Abstract
Background
Studies have reported antioxidant effect of oleuropein in isolated rat heart.
Objective
This study was conducted to investigate whether perfusion of isolated rat heart with oleuropein, before induction of ischemia or at the onset of reperfusion, had any effect on the hemodynamic parameters, infarct size and biochemical factors following ischemic – reperfusion injury.
Materials and methods
Forty-eight male Wistar rats were divided into 6 groups: the control groups (Con-P and Con-T groups), O10-P and O50-P groups perfused with 10 and 50 μg/g heart oleuropein 5 min before the induction of ischemia and O10-T and O50-T groups perfused with 10 and 50 μg/g heart oleuropein at the beginning of the reperfusion, respectively. All hearts were subjected to 30 min global ischemia and 90 min reperfusion. Hemodynamic parameters were monitored throughout the experiment. The creatine kinase (CK) and malondialdehyde (MDA) level of coronary outflow were assayed and the infarct size measured at the end of reperfusion.
Results
We found hemodynamic parameters namely heart rate, left ventricular end diastolic pressure (LVEDP), left ventricular developed pressure (LVDP), ±dp/dt and coronary outflow significantly improved in all groups that received oleuropein compared to the control groups. Also, the infarct size was smaller and the coronary outflow levels of CK and MDA were lower in the oleuropein groups compared to the control groups.
Conclusions
The findings suggest that perfusion of isolated rat heart with oleuropein would lead to improved myocardial dysfunction following ischemic-reperfusion injury. Our findings confirm the antioxidant potential of oleuropein.
Keywords: Creatine kinase, Malondialdehyde, LVEDP, LVDP
1. Introduction
Biologically active substances from plants attract the interest of many scientists in the era of modern pharmacotherapy worldwide [1], [2], [3]. It has been documented that the side effects of natural substances are much less than that of synthetic drugs [4]. Oleuropein is a natural substance present in olive leaves in a high concentration (6–9% of dry weight) [5], [6]. It is a polyphenolic constituent with high antioxidant capacity [7], [8], [9] comparable to a hydrosoluble analog of α-tocopherol or vitamin E [10]. Various studies shown that oleuropein has many biological benefits in animals and human beings including antioxidant [9], [11], [12], anti-inflammatory [13], antidiabetic [7], [14], anticancer [15], [16], hypoglycemic [17], hypolipidemic [17]. Almost all previous studies attribute the beneficial biological effects of olive leaf extracts to oleuropein [9], [14], [19], [20], [21], [22].
So far, few studies have been conducted on the effect of oleuropein on cardiovascular system in animal models to study cardioprotective effects [4], [23], [24], [25], [26], [27], [28]. However there is no study about the direct effect of oleuropein on myocardial dysfunction following ischemic – reperfusion injury. Because previous studies reported that oleuropein immediately after absorption in the gastrointestinal tract was converted to its conjugated and non-conjugated metabolites [24], [29], [30], [31], its plasma concentration will be very low. Petkov and Malonov (1978) for the first time, using the in vitro and in vivo animal models reported that oleuropein has anti-arrhythmic and vasodilatory effects in dogs, cats, rabbits and rats [4]. Manna and co-workers in 2004 noticed that perfusion of isolated rat hearts with oleuropein (20 μg/g heart) before the induction of ischemia and reperfusion injury had cardioprotective effects that were evident by reduced creatine kinase (CK) and malondialdehyde (MDA) and glutathione peroxidase levels in coronary outflow. They had no data on the effect of oleuropein on myocardial dysfunction, infarct size and the magnitude of arrhythmia [25]. Recently, Nekooian and colleagues (2014) indicated that pre-treatment of hypertensive rats with type 2 diabetes with oleuropein (20, 40 and 60 mg/kg) for 16 weeks attenuated cardiovascular complications [27].
This study, investigated whether perfusion of isolated rat hearts with oleuropein (10 and 50 μg/g heart) before the induction of ischemia or at the onset of reperfusion had any effects on cardiac dysfunction, infarct size and the magnitude of arrhythmia. There are main differences between this study and Manna's study [25]: first, they only used a single dose of oleuropein (20 μg/g heart); second, they only infused oleuropein before the induction of ischemia; and third, they did not study the effect of oleuropein on cardiac dysfunction, infarct size and the magnitude of arrhythmia.
2. Materials and methods
2.1. Animals
In this study, forty-eight male Wistar rats, weighing 300–350 g, were used. All animals were kept under the standard conditions (12 h light/dark cycle, 22 ± 2 °C, and humidity 55%) with free access to food and water in the animal house of Medical College of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. All procedures were according to the international guide for the and approved by the ethics committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (No. 1493).
2.2. Experimental groups
The study had five experimental groups.
Group 1 as the Con-P group: isolated rat hearts were perfused with Krebs – Henseleit solution before induction of ischemic – reperfusion injury (n = 8).
Group 2 as the O10-P group: isolated rat hearts were perfused with 10 μg/g heart oleuropein before induction of ischemic – reperfusion injury for 5 min (n = 8).
Group 3 as the O50-P group: isolated rat hearts were perfused with 50 μg/g heart oleuropein before induction of ischemic – reperfusion injury for 5 min (n = 8).
Group 4 as the Con-T group: isolated rat hearts were perfused with Krebs – Henseleit solution at the reperfusion period (n = 8).
