Abstract
The levels and roles of lipid mediators can be modified in response to nutritional stimuli. The aim of this study was to investigate shifts in oxylipin and sphingolipid profiles stimulated by a hypercholesterolemic (HC) diet along with the modulating effects of onion introduced as an antioxidant functional ingredient characterized in the diet (HCO). Oxylipin and sphingolipid profiles were determined in plasma and tissues from Wistar rats using LC-MS/MS. Plasma ω-3 and ω-6 PUFA-derived oxylipins decreased in rats after 7 weeks of HC feeding, but did not evidence a further shift with HCO diet. Onion ingredient supplementation modulated the hepatic concentrations of prostaglandins and enhanced ω-3 oxylipins in the liver of HCO-fed rats relative to the HC group. The HC diet induced shifts in plasma sphingolipids, increasing sphingoid bases, dihydroceramides and ceramides, whilst the sphingomyelin, hexosylceramide and lactosylceramide families decreased. The HCO diet modified some HC diet-induced changes in sphingolipids in liver and spleen tissue. Onion supplementation effected changes in lipid mediator levels in diet-induced hypercholesterolemic Wistar rats. The potential of onion as regulator of pro-inflammatory mediators, and possible enhancer of pro-resolution pathways, warrants further study of the interaction of functional ingredients with bioactive lipid mediators and their potential impact on inflammation, oxidative stress and organ dysfunction.
Keywords: Eicosanoids, Inflammation, Lipid profiling, Mass spectrometry, Oxylipins, Sphingolipids
Graphical abstract
Highlights
-
•
Onion supplementation ameliorated HC diet-related changes in hepatic prostaglandins.
-
•
HCO feeding increased ω-3-derived oxylipins in the liver of hypercholesterolemic rats.
-
•
Plasma sphingolipids reflected a characteristic pattern induced by the HC feeding.
-
•
The onion ingredient modulated specific sphingolipids within the liver and spleen.
-
•
The study of lipid mediators is useful to characterize the impact of diets on oxidative stress and inflammation.
1. Introduction
Specific dietary patterns have significant implications in the development of multiple alterations directly associated with inflammatory events [1], [2]. Inflammation, both acute and chronic, is triggered as a response to different disorders, including autoimmune, metabolic, intestinal or homeostatic imbalances that can be found in a large variety of diseases [3], [4], [5]. Several factors, including the genetic component as well as the microbial and environmental conditions, have been suggested to influence its initiation, progression and resolution [5]. In addition, there is a growing interest in dietary components as key antioxidants and inflammatory regulators [6], [7], [8], [9], [10]. However, the difficulty to define the direct effect of individual dietary components on certain pathologies and their translation on human health is well recognized [11], [12], [13], [14].
Hypercholesterolemia and inflammation are known to be closely linked processes, and they are clearly implicated in non-alcoholic fatty liver disease and atherosclerosis development [15]. Dietary cholesterol intake is known to induce an elevation of lipids and stimulate oxidative stress, which lead or promote the action of pro-inflammatory signaling cascades [16], [17], [18]. On the other hand, the bioactive nature of some foods like onion (Allium cepa L.), which is an important dietary source of antioxidant and anti-inflammatory compounds, has been related to the modulation of such cascades and could reduce the production of pro-inflammatory mediators. The discoveries about the inhibitory effect of garlic and onion exerted on the conversion of arachidonic acid (AA) into eicosanoids metabolism were summarized by Ali et al. [19]. More recently, another study also evidenced the potential activity of some compounds found in small yellow onion on the modulation of cyclooxygenase-1 (COX-1) and 12-lipoxygenase (12-LOX) activity [20]. In a recent report, Suleria et al. [21] stated that onion-derived phenolic compounds like flavonols and organosulfur compounds (specially thiosulfinates) play relevant anti-inflammatory effects, showing interactions to the inhibition of arachidonic acid metabolic pathways.
Oxylipins in general have attracted great interest as oxidation products of fatty acids such as arachidonic acid (AA), linoleic acid (LA), α-linolenic acid (α-LA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). This process is mainly initiated via three enzymatic pathways: cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP). It is well accepted that the levels of oxylipins in the open circulation and tissues may give insight into their role in the response to different physiological abnormalities, including a disequilibrium of the lipid levels, and different metabolic stresses accompanied by inflammation [22]. Likewise, sphingolipids are an important class of structural components and signaling molecules within the cell, whose metabolism can also be modified by constituents of the diet, such as cholesterol, with consequences for cell regulation and disease [23]. The biochemical synthesis of sphingolipids and their regulation are involved in the response processes of pathogenesis of metabolic and cardiovascular dysfunctions (e.g., type 2 diabetes, insulin resistance, obesity, metabolic syndrome, atherosclerosis and cardiomyopathy) [24]. Moreover, sphingolipids participate in multiple cellular signaling pathways such as the responses to cytokines and stress [25].
In addition, the presence and abundance of certain lipids in a specific tissue may give insight about the impact of pathological damage. This may lead to the discovery of new markers of injury and possible diagnosis when released and detected in blood [26], [27]. However, there are not many studies about the effect of functional food ingredients on the synthesis and actions of oxylipins and sphingolipids.
Recent findings by our group have shown that the consumption of onion processed for use as a functional ingredient, induced changes in the circulating fatty acids [28] and the recovery from the oxidative damage caused by a cholesterol overload in the antioxidant and vascular status of hypercholesterolemic Wistar rats [29]. Nonetheless, the functionality of this onion product as a powdered dietary ingredient on the modulation of signaling bioactive lipid mediators and its possible connection with findings addressing vascular benefits still remains unknown. Therefore, the aim of the present study was to determine the impact of diet enrichment with onion in plasma and tissue oxylipin and sphingolipid levels of hypercholesterolemic Wistar rats using ultra performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) targeted approaches.
2. Materials and methods
2.1. Onion ingredient preparation
Onions (Allium cepa L. var cepa, ‘Recas’) supplied by Cebacat (Asociación Catalana de Productores-Comercializadores de Cebolla) were harvested in Lleida (Spain) and stored at 4 °C until processing. Onions free from external damages were hand-peeled, cut into 10 mm diced-pieces, packaged in bags with very low gas permeability (Doypack®, Polyskin XL, Amcor Flexibles Hispania, S.L., Granollers, Barcelona, Spain) and treated with high-pressure to obtain a stable functional ingredient as previously described [30]. Briefly, the high-pressure treatment (400 MPa, 5 min, 25 °C) was applied in a High Pressure Iso-Lab System [High Pressure Iso-Lab System (model FPG7100:9/2C, Stansted Fluid Power Ltd., Essex, UK)]. The onion processed was directly frozen with liquid nitrogen and freeze-dried using a lyophilizer (model Lyoalfa, Telstar, S.A., Barcelona, Spain). A subsequent pulverization of the lyophilized diced onion was carried out with an ultra centrifugal mill ZM 200 (Retsch GmbH, Haan, Germany) obtaining a particle size ≤250 µm. The obtained onion powder was stored at −20±0.5 °C until dosage for the formulation of the diets. The nutritional and phytochemical composition, and antioxidant activity of the onion ingredient is presented in Supplementary Table S1.
