimicrob Agents Chemother. 2012;56(4):2158–2161. doi: 10.1128/AAC.05440-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savino F., Cordisco L., Tarasco V. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr. 2009;98:1582–1588. doi: 10.1111/j.1651-2227.2009.01419.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann K.M., Deutschmann A., Weitzer C. Antibiotic-associated hemorrhagic colitis caused by cytotoxin-producing Klebsiella oxytoca. Pediatrics. 2010;125:e960–e963. doi: 10.1542/peds.2009-1751. [DOI] [PubMed] [Google Scholar]
- 10.Savino F., Cordisco L., Tarasco V. Antagonistic effect of Lactobacillus strains against gas-producing coliforms isolated from colicky infants. BMC Microbiol. 2011;11:157. doi: 10.1186/1471-2180-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel G., Huprikar S., Factor S.H. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [PubMed: 18973455] [DOI] [PubMed] [Google Scholar]
- 12.Bratu S., Tolaney P., Karumudi U. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005;56:128–132. doi: 10.1093/jac/dki175. [PubMed: 15917285] [DOI] [PubMed] [Google Scholar]
- 13.Vali L., Dashti A.A., El-Shazly S., Jadaon M.M. Klebsiella oxytoca with reduced sensitivity to chlorhexidine isolated from a diabetic foot ulcer. Int J Infect Dis. 2015;34:112–116. doi: 10.1016/j.ijid.2015.03.021. [DOI] [PubMed] [Google Scholar]