Group 5 as the O10-T group: isolated rat hearts were perfused with 10 μg/g heart oleuropein for five min at the beginning of the reperfusion (n = 8).
Oleuropein was purchased from Extrasynthèse (Geney, France).
2.3. Isolation of the heart
Animals were anesthetized with Sodium Thiopental (75 mg/kg, i.p) and heparinized (1000 IU heparin, i.p) to prevent the occlusion of coronary vessels during the removal of the heart. Then, the chest was opened and the heart removed. To perfuse the coronary vessels, aorta was cannulated and perfused retrogradely with Krebs -Henseleit solution under Langendorf apparatus with constant pressure of 70–80 mmHg. Krebs -Henseleit solution contained (mM), NaCl (118), KCl (4.7), NaHCO3 (25), KH2PO4 (1.2), MgSO4 (1.2), CaCl2 (1.25) and glucose (11). Next, a full-fill water balloon was inserted into the left ventricle through the left atrium and connected to a transducer pressure (NARCO Bio – System, USA) to record intraventricular pressures including left ventricular end diastolic pressure (LVEDP), left ventricular developed pressure (LVDP), ventricular pressure time changes (max and min dp/dt) and cardiac contractility. The pressures were monitored using power lab data acquisition system (ADInstrument, Australia) with Lab Chart pro 7 software. Subsequently, the volume of the balloon was gradually increased to adjust the LVEDP to 4–7 mmHg. To monitor the electrical activity of the heart, two electrodes were placed on the base and top of the ventricles. Finally, a warm water jacket was placed around the heart to maintain the temperature at approximately 37 °C [49].
2.4. Induction of ischemic reperfusion injury
To induce global ischemic – reperfusion injury, twenty min after stabilization of the heart under the Langendorff apparatus, the coronary flow was completely occluded for 30 min and then reperfused for 90 min.
2.5. The effect of oleuropein on coronary outflow
To determine the effect of oleuropein on the rate of coronary outflow, it was measured manually 1 min before administration of oleuropein, before the induction of ischemia and 5, 10, 30, 60 and 90 min during reperfusion.
2.6. Measurement of CK activity and MDA in coronary outflow
Following 10 min of reperfusion, a sample of coronary outflow was collected to measure CK activity as a marker of ischemic injury and MDA level as a marker of lipid peroxidation [25], [32]. CK activity was measured using a standard kit (Man CO, Iran) with catalog number of 101031 and MDA level measured manually. In brief, 100 μl of coronary outflow was mixed with 100 μl of SDS 8.1% and 750 μl acetic acid 20% and the volume was made up to 2 ml with distilled water. Then, 10 μl of Butylated hydroxytoluene 1%, 70 μl thiobarbituric acid 0.8% were added and vortexed. Next, this solution was heated to 95 °C for 1 h and at 4 °C (refrigerator) for 10 min, respectively. The solution was then centrifuged at 3000 g for 15 min. Finally 2.5 ml N-butanol – pyridine (15:1) was added and the absorbance of supernatant was read at 532 nm. The MDA concentration was calculated using tetraethoxypropane standard curve as a positive control substance.
2.7. Measurement of the infarct size
At the end of 90 min reperfusion, left ventricle was separated and frozen at −24 °C for 24 h. Then, it was cut into sections of 2 mm and stained with triphenyltetrazolium chloride (TTC) 1% at 37 °C for 30 min. TTC causes the viable tissues to obtain red color and the dead tissues to appear white in color. To increase the contrast between the colors, the sections were incubated in formalin 10% for 1 h. Finally, photos were taken from both sides of sections and the infarct size was determined as a percent of total area of the left ventricle using Photoshop 8 CS image-analysis software.
2.8. Statistical analysis
Data were analyzed using GraphPad Prism 5 (San Diego, CA) and expressed as mean ± SEM and the percentage of incidence. One – way and two – way analysis of variance (ANOVA) was used to analyze the difference between means and Fisher exact test to analyze the percentage of incidence. p < 0.05 were considered statistically significant.
3. Results
3.1. The effect of perfusion on cardiac hemodynamic parameters
There was no significant difference in heart rate between Con-P group and O10-P and O50-P groups which received 10 and 50 μg/g heart oleuropein before ischemia, respectively (Table 1).
Table 1.
Effect of perfusion of isolated rat hearts with oleuropein before induction of ischemic – reperfusion injury on myocardial function parameters.