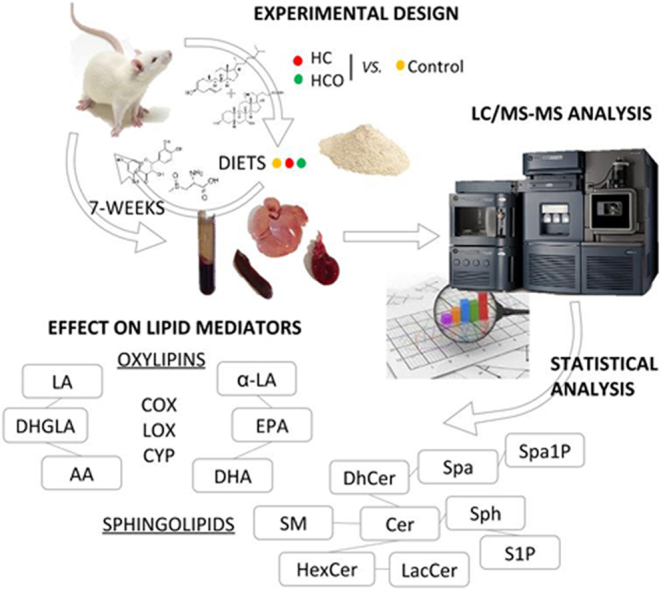
2.2. Experimental design
The present study was performed in compliance with the Directive 2010/63/UE regarding the protection of animals used for scientific purposes and was approved by the Spanish Ministry of Science and Innovation Advisory Committee [project AGL2010-15910 (subprogram ALI)] and by an Ethics Committee of the Complutense University of Madrid (Spain).
Eight weeks-old male Wistar rats (n=24) with a weight of 245±5 g were supplied by Harlan Laboratories Models (Harlan, SL, Barcelona, Spain) and transferred to the Faculty of Medicine in the Complutense University of Madrid (Spain) to conduct the experiments. Animals were acclimatized for 1 week and then randomly assigned to 3 experimental groups in individual metabolic cages placed under controlled conditions of light (12 h light/dark), and temperature (22±1 °C). Each group (n=8) received water ad libitum and had free access to one of the following three diets: control (C) diet, composed of a homogeneous mixture of 100% rodent diet (based on the AIN-93M diet); high-cholesterol (HC) diet, composed of control diet with 2% cholesterol and 0.5% cholic acid and high-cholesterol enriched with onion (HCO) diet was identical to the HC diet, but with 10% onion powder, balancing the dietary fibre with cellulose powder. The amount of maize starch in the HC and HCO diets was adjusted to compensate for the addition of cholesterol and cholic acid in the HC diet, and onion powder in the HCO diet. The exact composition of each diet can be found in Supplementary Table S2.
Animal feeding and water was daily replaced to monitor a normal rate of consumption, deposition of feces and urine throughout the 7 weeks of experimental feeding. Body weight was weekly evaluated ensuring the correct growth of all animals until euthanasia.
2.3. Blood and tissues sampling
Animals in fasting conditions were anaesthetized and euthanized by extracting blood by cardiac puncture until exsanguination, taking randomly one animal at a time, of each one of three groups. Blood collection was carried out in BD Vacutainer® tubes containing EDTA as anticoagulant. Plasma was obtained from each sample by centrifuging at 1,500g for 15 min at 4 °C and stored at −80 °C in aliquots.
Organ collection of heart, liver and spleen was conducted in aseptic conditions just after exsanguination. The organs of each animal were carefully collected and fat was removed. Dissected parts were individually frozen in liquid nitrogen and immediately stored at −80 °C.
Homogenates from laminar samples of the heart, the central lobe of liver and the spleen were prepared for extraction and analysis. Approximately, 50 mg and 20 mg of tissue (for oxylipins or sphingolipids, respectively) were weighed and homogenized in 200 µL of methanol with a Bullet Blender®. Homogenization was performed using 0.5 mm Zirconium Oxide Beads (liver and spleen tissues) or Stainless Steel Beads 0.9–2.0 mm (heart tissues). In order to avoid inter assay variations that could affect the comparison of data from different groups, all samples of the same type of tissue were manipulated on dry ice, weighed in a controlled room temperature (4 °C) and kept in Eppendorf® tubes at −80 °C the same day. Then, each set of samples was extracted separately and analyzed on consecutive days.
2.4. UPLC-MS/MS determination of oxylipins
Extraction of oxylipins from plasma and liver homogenate was carried out as previously described [31] with minor modifications. Briefly, 10 µL of internal standards solution were spiked to 250 µL of plasma or 50 mg of liver tissue homogenate. After dilution of samples with 2.5 mL of 0.1% acetic acid in water, solid phase extraction was performed using Oasis HBL 60 mg cartridge columns (Waters, Milford, MA) [31] and dry extracts were stored at −80 °C for a maximum of two days. On the day of analysis, extracts were reconstituted in 100 µL of MeOH and filtered using 0.1 µm membrane spin filters (Merck Millipore, Darmstadt, Germany). The chromatographic separation was performed on an Acquity UPLC separation module (Waters) equipped with a 2.1x150 mm BEH C18 column with a 1.7 µm particle size (Waters). Oxylipins were determined using a Xevo TQ-S mass spectrometer (Waters). Chromatographic and mass spectrometry parameters are detailed elsewhere [31]. A list of detected oxylipins and their SRM transitions is provided in Supplementary Table S3.
2.5. UPLC-MS/MS determination of sphingolipids
Ceramides, sphingomyelins, hexosylceramides, lactosylceramides and dihydroceramides from the NS (non-hydroxylated/sphingosine) class were determined in plasma, liver, heart and spleen homogenates of Wistar rats as previously described [32] with slight modifications. Briefly, 10 µL of internal standard containing solution were added to 100 µL of plasma or 20 mg of tissue homogenate. Samples were then extracted following a modified Bligh and Dyer procedure by sequentially adding 380 µL of CHCl3:MeOH (1:2), 125 µL of CHCl3 and 125 µL of H2O for plasma. In the case of tissues, 100 µL of H2O, 130 µL of CHCl3, 50 µL of MeOH, 125 µL of CHCl3 and 125 µL of H2O were sequentially added. After each addition, samples were vortexed. Then, samples were centrifuged for 15 min at 10,000g, the CHCl3 layer was transferred to a new vial where it was reduced to dryness in a SPEEDVAC® Concentrator from Genevac (Ipswich, UK) and stored at −80 °C until analysis. On the day of analysis, extracts were reconstituted in MeOH, filtered using 0.1 µm membrane spin filters and analyzed using UPLC-MS/MS as previously described [32]. For plasma only, 100 µL spiked with 10 µL of internal standard mix, were precipitated with 500 mL of MeOH and 250 µL of CH2Cl2. After vortexing and centrifugation for 15 min at 10,000g, the supernatant was transferred to another vial and brought to dryness in a SPEEDVAC® Concentrator. Samples were reconstituted in MeOH:H2O (75:25), filtered using 0.1 µm membrane spin filters and 7.5 µL were injected onto the UPLC-MS/MS system. For both sphingolipid platforms, chromatographic and mass spectrometry details have been published elsewhere [32].