| Base |
PO5 |
Ie |
R5 |
R10 |
R30 |
R60 |
R90 |
|
|---|---|---|---|---|---|---|---|---|
| HR | ||||||||
| Con-P | 265 ± 11 | 264 ± 8 | 0 ± 0 | 207 ± 28 | 239 ± 14 | 226 ± 16 | 222 ± 9 | 217 ± 14 |
| O10 | 263 ± 18 | 263 ± 18 | 0 ± 0 | 218 ± 13 | 231 ± 9 | 233 ± 7 | 233 ± 8 | 223 ± 7 |
| O50 | 275 ± 10 | 281 ± 9 | 0 ± 0 | 233 ± 15 | 230 ± 16 | 252 ± 14 | 245 ± 17 | 246 ± 16 |
| LVEDP | ||||||||
| Con-P | 5.3 ± 0.3 | 5.8 ± 0.3 | 26 ± 2.8 | 61 ± 5.2 | 59 ± 5.6 | 53 ± 5.1 | 50 ± 3.5 | 44 ± 2.4 |
| O10 | 6.4 ± 0.4 | 6 ± 0.5 | 18 ± 4.2 | 41 ± 3.9 b | 35 ± 3.3 b | 31 ± 3 b | 30 ± 3.9 | 29 ± 3.9 b |
| O50 | 4.4 ± 0.8 | 4.2 ± 0.8 | 20 ± 5.8 | 35 ± 5.9 b | 30 ± 5.5 b | 39 ± 4.6 b | 35 ± 6.5 b | 32 ± 5.9 b |
| LVDP | ||||||||
| Con-P | 101 ± 2.9 | 101 ± 83 | 0 ± 0 | 66 ± 11 | 49 ± 4.9 | 47 ± 6.6 | 44 ± 4.9 | 66 ± 11 |
| O10 | 95 ± 3.7 | 95 ± 5.2 | 0 ± 0 | 70 ± 9.8 | 67 ± 6 | 65 ± 6.7 a | 63 ± 607 a | 60 ± 5 a |
| O50 | 97 ± 8.7 | 99 ± 9 | 0 ± 0 | 64 ± 8.5 | 58 ± 8.5 | 54 ± 4.6 | 54 ± 7.9 a | 52 ± 7.6 a |
| max dp/dt | ||||||||
| Con-P | 3743 ± 338 | 3846 ± 291 | 0 ± 0 | 2565 ± 370 | 2295 ± 172 | 2199 ± 239 | 1947 ± 154 | 1922 ± 365 |
| O10 | 3922 ± 267 | 3782 ± 301 | 0 ± 0 | 3420 ± 413 | 3144 ± 236 a | 3112 ± 200 a | 3062 ± 263 a | 2716 ± 250 |
| O50 | 3548 ± 138 | 3616 ± 138 | 0 ± 0 | 2863 ± 226 | 3211 ± 272 a | 3013 ± 189 | 2665 ± 204 a | 2268 ± 192 |
| min dp/dt | ||||||||
| Con-P | −2972 ± 110 | −3013 ± 104 | 0 ± 0 | −2045 ± 164 | −1885 ± 101 | −1906 ± 129 | 1783 ± 116 | −1551 ± 280 |
| O10 | −2859 ± 224 | −3190 ± 234 | 0 ± 0 | −2607 ± 231a | 2545 ± 239 a | 2376 ± 201 a | −2376 ± 214 a | −2167 ± 174 a |
| O50 | −2920 ± 149 | −2827 ± 131 | 0 ± 0 | −2657 ± 288 a | −2366 ± 204 a | −2103 ± 227 | −2174 ± 238 a | −2034 ± 188 a |
| CF | ||||||||
| Con-P | 11.8 ± 0.5 | 11.8 ± 0.4 | 0 ± 0 | 7 ± 0.7 | 6.7 ± 0.6 | 6.3 ± 0.7 | 5.3 ± 0.8 | 4.6 ± 0.9 |
| O10 | 12 ± 0.8 | 12.3 ± 0.4 | 0 ± 0 | 10.1 ± 1 a | 9.3 ± 0.9 a | 8.5 ± 0.9 a | 8 ± 0.8 a | 7.3 ± 0.7 a |
| O50 | 11.9 ± 0.7 | 12.3 ± 0.7 | 0 ± 0 | 10.2 ± 1.2 b | 8.7 ± 0.8 a | 9.3 ± 1.2 a | 8.6 ± 1.1 a | 7.8 ± 1.1 a |
Data shown that as Mean ± SEM.
HR, heart rate; LVEDP, left ventricular end diastolic pressure; LVDP, left ventricular developed pressure and CF, coronary flow (G). Con-P, pre-treatment control group; O10-P and O50-P mean groups that the hearts were received 10 and 50 μg/g heart oleuropein for 5 min before ischemia, respectively. PO5 means 5 min after oleuropein infusion, respectively. I and R mean ischemic and reperfusion times, respectively. a; p < 0.05 and b; p < 0.01 vs control group.
Compared with the Con-P group, LVEDP, only during reperfusion period, was significantly lower in O10-P and O50-P groups. There was no significant difference between O10-P and O50-P groups (Table 1).
Except at the first 5 min of reperfusion period, LVDP, coronary outflow and contractility index were significantly higher at the rest of reperfusion in O10-P and O50-P groups than the Con-P group, especially in O50-P groups (Table 1).
Compared with the Con-P group, during the reperfusion period, max dp/dt was considerably more positive and min dp/dt was significantly more negative in O10-P and O50-P groups. There was no significant difference between O10-P and O50-P groups (Table 1).
3.2. The effect of perfusion on cardiac hemodynamic parameters
There was no significant difference in heart rate between Con-T group and O10-T and O50-T groups which received 10 and 50 μg/g heart oleuropein at the beginning of reperfusion, respectively (Table 2).
Table 2.