2.6. Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics Version 22 (SPSS Inc., Chicago, IL, USA) and Graph Pad Prism 5.0 for Windows (GraphPad Software, San Diego, CA, USA). Normality was tested via a Shapiro-Wilk test. One-way ANOVA with Tukey's post hoc comparisons and Kruskal-Wallis test with Dunn's post hoc comparisons were used for multiple group comparisons of normally and non-normally distributed data, respectively. Q-values were calculated using the Storey method [33], only compounds presenting a significant p-value (<0.05) and q-value were considered as significant.
3. Results and discussion
3.1. Animal growth and biochemical parameters
The main effects of the HC and HCO diets on animal growth and other physiological and biochemical parameters are summarized in Supplementary Table S4. Further details of the effect of the onion ingredient on antioxidant status, lipid profile and other metabolic routes of the hypercholesterolemic model of Wistar rats can be found elsewhere [28], [29], [34].
3.2. Oxylipin metabolism
Oxylipins are bioactive lipids mostly de novo synthesized by the oxidation of polyunsaturated fatty acids (PUFAs). These PUFAs are normally stored in membrane-bound phospholipids, and are released by the action of phospholipases following an enzymatic conversion into biologically active derivatives through COX, LOX and CYP routes. However, the metabolism of oxylipins can also be modified by diet constituents such as the intake of cholesterol or the consumption of specific fatty acids. Hypercholesterolemia has been associated with a major synthesis of thromboxane A2 (TxA2) in atherosclerotic lesions [35], [36] and recent in-depth studies regarding the metabolic syndrome, diabetes and other disorders have also suggested the emerging role of lipid mediators [37], [38]. Thus, each pathological condition has a characteristic pattern of inflammatory response and corresponding resolution, and failures in these mechanisms can lead to chronic inflammation.
The fact that feeding supplementation with onion might cause a differential inhibition on COX and LOX activities has been previously postulated by analyzing the effects of different onion preparations on platelet aggregation and the subsequent eicosanoid synthesis [19]. However, the effects of onion-derived products supplementation on the oxylipin profile have never been explored.
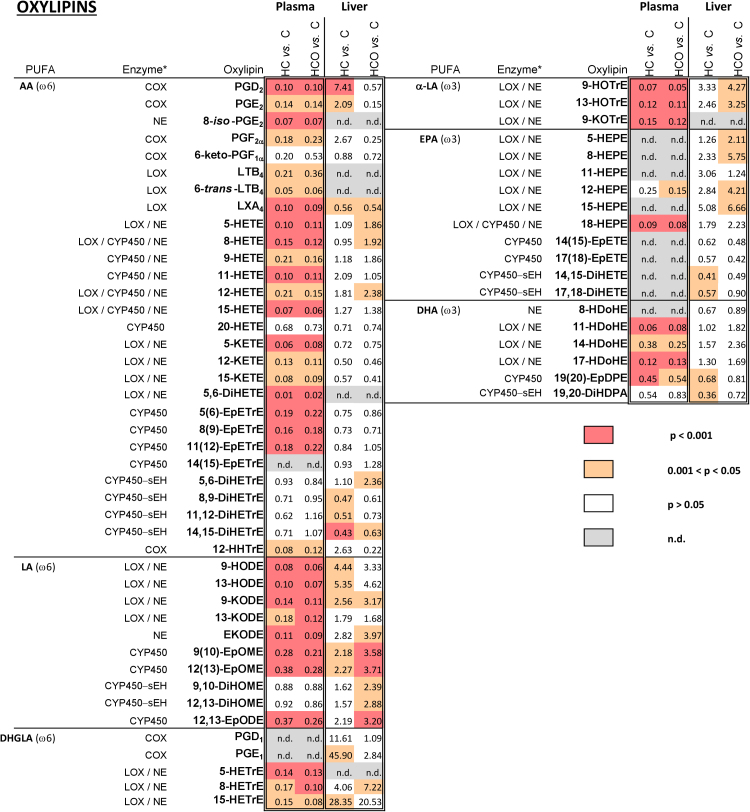
In the present study, a total of 62 oxylipins (shown in Supplementary Table S3) derived from AA, LA, DHGLA, α-LA, EPA and DHA metabolic pathways were detected and quantified in the plasma and livers from Wistar rats fed with the three different diets. The comparison between HC and C groups showed a shift in the oxylipin profile induced after 7 weeks of cholesterol and cholic acid intake. Besides, the comparative analysis of the HCO group and the C group evidenced either the preventive or enhancer effect exerted by the supplementary use of the onion ingredient in the oxylipin profile. As the effect of onion enrichment (HCO group) relative to the HC group may be subtle, the significance and absolute values of the changes produced by feeding either HC or HCO vs. C group are the key to elucidate the prevention exerted by the functional ingredient. Quantified metabolites in both plasma and liver tissues are shown in Fig. 1, where the fold-change obtained between the HC and HCO groups relative to C group is shown, together with their respective precursor PUFA and enzymatic pathway.
Fig. 1.
Changes in oxylipins found in plasma and livers of Wistar rats after 7 weeks of experimental feeding. Control (C), high-cholesterol (HC) and high-cholesterol enriched with onion (HCO). Values greater and lower than 1 represent either an increase or a decrease relative to the control, respectively. n.d.: not determined. *Main enzymes/pathway responsible for oxylipin formation. In the absence of chiral determination, it is not possible to conclusively identify the synthetic source of the mono-hydroxy derivatives. Both potential enzymatic and non-enzymatic synthetic routes are indicated. AA: Arachidonic acid; LA: Linoleic acid; DHGLA: Dihomo-gamma-Linolenic acid; a-LA: alpha-Linolenic acid; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; COX: Cyclooxygenase; LOX: Lipoxygenase; CYP450: Cytochrome P450; NE: Non-enzymatic oxidation; sEH: soluble epoxide hydrolase. An oxylipin nomenclature list is provided in Supplementary Table S3.
3.2.1. Decreases in HC diet-induced circulating oxylipins are not ameliorated by onion supplementation
In the present study, a generalized decrease of AA (20:4n-6)–derived oxylipins was observed in the plasma of hypercholesterolemic rats, whether fed or not with onion supplementation (HC-fed and HCO-fed rats – Fig. 1). This is in accordance with the decrease in the plasma AA levels of HC- and HCO-fed rats found in the same animals previously reported [28], evidencing a possible connection among the circulating levels of AA and the phospholipid-bound AA derived products.
Likewise, the same trend was found for ω-6 oxylipins generated from LA and DHGLA. LA (18:2n-6) is converted into GLA (18:3n-6), which is a precursor for DHGLA (C20:3n-6) and can be converted to AA (20:4n-6); both DHGLA and AA can be used as substrates of the enzyme COX. DHGLA can also be converted by COX-1/2 to prostaglandins of the 1-series (PGE1) and/or metabolized by the 15-LOX into 15-(S)-hydroxy-8,11,13-eicosa-trienoic acid (15-HETrE), which have been described to be involved in the suppression of chronic inflammation [39]. In this study, LA and DHGLA derived metabolites were significantly decreased in plasma by feeding the HC diet, with the exception of the diols 9,10-DiHOME and 12,13-DiHOME, whose decreased was less prominent.
In addition, ω-3 fatty acids are associated with the prevention and modulation of multiple diseases, including the improvement of inflammatory responses [40]. The effects of EPA (20:5n-3) and DHA (22:6n-3) have been linked with the attenuation of atherosclerosis, the stabilization of atherosclerotic plaques and the reduction of cardiovascular disease risk. However, a better understanding of the variations in the metabolism of these ω-3 and their oxylipin products under different pathological circumstances is needed. In this case, the hypercholesterolemic rats also evidenced significantly lower plasma concentration of α-LA derivatives (9-HOTrE, 13-HOTrE and 9-KOTrE), EPA derivatives (12- and 18-HEPE) as well as both the non-enzymatic/LOX products (11-, 14- and 17-HDoHE) and CYP450 metabolized DHA derivatives (19(20)-EpDPE and 19,20-DiHDPA). Thus, feeding the HCO diet to the rats neither produced nor attenuated significant changes induced in plasma ω-3 oxylipins by the HC diet.
On balance, all these results show that HC feeding modified both the fatty acid pattern and its related oxylipins profile. However, the onion ingredient did not affect the observed decreases in circulating oxylipins in the HC-fed rats. A limitation in the results is that it is possible that responses in the oxylipin pattern found in this hypercholesterolemic model may not be reproduced in other species (e.g. [41],). Thus, the modulation of oxylipins induced by supplementation with onion should be studied for each individual model, taking also into account the specific influence and response to the basal diets.
3.2.2. Increase induced by HC diet in liver COX metabolites are ameliorated by onion supplementation
Certain AA-derived eicosanoids presented an opposite trend relative to their parent fatty acid in liver (Fig. 1 [28];). Some of these oxylipins also presented an opposite trend compared with plasma after both HC and HCO diet consumption (e.g., PGD2, PGE2, 5-, 11-, 12- and 15-HETEs, 5,6-DiHETrE and 12-HHTrE).
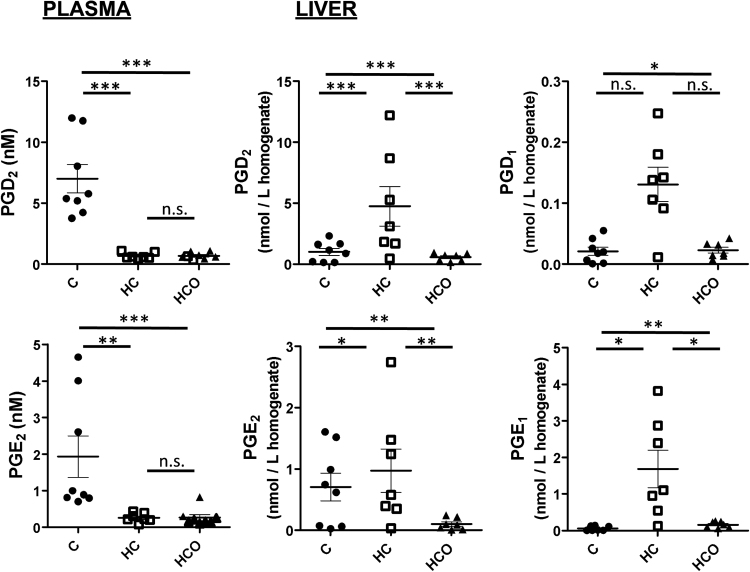
PGD2, PGE2 and TxA2 are known to be the main PGs synthesized by the Kupffer cells present in liver [42], and their activation has been related to the initiation of both a phagocytic and an inflammatory response, involving the release of ROS and various mediators of cell injury such as cytokines [43]. The inflammatory process triggered in liver of HC-fed rats led to higher PGD2 and PGE2 concentrations, while the use of the onion ingredient attenuated their concentrations in the HCO-fed group (Fig. 2). Moreover, the increased levels of PGD1 and PGE1, as homologous COX metabolites derived from DHGLA, detected in the HC group were also significantly prevented by the intake of the onion ingredient (Fig. 2). However, as livers were not perfused during tissue collection, platelet activation could not be outruled as the source of these changes. This drawback in sample collection needs to be considered when interpreting the results as the activation of the blood cells could have resulted in a release of PGE2 and PGD2. Nonetheless, differences observed between groups of samples and the opposite trend measured in plasma for the same compounds suggest that detected changes origin from the liver.
Fig. 2.
Comparison of prostaglandins levels found in plasma and liver tissues from Wistar rats after 7 weeks of experimental feeding. Control (C), high-cholesterol (HC) and high-cholesterol enriched with onion (HCO). *P<0.05; **P<0.01; ***P<0.001; n.s.: not significant.
The endogenous synthesis of these PGs is known to be mediated by the activity of the COX enzymes, emphasizing the pro-inflammatory role of the isoform COX-2, which is rapidly induced by inflammatory and mitogenic stimuli [44]. In addition, the transcription factor nuclear factor-κB (NF-κB), which is known as a key player in the expression of atherogenic and inflammatory genes such as the COX-2 gene, can be activated by ROS and oxidized LDL. Thus, a common link might be established between the inhibition of NF-κB, the subsequent COX-2 activity and the final PGs concentration. In support of these findings, the ability of lipophilic extracts and oils from onion inhibiting the fatty acid oxygenase has been mentioned previously [45], [46]. However, insufficient studies have been performed to date to support the use of onion in the diet as a suitable natural COX inhibitor. Moreover, the examination of fistular onion stalk extracts and aqueous extracts of onion showed the inhibition of several inflammatory signaling pathways including the NF-kB [47], [48].
Thus, the present study provides new insights about the effects of onion supplementation in the hepatic concentrations of PGs in diet-induced hypercholesterolemic Wistar rats.
Furthermore, an increased concentration for all measured LA and DHGLA derived-oxylipins in liver was also observed in the HC group, whilst significant changes were observed in the HCO group with enhanced 9(10)-EpOME, 12(13)-EpOME, 12,13-DiHOME levels compared with the C and HC groups (Fig. 1).