Effect of perfusion of isolated rat hearts with oleuropein at the beginning of reperfusion on myocardial function parameters.
| Base |
Ie |
R5 |
R10 |
R30 |
R60 |
R90 |
|
|---|---|---|---|---|---|---|---|
| HR | |||||||
| Con-T | 292 ± 15 | 0 ± 0 | 252 ± 29 | 249 ± 21 | 242 ± 11 | 255 ± 23 | 246 ± 37 |
| O10 | 285 ± 28 | 0 ± 0 | 219 ± 10 | 240 ± 18 | 241 ± 11 | 233 ± 10 | 239 ± 13 |
| O50 | 282 ± 16 | 0 ± 0 | 232 ± 8.4 | 263 ± 14 | 227 ± 9 | 225 ± 12 | 220 ± 8 |
| LVEDP | |||||||
| Con-T | 5.3 ± 0.5 | 26 ± 4.1 | 58 ± 7.2 | 51 ± 6.2 | 48 ± 5.1 | 40 ± 3.3 | 37 ± 2.5 |
| O10 | 4.2 ± 0.7 | 21 ± 6 | 47 ± 4.3 a | 35 ± 5.6 a | 30 ± 5 b | 27 ± 3.2 b | 25 ± 2.2 |
| O50 | 4.4 ± 0.7 | 23 ± 3.2 | 42 ± 2.1 | 32 ± 3.5 b | 24 ± 3.3 c | 22 ± 3 c | 21 ± 2.6 c |
| LVDP | |||||||
| Con-T | 114 ± 8.7 | 0 ± 0 | 83 ± 2.7 | 76 ± 4 | 72 ± 3.5 | 61 ± 3.3 | 53 ± 3.1 |
| O10 | 122 ± 5.4 | 0 ± 0 | 104 ± 6.7 | 95 ± 5.5 b | 85 ± 5.3 a | 79 ± 6.1 a | 72 ± 6.1 b |
| O50 | 112 ± 5.1 | 0 ± 0 | 113 ± 3.3 | 102 ± 5.6 b | 97 ± 6.6 b | 91 ± 6.3 b | 81 ± 6.4 b |
| max dp/dt | |||||||
| Con-T | 4493 ± 357 | 0 ± 0 | 3065 ± 345 | 2639 ± 271 | 2289 ± 268 | 2139 ± 185 | 2182 ± 256 |
| O10 | 5260 ± 292 | 0 ± 0 | 4111 ± 357 a | 3857 ± 428 b | 3547 ± 375 a | 3630 ± 353 b | 3543 ± 345 a |
| O50 | 4415 ± 280 | 0 ± 0 | 4471 ± 348 a | 4552 ± 236 a | 4456 ± 279 | 4171 ± 474 c | 4152 ± 355 c |
| min dp/dt | |||||||
| Con-T | −3972 ± 206 | 0 ± 0 | −2384 ± 293 | −2278 ± 256 | −2106 ± 122 | −1703 ± 131 | −1701 ± 194 |
| O10 | −4341 ± 245 | 0 ± 0 | −3536 ± 153 b | −3175 ± 228 a | −2926 ± 141 b | −2869 ± 179 b | −2639 ± 234 |
| O50 | −4386 ± 230 | 0 ± 0 | −3920 ± 153 c | −3848 ± 221 c | −3474 ± 195 c | −3365 ± 228 c | −3099 ± 229 |
| CF | |||||||
| Con-T | 12.6 ± 0.6 | 0 ± 0 | 7.3 ± 0.4 | 6.7 ± 0.4 | 6.3 ± 0.9 | 5.2 ± 0.5 | 4.5 ± 0.7 |
| O10 | 13.7 ± 0.5 | 0 ± 0 | 10.5 ± 0.5 a | 9.9 ± 0.6 b | 9.2 ± 0.4 a | 8.4 ± 0.4 b | 8.3 ± 0.4 b |
| O50 | 14.2 ± 0.6 | 0 ± 0 | 11.1 ± 0.7 b | 10.5 ± 0.5 b | 9.9 ± 0.4 b | 9.7 ± 0,5 b | 9.1 ± 0.3 b |
Data shown that as Mean ± SEM.
HR, heart rate; LVEDP, left ventricular end diastolic pressure; LVDP, left ventricular developed pressure and CF, coronary flow (G). Con-T, treatment control group; O10-T and O50-T mean groups that the hearts were received 10 and 50 μg/g heart oleuropein for 5 min at the early of reperfusion, respectively. I and R mean ischemic and reperfusion times, respectively. a; p < 0.05, b; p < 0.01 and c; p < 0.001 vs control group.
Following the perfusion of ischemic myocardium with oleuropein in O10-T and O50-T groups, LVEDP, during all reperfusion period, was significantly lower than the Con-T group. There was no significant difference between O10-T and O50-T groups (Table 2).
Compared with the Con-T group, LVDP, contractility index and coronary outflow, during all reperfusion period, were significantly higher in O10-T and O50-T groups which received oleuropein at the beginning of reperfusion. These three parameters had not significant difference between O10-T and O50-T groups during reperfusion period (Table 2).
Following the perfusion of ischemic myocardium with oleuropein in O10-T and O50-T groups, max dp/dt was significantly more positive and min dp/dt was more negative than the Con-T group. There was no significant difference between O10-T and O50-T groups (Table 2).