The hepatic α-LA, EPA, DHA (ω-3)-derived oxylipin profiles were also distinctly modified by the HC and HCO diets. Seen as a whole, the oxylipins concentration was increased in both the HC and HCO groups, whereas differential changes were detected for EPA- and DHA-derived oxylipins metabolized by the CYP enzyme system. In these cases, the HC diet induced a decrease in oxylipins. Feeding the HCO diet to the rats did not produce significant changes compared with the HC diet, but it did modulate the trend seen in the HC-fed rats. The inclusion of the onion ingredient in the HCO diet generally increased the concentration of ω-3–derived oxylipins compared with the HC feeding (Fig. 1), which also suggest the possible influence of this functional ingredient by enhancing the mechanisms involved in the resolution of inflammation. EPA- and DHA- may lead to the production of different mediators such as resolvins and protectins which act as potent resolution agonists at multiple levels [49]. However, further studies are needed to determine the potential impact of onion upregulating the pro-resolution pathways. Taken together, the present results suggest that dietary incorporation of onion functional ingredients may help manage liver inflammation counteracting the effects of hypercholesterolemia by a dual anti-inflammatory and pro-resolution action on liver oxylipins.
3.3. Sphingolipid metabolism
In this study, sphingolipids belonging to the ceramide NS class (i.e., non hydroxylated fatty acid/sphingosine backbone) have been analyzed, describing the 'hallmarks' of inflammation associated to hypercholesterolemia in plasma and different tissues. In addition, the modulation exerted by the onion ingredient was examined.
3.3.1. Plasma sphingolipids reflect a characteristic pattern induced by the HC feeding
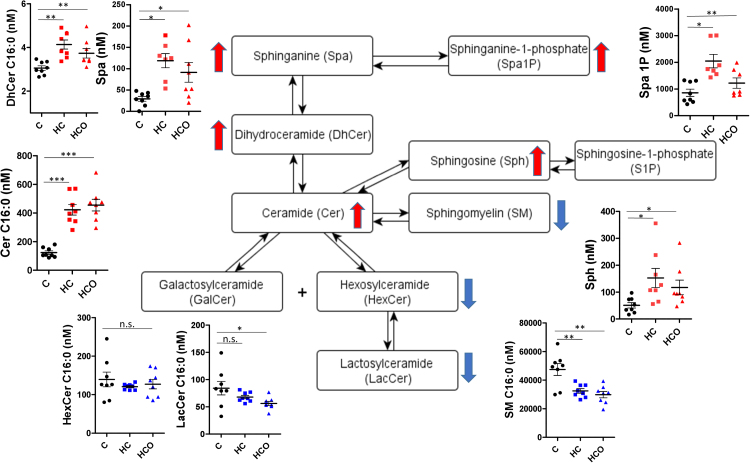
Diet-inducted hypercholesterolemia modified the circulating sphingolipid pattern (Fig. 3). Levels of sphingoid bases –sphinganine (Spa) and sphinganine-1-phosphate (Spa1P)–, dihydroceramides (DhCer) and ceramides (Cer) were increased, while levels of hexosylceramides (HexCer), lactosylceramides (LacCer) and sphingomyelins (SM) were decreased in the HC and HCO groups compared with the C group. Specific information of significantly altered fatty acyl chains is detailed in Fig. 4. As a general example of the plasma sphingolipid pattern, changes produced by HC and HCO feeding for C16:0 species is shown in Fig. 3.
Fig. 3.
Plasma sphingolipid pattern after 7 weeks of experimental feeding. Control (C), high-cholesterol (HC) and high-cholesterol enriched with onion (HCO). Red indicates an increase and blue a decrease in relative sphingolipid levels. C16:0 species are shown as an example of changes produced in plasma by HC and HCO diets. *P<0.05; **P<0.01; ***P<0.001; n.s.: not significant. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
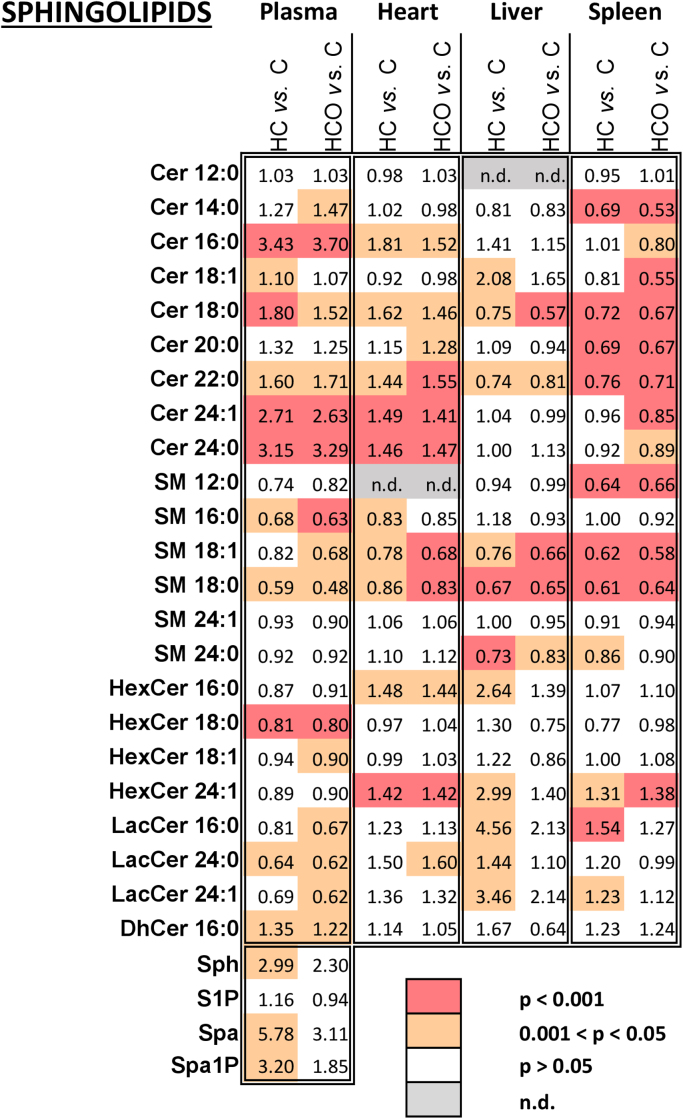
Fig. 4.
Sphingolipid shifts produced in plasma and tissues after 7 weeks of experimental feeding. Control (C), high-cholesterol (HC) and high-cholesterol enriched with onion (HCO). Values greater and lower than 1 represent either an increase or a decrease relative to the control, respectively. n.d.: not determined. Cer: Ceramide; SM: Sphingomyelin; DhCer: Dihydroceramide; HexCer: Hexosylceramide; LacCer: Lactosylceramide; Sph: Sphingosine; S1P: Sphingosine-1-phosphate; Spa: Sphinganine; Spa1P: Sphinganine-1-phosphate.