3.3. The effect of perfusion of isolated rat heart with oleuropein before induction of ischemia and/or at the early of reperfusion on CK activity and MDA level of coronary outflow and infarct size
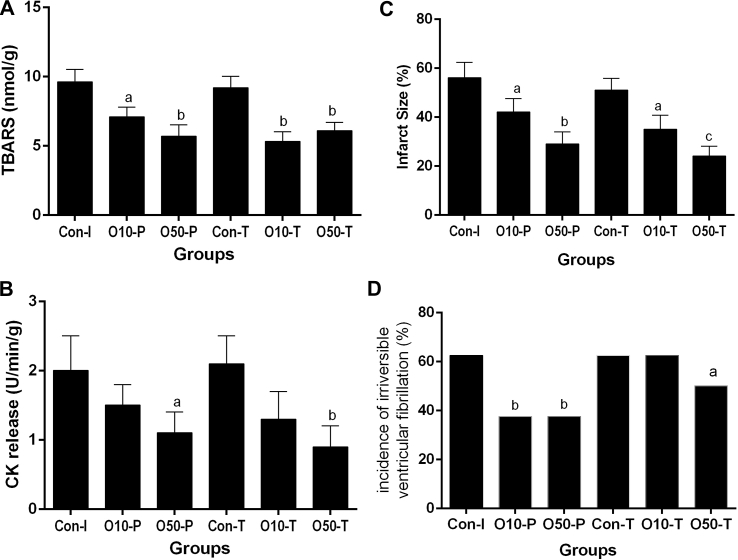
MDA level of coronary outflow was decreased in all groups received oleuropein compared with the control group. There was not any significant difference between oleuropein received groups, although it was lower in O10-T and O50-T groups (Fig. 1A).
Fig. 1.
The effect of perfusion of isolated rat hearts with oleuropein before induction of ischemia and/or at the beginning of reperfusion on the MDA level (A) and CK activity of coronary outflow (B), infarct size (C) and the incidence percentage of irreversible ventricular fibrillation (D). Con-P, pre-treatment control group; Con-T, treatment control group; O10-P and O50-P mean groups received 10 and 50 μg/heart oleuropein for 5 min before ischemia, respectively. O10-T and O50-T mean groups received 10 and 50 μg/g heart oleuropein for 5 min at the beginning of reperfusion, respectively. a; p < 0.05, b; p < 0.01 and c; p < 0.001 vs control group.
CK activity of coronary outflow decreased in all groups receiving oleuropein compared with the control group that was only significant in O50-P and O50-T groups (Fig. 1B).
The infarct size decreased in all groups receiving oleuropein compared with the control group that was only significant in O50-P and O50-T groups (Fig. 1C).
Compared with the control group, the incidence percentage of irreversible ventricular fibrillation decreased in O10-T, O50-T and O50-T groups, significantly (Fig. 1D).
4. Discussion
The main findings of this study indicated that perfusion of isolated rat heart with oleuropein (before and/or after ischemia) is cardioprotective against ischemic – reperfusion injury. These effects were evident with the decreased LVEDP, infarct size, oxidative stress and magnitude of reperfusion arrhythmia and increased LVDP, cardiac contractility and coronary outflow.
Reperfusion of acute post-infarction ischemic myocardium is a prerequisite to salvage it. However, restoration of coronary flow of ischemic area is accompanied by a series of events that lead to additional cellular injury. It is known as “reperfusion injury” [33], [34], [35]., The main cause of reperfusion injury is attributed to the excess generation of free radicals, especially early in reperfusion [36] Reactive oxygen species (ROS) including superoxide anions, hydrogen peroxide and hydrogen radicals are the main free radical species [37], [38]. In view of this, development of new strategy to prevent or attenuate reperfusion injury to maximize myocardial salvage in patients with the acute ischemic heart diseases is necessary.
Many studies have shown that antioxidant therapy with a variety of agents like superoxide dismutase, N-acetylcysteine, vitamin E and a combination of them decrease the magnitude of ischemic-reperfusion injury in experimental settings [14], [39]. On the other hand, several studies have reported the failure of antioxidant therapy against ischemic – reperfusion injury in animal models [38]. Nevertheless, we wanted to know, in the present study, whether oleuropein as a potent natural phenolic antioxidant had any direct effects on the myocardial dysfunction, the magnitude of arrhythmia and the infarct size following ischemic reperfusion injury in isolated rat heart or not? Almost all previous studies on the absorption of oleuropein in the intestine have reported that it is absorbed from the gut and rapidly converted to its weaker antioxidant metabolites, [24], [29], [30], [31]. Hence, the free plasma concentration of oleuropein is very low. So we studied the direct effect of oleuropein but not its metabolites in isolated rat hearts.
Oleuropein is the main phenolic compound of olive leaves that constitutes to about 264 mg/g dry weight (as equivalents of tyrosol) of it [18]. Many studies indicated that oleuropein has a wide range of health benefits [8], [25], [40], [41]. It has been reported that the antioxidant activity of oleuropein is comparable to that of hydrosoluble α-tocopherol [26], [42]. Thus, antioxidant and anti-inflammatory properties of oleuropein made it an adequate candidate to investigate its potential protective effects in the heart [27]. The findings of the present study suggest that perfusion of isolated rat hearts with oleuropein (before or after ischemia) had no effects on the physiological functional parameters of myocardium that was obvious by no significant changes in the heart rate, LVEDP, max and min dp/dt and contractility. On the other hand, oleuropein lead to improved cardiac dysfunction following ischemic – reperfusion injury that apparent by decreased LVEDP, and increased max and min dp/dt, LVDP and contractility (Table 1). These cardioprotective effects of oleuropein can be attributed to its antioxidant properties. As we know, the production of free radicals is considerably increased following the reperfusion that might lead to high intracellular calcium concentration or “calcium overload” [43]. In the present study, perfusion of isolated rat hearts with oleuropein (before ischemia or early in reperfusion) attenuated the reperfusion induced – calcium overload that was evident by decreased LVEDP during reperfusion of ischemic myocardium (Table 1). Until now, there has been no report about the effect of oleuropein on gating of calcium channels. Thus, there is need to investigate it in the future studies.
In the present study, the decreased MDA level in the coronary outflow of groups that received oleuropein (Fig. 1A) is a marker of lower oxidative stress during reperfusion that are consistent with previous studies [25]. Manna and co-workers in 2004 also reported that the perfusion of the isolated rat heart with oleuropein could reduce MDA level of coronary outflow [25]. Andreadou and co-workers in 2006 reported that oleuropein lead to the decreased plasma level of MDA as a marker of lipid peroxidation in rats receiving doxorubicin [29].
The results of our study indicated that perfusion of isolated rat hearts with oleuropein especially early in reperfusion causes the increased myocardial contractility. Thus, oleuropein might lead to the facilitation of influx and outflow of calcium during systole and diastole, respectively. Andreadou and co-workers in 2006 observed that administration of oleuropein to rats with doxorubicin-induced cardiotoxicity caused improved myocardial contractility [44] that is in accordance with the results of our study in vitro.
Previous studies using the anesthetized rabbit model indicated that feeding of rabbits with oleuropein-rich diet had anti-infarct effect [29]. In the present study, we also observed that perfusion of isolated rat heart with oleuropein (before ischemia and/or early in reperfusion) had an anti-infarct effect. The anti-infarct effect of oleuropein is consistent with the decreased level of CK activity in coronary outflow. The CK activity is considered as a marker of myocardial injury [25]. Manna and co-worker reported that perfusion of isolated rat heart with 20 μg/g heart oleuropein lead to the decreased CK activity in coronary outflow, but they did not point toward the infarct size [25].
It has been documented that following the restoration of the occluded coronary flow, despite patent epicardial coronary artery, the myocardial perfusion to the ischemic area decreases. This is known as no – reflow phenomenon that are mainly attributed to the arteriolar and capillary structural changes during reperfusion [37], [45]. The findings of the present study show that perfusion of isolated hearts with solution containing oleuropein before induction of the ischemic-reperfusion injury had no effect on the contractile tone of coronary vessels (no vasodilatory effect) that are inconsistent with the findings of Petkov and Malonov 's work in 1978 [4]. This inconsistency maybe due to the differences in the doses and the models used. However, perfusion of myocardium with oleuropein (10 and 50 μg/g heart, before ischemia or early in reperfusion) increased the rate of coronary outflow. According to the above information, it appears oleuropein increased the rate of coronary outflow through the protection of vascular endothelial cells against ischemic-reperfusion injury and attenuation of the no-reflow phenomenon. Parzonko and co-workers in 2013 reported that oleuropein protected the endothelial progenitor cells against angiotensin II cytotoxicity in vitro [46] that is in agreement with the data of our study. Two studies using human settings shown that olive leaf extract enriched with oleuropein had antihypertensive effect that was comparable with captopril as a standard synthetic antihypertensive drug [22], [47]. In Manna's study that is almost like our study, there was no measurement of coronary outflow [25]. There is a need for more investigations in this context in the future.
Another dangerous complication of reperfusion of the ischemic myocardium is the incidence of life – threatening arrhythmia namely ventricular tachycardia and ventricular fibrillation [48]. In the present study, we observed that perfusion of isolated rat hearts with oleuropein especially with high dose of oleuropein (50 μg/g heart) significantly decreased the incidence of irreversible ventricular fibrillation during reperfusion (Fig. 1D). There have been very studies on oleuropein and cardiac arrhythmia. In 1978, Petkov and Malonov, for the first time, reported that oleuropein had anti-arrhythmic effects against aconitine, calcium chloride, barium chloride and ischemic-induced arrhythmia in different animal models [4]. We have previously indicated that pre-treatment of rats with a single dose of oleuropein (100 mg/kg, i.p), before the excision of the heart, induced cardioprotective effects that continued for about 3h [49]. We also observed that oral administration of oleuropein (20 mg/kg/day) for 28 days could protect the heart against aconitine-induced arrhythmia, but not for 3, 7 and 14 days [26]. There are two likely reasons for these differences: first, it appears the oral dose was low and second, oleuropein after absorption was converted to its other metabolites with weaker effects.