Cer has been highlighted as central mediator and both a precursor and product of other bioactive sphingolipids including sphingosine (Sph), sphingosine-1-phosphate (S1P), SM as well as glycosylated forms such as the HexCer or LacCer. Evidence from animal models suggests that Cer accumulation plays a critical role in the pathogenesis of atherosclerosis and heart dysfunction [50]. The activation of the SM-Cer pathway is known to play a pivotal role in LDL oxidation, which is directly linked to atherogenesis [24], [51]. In agreement with the present results, Ichi et al. [52] demonstrated that a cholesterol-enriched diet is able to affect the Cer metabolism in male Wistar rats, with increases in several Cer species (particularly C24:0 and C24:1) compared to a control diet. Thus, the higher levels of circulating ceramides found in the HC group compared with the C group could be the result of an increase in – de novo synthesis (as indicated by higher Spa levels) and/or an augmented conversion from SM. Circulating SM concentrations that have been reported increased in atherogenic events [24], [53] presented lower levels within the HC group of the present study. Thus, the increment in ceramides could also have been produced predominantly by stimulation in the conversion of SM to Cer by acid sphingomyelinase (aSMase) as a possible defense mechanism to avoid more serious pathological states.
Cer in circulation may also reduce NO bioavailability promoting atherosclerosis though direct effect on endothelial dysfunction. Likewise, the inhibition of Cer synthesis has shown to reverse endothelial dysfunction in numerous studies as seen in the model of streptozotocin-induced diabetic rats [54]. Consistent with these findings, it was recently shown that supplementation with cholesterol and cholic acid in this animal model caused impairments in endothelium-dependent relaxation and NADPH oxidase activity, whereas the activities were restored by the HCO diet [29]. However, these beneficial effects found in the vasculature as well as in other biomarkers of hypercholesterolemic rats fed with the onion ingredient could not be associated to direct changes in the plasma sphingolipids analyzed in this study. In this case, the supplementation with the onion ingredient (HCO diet) was not able to prevent the changes promoted in plasma by the HC feeding (Fig. 3). Nevertheless, it must be mentioned that increases in the sphingoid bases were generally higher in the HC group compared with the HCO group, both relative to the control. Spa and Spa1P levels were found to be lower in the rats fed the HCO diet, which suggests that onion ingredient supplementation interfered in the de novo synthesis of sphingolipids (Fig. 4).
3.3.2. Regulation of sphingolipids differs among tissues depending on HC and HCO diets
Examination of the changes induced by the HC diet in the levels of sphingolipids and their distribution within the heart, liver and spleen of male Wistar rats has pointed to significant shifts in specific metabolites. The targeted sphingolipid approach revealed that the HC diet increased heart levels of most Cer, while no significant differences were observed in the HCO relative to the HC group. In contrast, C16:0, C18:0 and C18:1-SM were significantly decreased while C16:0 and C24:1-HexCer were significantly increased (Fig. 4).
A different modulation was observed for liver and spleen Cer of hypercholesterolemic rats (both HC- and HCO-fed). The level of hepatic Cer (e.g., C18:1) in the HC-fed rats were higher than in the C group, while specific liver Cer (C18:0 and C22:0) were significantly decreased. In addition, a significant decrease in spleen Cer was found in the HC group relative to the C group. In this case, the HCO diet produced greater decreases in the levels of C16:0, C18:1 and C24:1-Cer than the HC diet.
These results suggest that a specific inhibition of Cer synthesis in the liver and spleen could be associated with the intake of the onion ingredient. However, decreases in specific hepatic Cer could be interpreted as a metabolic response to offset the diet-induced hepatic steatosis and inflammation, rather than a plausible direct impact on the metabolite synthesis or transformation.
On the other hand, the levels of SM species, in particular those containing C18:0 and C18:1 fatty acyl chains, were significantly lower in the HC group, whereas HexCer and LacCer were generally increased in both liver and spleen tissues. Moreover, a significant amelioration in the increase of HexCer and LacCer in the HCO-fed rats compared to the HC group, was found in liver, which all together suggest a possible influence of the onion ingredient.
In concordance with the present results, the Cer/cholesterol accumulation in the liver has been described to contribute to a wide range of pathologies, including the transition from steatosis to steatohepatitis, which can further progress to cirrhosis and hepatocellular carcinoma [55]. Likewise, alterations in other sphingolipids as the level of glycosylceramides (such as HexCer and LacCer) have been noted in cells and tissues in response to cardiovascular disease, diabetes, skin disorders, cancer and renal dysfunction [56], [57]. However, recent studies have also reported certain inconsistency in the changes produced in glycosylated forms between different tissues and plasma. These changes have been explained by the fact that more than one class of sphingolipids could modulate the same action, differing in both tissue specificity and mechanism of action [58].
Therefore, since the modulation of the cholesterol feeding effects may be considered a potential target for the treatment of hypercholesterolemia-induced diseases, the modifications stimulated in Cer and other sphingolipids in the liver and spleen by introducing the onion ingredient found in the present study should be further investigated. Specifically, the examination of enzyme activities involved in the sphingolipid biosynthesis, in order to distinguish their contribution in the pathway along with the contribution of fatty acid substrates in the progress of hypercholesterolemia and its prevention should be studied.
4. Conclusions
The HC diet caused a differential modulation in oxylipins and sphingolipids, which differed in each family of downstream PUFA-metabolites and biological matrix. HC feeding modified the fatty acid pattern and subsequent eicosanoid profile. The onion ingredient did not revert the decrease in circulating oxylipins in the HC-fed rats. However, onion supplementation did ameliorate HC diet-related changes in the hepatic concentrations of PGs and modified the concentration of some CYP450/LOX derived oxylipins in the liver of HCO-fed rats. An increase in ω-3-derived oxylipins was observed in the HCO group relative to C group compared with the changes found in the HC group relative to C group, which encourages further investigation of the effect of onion on pro-resolution pathways. However, the availability of PUFAs as a substrate also affects the circulating levels of oxylipins, and a number of oxylipin species may be produced via multiple enzymatic/non-enzymatic pathways. Accordingly, while these initial results are interesting, a focused study of the synthetic pathways is necessary for an integrative interpretation of the data.
The analysis of plasma sphingolipids reflected a characteristic pattern induced by the HC feeding, where circulating levels of Cer and sphingoid bases increased and HexCer, LacCer and SM decreased. The onion ingredient modulated specific sphingolipids within the liver and spleen, which calls for further studies in tissues to improve the understanding of the effect of a nutritional diet on sphingolipid metabolism.
Taken together, these results suggest that use of onion as functional ingredient may be useful to ameliorate the impact of hypercholesterolemia in liver inflammation. Therefore, the study of the interaction of functional food ingredients with bioactive lipid mediators as part of the hallmark of metabolic impairments is a useful strategy to characterize both their preventive effects and the potential impact of diets on inflammation, oxidative stress and organ dysfunction.
Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation [AGL2010-15910 (subprogram ALI)]. CEW was funded by the Swedish Heart Lung Foundation. DG-P also acknowledge the grant from the Margit and Folke Pehrzon Foundation.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.12.002.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.McGillicuddy F.C., Roche H.M. Nutritional status, genetic susceptibility, and insulin resistance−important precedents to atherosclerosis. Mol. Nutr. Food Res. 2012;56:1173–1184. doi: 10.1002/mnfr.201100785. [DOI] [PubMed] [Google Scholar]
- 2.Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. 2010;45:907–914. doi: 10.1007/s11745-010-3408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olefsky J.M., Glass C.K. Macrophages, inflammation and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 4.Hansson G.K., Robertson A.K., Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 5.Schmid-Schönbein G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006;8:93–131. doi: 10.1146/annurev.bioeng.8.061505.095708. [DOI] [PubMed] [Google Scholar]
- 6.Meijer K., Vonk R.J., Priebe M.G., Roelofsen H. Cell-based screening assay for anti-inflammatory activity of bioactive compounds. Food Chem. 2015;166:158–164. doi: 10.1016/j.foodchem.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson J.F., Ryan M.F., Gibney E.R., Brennan L., Roche H.M., Reilly M.P. Dietary isoflavone intake is associated with evoked responses to inflammatory cardiometabolic stimuli and improved glucose homeostasis in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2014;24:996–1003. doi: 10.1016/j.numecd.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan N., Khymenets O., Urpí-Sardà M., Tulipani S., Garcia-Aloy M., Monagas M., Mora-Cubillos X., Llorach R., Andres-Lacueva C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients. 2014;6:844–880. doi: 10.3390/nu6020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suhaila M. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci. Technol. 2014;35:114–128. [Google Scholar]
- 10.Hardman W.E. Diet components can suppress inflammation and reduce cancer risk. Nutr. Res. Pract. 2014;8:233–240. doi: 10.4162/nrp.2014.8.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorburn A.N., Macia L., Mackay C.R. Diet, metabolites, and "Western-lifestyle" inflammatory diseases. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.D'Haens G.R., Sartor R.B., Silverberg M.S., Petersson J., Rutgeerts P. Future directions in inflammatory bowel disease management. J. Crohns Colitis. 2014;8:726–734. doi: 10.1016/j.crohns.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Chilton F.H., Murphy R.C., Wilson B.A., Sergeant S., Ainsworth H., Seeds M.C., Mathias R.A. Diet-gene interactions and PUFA metabolism: a potential contributor to health disparities and human diseases. Nutrients. 2014;6:1993–2022. doi: 10.3390/nu6051993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson W.G., Demarzo A.M., Yegnasubramanian S. The diet as a cause of human prostate cancer. Cancer Treat. Res. 2014;159:51–68. doi: 10.1007/978-3-642-38007-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleemann R., Verschuren L., van Erk M.J., Nikolsky Y., Cnubben N.H., Verheij E.R., Smilde A.K., Hendriks H.F., Zadelaar S., Smith G.J., Kaznacheev V., Nikolskaya T., Melnikov A., Hurt-Camejo E., van der Greef J., van Ommen B., Kooistra T. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8:R200. doi: 10.1186/gb-2007-8-9-r200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorkbacka H., Kunjathoor V.V., Moore K.J., Koehn S., Ordija C.M., Lee M.A., Means T., Halmen K., Luster A.D., Golenbock D.T., Freeman M.W. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 17.Gleim S., Stitham J., Tang W.H., Martin K.A., Hwa J. An eicosanoid-centric view of atherothrombotic risk factors. Cell. Mol. Life Sci. 2012;69:3361–3380. doi: 10.1007/s00018-012-0982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E.J., Kim B.H., Seo H.S., Lee Y.J., Kim H.H., Son H.H., Choi M.H. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS One. 2014;9:e97841. doi: 10.1371/journal.pone.0097841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M., Thomson M., Afzal M. Garlic and onions: their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot. Essent. Fat. Acids. 2000;62:55–73. doi: 10.1054/plef.1999.0124. [DOI] [PubMed] [Google Scholar]
- 20.Simin N., Orcic D., Cetojevic-Simin D., Mimica-Dukic N., Anackov G., Beara I., Mitic-Culafic D., Bozin B. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp flavum, Alliaceae) Food Sci. Technol. 2013;54:139–146. [Google Scholar]
- 21.Suleria H.A.R., Butt M.S., Anjum F.M., Saeed F., Khalid N. Onion: nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015;55:50–66. doi: 10.1080/10408398.2011.646364. [DOI] [PubMed] [Google Scholar]
- 22.Balgoma D., Checa A., Sar D.G., Snowden S., Wheelock C.E. Quantitative metabolic profiling of lipid mediators. Mol. Nutr. Food Res. 2013;57:1359–1377. doi: 10.1002/mnfr.201200840. [DOI] [PubMed] [Google Scholar]
- 23.Vesper H., Schmelz E.M., Nikolova-Karakashian M.N., Dillehay D.L., Lynch D.V., Merrill A.H., Jr Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 1999;129:1239–1250. doi: 10.1093/jn/129.7.1239. [DOI] [PubMed] [Google Scholar]
- 24.Cowart L.A. Springer; New York: 2011. Sphingoshlipid Metabolism and Analysis in Metabolic Disease. [Google Scholar]
- 25.Yang J., Yu Y.N., Sun S.Y., Duerksen-Hughes P.J. Ceramide and other sphingolipids in cellular responses. Cell Biochem. Biophys. 2004;40:323–350. doi: 10.1385/CBB:40:3:323. [DOI] [PubMed] [Google Scholar]
- 26.Sheth S.A., Iavarone A.T., Liebeskind D.S., Won S.J., Swanson R.A. Targeted lipid profiling discovers plasma biomarkers of acute brain injury. PLoS One. 2015;10:e0129735. doi: 10.1371/journal.pone.0129735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upadhyay R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids. 2015;2015:971453. doi: 10.1155/2015/971453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colina-Coca C., Rodríguez-Alcalá L.M., Fontecha J., González-Peña D., de Ancos B., Sánchez-Moreno C. Effects of hypercholesterolemic diet enriched with onion as functional ingredient on fatty acid metabolism in Wistar rats. Food Res. Int. 2014;64:546–552. doi: 10.1016/j.foodres.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 29.González-Peña D., Angulo J., Vallejo S., Colina-Coca C., de Ancos B., Sánchez-Ferrer C.F., Peiró C., Sánchez-Moreno C. High-cholesterol diet enriched with onion affects endothelium-dependent relaxation and NADPH oxidase activity in mesenteric microvessels from Wistar rats. Nutr. Metab. 2014;11:57. doi: 10.1186/1743-7075-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Peña D., Colina-Coca C., Char C.D., Cano M.P., de Ancos B., Sánchez-Moreno C. Hyaluronidase inhibiting activity and radical scavenging potential of flavonols in processed onion. J. Agric. Food Chem. 2013;61:4862–4872. doi: 10.1021/jf3054356. [DOI] [PubMed] [Google Scholar]