5. Conclusions
The results of the present study indicated that perfusion of isolated rat heart with oleuropein had anti-stunning, anti-infarct, anti-arrhythmic, and antioxidant effects. Thus, further studies are needed to assess if intracoronary administration of oleuropein before the cardiac surgery procedures especially prior to coronary artery bypass graft or percutaneous angioplasty or thrombolytic therapy could reduce the magnitude of the complications of ischemic–reperfusion injury in human settings.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful to the Vice-Chancellor of Shahid Sadoughi University of Medical Sciences, Yazd, Iran that provided us a grant.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Fatehi M., Farifteh F., Fatehi-Hassanabad Z. Antispasmodic and hypotensive effects of Ferula asafoetida gum extract. J Ethnopharmacol. 2004;91:321–324. doi: 10.1016/j.jep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Dehpour A.A., Ebrahimzadeh M.A., Seyed Fazel N., Seyed Mohammad N. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. 2009;60(4):405–412. [Google Scholar]
- 3.Abd El-Razek M.H. A new ester isolated from Ferula assa-foetida L. Biosci Biotechnol Biochem. 2007;71(9):2300–2303. doi: 10.1271/bbb.70065. [DOI] [PubMed] [Google Scholar]
- 4.Petkov V., Manolov P. Pharmacological studies on substances of plant origin with coronary dilatating and antiarrhythmic action. Am J Chin Med. 1978 1;06(02):123–130. doi: 10.1142/s0147291778000198. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez M., Zarzuelo A., Gamez M.J., Utrilla M.P., Jimenez J., Osuna I. Hypoglycemic activity of olive leaf. Planta Med. 1992;58(06):513–515. doi: 10.1055/s-2006-961538. [DOI] [PubMed] [Google Scholar]
- 6.Somova L.I., Shode F.O., Ramnanan P., Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003;84(2–3):299–305. doi: 10.1016/s0378-8741(02)00332-x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Azzawie H.F., Alhamdani M.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006;78:1371–1377. doi: 10.1016/j.lfs.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 8.El S.N., Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67:632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee O.H., Lee B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol. 2010;101:3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Dekanski D., Ristic S., Mitrovic D.M. Antioxidant effect of dry olive (Olea europaea L.) leaf extract on ethanol-induced gastric lesions in rats. Mediterr J Nutr Metab. 2009;2(3):205–211. [Google Scholar]
- 11.Czerwinska M., Kiss A.K., Naruszewicz M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012 1;131(3):940–947. [Google Scholar]
- 12.Domitrovic R., Jakovac H., Marchesi V.V., Sain I., Romic Z., Rahelic D. Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol Res. 2012;65(4):451–464. doi: 10.1016/j.phrs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Svobodova M., Andreadou I., Skaltsounis A.L., Kopecky J., Flachs P. Oleuropein as an inhibitor of peroxisome proliferator-activated receptor gamma. Genes Nutr. 2014;9(1):376. doi: 10.1007/s12263-013-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cumaoglu A., Rackova L., Stefek M., Kartal M., Maechler P., Karasu C. Effects of olive leaf polyphenols against H(2)O(2) toxicity in insulin secreting beta-cells. Acta Biochim Pol. 2011;58(1):45–50. [PubMed] [Google Scholar]
- 15.Han J., Talorete T.P., Yamada P., Isoda H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology. 2009;59:45–53. doi: 10.1007/s10616-009-9191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chimento A., Casaburi I., Rosano C., Avena P., De L.A., Campana C. Oleuropein and hydroxytyrosol activate GPER/GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol Nutr Food Res. 2014;58(3):478–489. doi: 10.1002/mnfr.201300323. [DOI] [PubMed] [Google Scholar]
- 17.Priore P., Siculella L., Gnoni G.V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J Nutr Biochem. 2014;25(7):683–691. doi: 10.1016/j.jnutbio.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.de Bock M., Thorstensen E.B., Derraik J.G., Henderson H.V., Hofman P.L., Cutfield W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol Nutr Food Res. 2013;57(11):2079–2085. doi: 10.1002/mnfr.201200795. [DOI] [PubMed] [Google Scholar]
- 19.Jemai H., Bouaziz M., Fki I., El Feki A., Sayadi S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chemico-biological Interact. 2008;176:88–98. doi: 10.1016/j.cbi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Scheffler A., Rauwald H.W., Kampa B., Mann U., Mohr F.W., Dhein S. Olea europaea leaf extract exerts L-type Ca(2+) channel antagonistic effects. J Ethnopharmacol. 2008;120:233–240. doi: 10.1016/j.jep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Singh I., Mok M., Christensen A.M., Turner A.H., Hawley J.A. The effects of polyphenols in olive leaves on platelet function. Nutr metabolism Cardiovasc Dis. 2008;18:127–132. doi: 10.1016/j.numecd.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Susalit E., Agus N., Effendi I., Tjandrawinata R.R., Nofiarny D., Perrinjaquet-Moccetti T. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18(4):251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Andreadou I., Papaefthimiou M., Zira A., Constantinou M., Sigala F., Skaltsounis A.L. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 2009;22:585–592. doi: 10.1002/nbm.1370. [DOI] [PubMed] [Google Scholar]