- 31.Balgoma D., Yang M., Sjodin M., Snowden S., Karimi R., Levanen B., Merikallio H., Kaarteenaho R., Palmberg L., Larsson K., Erle D.J., Dahlen S.E., Dahlen B., Skold C.M., Wheelock A.M., Wheelock C.E. Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD. Eur. Respir. J. 2016;47:1645–1656. doi: 10.1183/13993003.01080-2015. [DOI] [PubMed] [Google Scholar]
- 32.Checa A., Khademi M., Sar D.G., Haeggstrom J.Z., Lundberg J.O., Piehl F., Olsson T., Wheelock C.E. Hexosylceramides as intrathecal markers of worsening disability in multiple sclerosis. Mult. Scler. 2015;21:1271–1279. doi: 10.1177/1352458514561908. [DOI] [PubMed] [Google Scholar]
- 33.Storey J.D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González-Peña D., Dudzik D., Colina-Coca C., de Ancos B., García A., Barbas C., Sánchez-Moreno C. Multiplatform metabolomic fingerprinting as a tool for understanding hypercholesterolemia in Wistar rats. Eur. J. Nutr. 2016;55:997–1010. doi: 10.1007/s00394-015-0914-1. [DOI] [PubMed] [Google Scholar]
- 35.Cyrus T., Ding T., Pratico D. Expression of thromboxane synthase, prostacyclin synthase and thromboxane receptor in atherosclerotic lesions: correlation with plaque composition. Atherosclerosis. 2010;208:376–381. doi: 10.1016/j.atherosclerosis.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Novgorodtseva T.P., Karaman Y.K., Zhukova N.V., Lobanova E.G., Antonyuk M.V., Kantur T.A. Composition of fatty acids in plasma and erythrocytes and eicosanoids level in patients with metabolic syndrome. Lipids Health Dis. 2011;10:82. doi: 10.1186/1476-511X-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardwick J.P., Eckman K., Lee Y.K., Abdelmegeed M.A., Esterle A., Chilian W.M., Chiang J.Y., Song B.-J. Eicosanoids in metabolic syndrome. Adv. Pharmacol. 2013;66:157–266. doi: 10.1016/B978-0-12-404717-4.00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tourdot B.E., Ahmed I., Holinstat M. The emerging role of oxylipins in thrombosis and diabetes. Front. Pharmacol. 2014;4:176. doi: 10.3389/fphar.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Lin H., Gu Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorente-Cebrián S., Costa A.G.V., Navas-Carretero S., Zabala M., Laiglesia L.M., Martínez J.A., Moreno-Aliaga M.J. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J. Physiol. Biochem. 2015;71:341–349. doi: 10.1007/s13105-015-0395-y. [DOI] [PubMed] [Google Scholar]
- 41.Bojic L.A., McLaren D.G., Harms A.C., Hankemeier T., Dane A., Wang S.P., Rosa R., Previs S.F., Johns D.G., Castro-Perez J.M. Quantitative profiling of oxylipins in plasma and atherosclerotic plaques of hypercholesterolemic rabbits. Anal. Bioanal. Chem. 2016;408:97–105. doi: 10.1007/s00216-015-9105-4. [DOI] [PubMed] [Google Scholar]
- 42.Kuiper J., Zijlstra F.J., Kamps J.A., van Berkel T.J. Identification of prostaglandin D2 as the major eicosanoid from liver endothelial and Kupffer cells. Biochim. Biophys. Acta. 1988;959:143–152. doi: 10.1016/0005-2760(88)90025-2. [DOI] [PubMed] [Google Scholar]
- 43.Tolman K.G. Eicosanoids and the liver. Prostaglandins Other Lipid Mediat. 2000;61:163–174. doi: 10.1016/s0090-6980(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 44.Breinig M., Schirmacher P., Kern M.A. Cyclooxygenase-2 (COX-2) – A therapeutic target in liver cancer? Curr. Pharm. Des. 2007;13:3305–3315. doi: 10.2174/138161207782360627. [DOI] [PubMed] [Google Scholar]
- 45.Bayer T., Wagner H., Wray V., Dorsch W. Inhibitors of cyclo-oxygenase and lipoxygenase in onions. Lancet. 1988;2:906. doi: 10.1016/s0140-6736(88)92503-2. [DOI] [PubMed] [Google Scholar]
- 46.Vanderhoek J.Y., Makheja A.N., Bailey J.M. Inhibition of fatty-acid oxygenases by onion and garlic oils - Evidence for the mechanism by which these oils inhibit platelet-aggregation. Biochem. Pharmacol. 1980;29:3169–3173. doi: 10.1016/0006-2952(80)90581-x. [DOI] [PubMed] [Google Scholar]
- 47.He B., Hao J., Sheng W., Xiang Y., Zhang J., Zhu H., Tian J., Zhu X., Feng Y., Xia H. Fistular onion stalk extract exhibits anti-atherosclerotic effects in rats. Exp. Ther. Med. 2014;8:785–792. doi: 10.3892/etm.2014.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava K.C. Aqueous extracts of onion, garlic and ginger inhibit platelet-aggregation and alter arachidonic-acid metabolism. Biomed. Biochim. Acta. 1984;43:S335–S346. [PubMed] [Google Scholar]
- 49.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park T.S., Goldberg I.J. Sphingolipids, lipotoxic cardiomyopathy, and cardiac failure. Heart Fail. Clin. 2012;8:633–641. doi: 10.1016/j.hfc.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auge N., Andrieu N., NegreSalvayre A., Thiers J.C., Levade T., Salvayre R. The sphingomyelin-ceramide signaling pathway is involved in oxidized low density lipoprotein-induced cell proliferation. J. Biol. Chem. 1996;271:19251–19255. doi: 10.1074/jbc.271.32.19251. [DOI] [PubMed] [Google Scholar]
- 52.Ichi I., Nakahara K., Kiso K., Kojo S. Effect of dietary cholesterol and high fat on ceramide concentration in rat tissues. Nutrition. 2007;23:570–574. doi: 10.1016/j.nut.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Jiang X.C., Goldberg I.J., Park T.S. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. In: Cowart L.A., editor. Sphingolipids and Metabolic Disease. Springer; New York: 2011. pp. 19–39. [DOI] [PubMed] [Google Scholar]
- 54.Chun L., Junlin Z., Aimin W., Niansheng L., Benmei C., Minxiang L. Inhibition of ceramide synthesis reverses endothelial dysfunction and atherosclerosis in streptozotocin-induced diabetic rats. Diabetes Res. Clin. Pract. 2011;93:77–85. doi: 10.1016/j.diabres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Morales A., Mari M., Garcia-Ruiz C., Colell A., Fernandez-Checa J.C. Hepatocarcinogenesis and ceramide/cholesterol metabolism. Anticancer Agents Med. Chem. 2012;12:364–375. doi: 10.2174/187152012800228689. [DOI] [PubMed] [Google Scholar]
- 56.Messner M.C., Cabot M.C. Springer; New York: 2010. Glucosylceramide in humans, Sphingolipids as Signaling and Regulatory Molecules; pp. 156–164. [Google Scholar]
- 57.Subathra M., Korrapati M., Howell L.A., Arthur J.M., Shayman J.A., Schnellmann R.G., Siskind L.J. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am. J. Physiol. – Ren. Physiol. 2015;309:F204–F215. doi: 10.1152/ajprenal.00150.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chavez J.A., Siddique M.M., Wang S.T., Ching J., Shayman J.A., Summers S.A. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem. 2014;289:723–734. doi: 10.1074/jbc.M113.522847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material