- 24.Andreadou I., Sigala F., Iliodromitis E.K., Papaefthimiou M., Sigalas C., Aligiannis N. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol. 2007;42:549–558. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Manna C., Migliardi V., Golino P., Scognamiglio A., Galletti P., Chiariello M. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J Nutr Biochem. 2004;15:461–466. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Esmailidehaj M., Mirhosseini S.J., Rezvani M.E., Rasoulian B., Mosaddeghmehrjardi M.H., Haghshenas D. Prolonged oral administration of oleuropein might protect heart against aconitine-induced arrhythmia. Iran J Pharm Res. 2012;11(4):1255–1263. [PMC free article] [PubMed] [Google Scholar]
- 27.Nekooeian A.A., Khalili A., Khosravi M.B. Effects of oleuropein in rats with simultaneous type 2 diabetes and renal hypertension: a study of antihypertensive mechanisms. J Asian Nat Prod Res. 2014;23:1–10. doi: 10.1080/10286020.2014.924510. [DOI] [PubMed] [Google Scholar]
- 28.Janahmadi Z., Nekooeian A.A., Moaref A.R., Emamghoreishi M. Oleuropein offers cardioprotection in rats with acute myocardial infarction. Cardiovasc Toxicol. 2015;15(1):61–68. doi: 10.1007/s12012-014-9271-1. [DOI] [PubMed] [Google Scholar]
- 29.Andreadou I., Iliodromitis E.K., Mikros E., Constantinou M., Agalias A., Magiatis P. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J Nutr. 2006;136(8):2213–2219. doi: 10.1093/jn/136.8.2213. [DOI] [PubMed] [Google Scholar]
- 30.Brahmi F., Mechri B., Dabbou S., Dhibi M., Hammami M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind Crops Prod. 2012;38(0):146–152. [Google Scholar]
- 31.Oi-Kano Y., Kawada T., Watanabe T., Koyama F., Watanabe K., Senbongi R. Oleuropein, a phenolic compound in extra virgin olive oil, increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J Nutr Sci Vitaminol. 2008;54(5):363–370. doi: 10.3177/jnsv.54.363. [DOI] [PubMed] [Google Scholar]
- 32.Poudyal H., Campbell F., Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr. 2010;140(5):946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 33.Ebel D., Schlack W., Comfere T., Preckel B., Thamer V. Effect of propofol on reperfusion injury after regional ischaemia in the isolated rat heart. Br J Anaesth. 1999;83:903–908. doi: 10.1093/bja/83.6.903. [DOI] [PubMed] [Google Scholar]
- 34.Williams R.S., Benjamin I.J. Protective responses in the ischemic myocardium. J Clin Invest. 2000;106:813–818. doi: 10.1172/JCI11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu M., Wu M., Wang J.F., Qiao Y.J., Wang Z. Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes. Biochem Biophys Res Commun. 2007 23;354(4):929–936. doi: 10.1016/j.bbrc.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 36.Guo R., Gao X.Y., Wang W., Wang H.J., Zhang F., Zhang Y. Tempol reduces reperfusion-induced arrhythmias in anaesthetized rats. Pharmacol Res. 2005;52:192–198. doi: 10.1016/j.phrs.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Williams T.M., Waksman R., De S.K., Jacques A., Mahmoudi M. Ischemic preconditioning-an unfulfilled promise. Cardiovasc Revasc Med. 2015;16(2):101–108. doi: 10.1016/j.carrev.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Becker L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004 15;61(3):461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Mohagheghi F., Bigdeli M.R., Rasoulian B., Hashemi P., Pour M.R. The neuroprotective effect of olive leaf extract is related to improved blood-brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine. 2011;18:170–175. doi: 10.1016/j.phymed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Khalatbary A.R., Zarrinjoei G.R. Anti-inflammatory effect of oleuropein in experimental rat spinal cord trauma. Iran Red Crescent Med J. 2012;14(4):229–234. [PMC free article] [PubMed] [Google Scholar]
- 41.Vissers M.N., Zock P.L., Roodenburg A.J., Leenen R., Katan M.B. Olive oil phenols are absorbed in humans. J Nutr. 2002;132(3):409–417. doi: 10.1093/jn/132.3.409. [DOI] [PubMed] [Google Scholar]
- 42.Mancebo-Campos V., Salvador M.D., Fregapane G. Antioxidant capacity of individual and combined virgin olive oil minor compounds evaluated at mild temperature (25 and 40 degrees C) as compared to accelerated and antiradical assays. Food Chem. 2014;150:374–381. doi: 10.1016/j.foodchem.2013.10.162. [DOI] [PubMed] [Google Scholar]
- 43.Talukder M.A., Zweier J.L., Periasamy M. Targeting calcium transport in ischaemic heart disease. Cardiovasc Res. 2009;84(3):345–352. doi: 10.1093/cvr/cvp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreadou I., Mikros E., Ioannidis K., Sigala F., Naka K., Kostidis S. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J Mol Cell Cardiol. 2014;69:4–16. doi: 10.1016/j.yjmcc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Rezkalla S.H., Kloner R.A. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72(7):950–957. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 46.Parzonko A., Czerwinska M.E., Kiss A.K., Naruszewicz M. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine. 2013;20(12):1088–1094. doi: 10.1016/j.phymed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Perrinjaquet-Moccetti T., Busjahn A., Schmidlin C., Schmidt A., Bradl B., Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytotherapy Res. 2008;22:1239–1242. doi: 10.1002/ptr.2455. [DOI] [PubMed] [Google Scholar]
- 48.Canyon S.J., Dobson G.P. Protection against ventricular arrhythmias and cardiac death using adenosine and lidocaine during regional ischemia in the in vivo rat. Am J physiol Heart circulatory physiol. 2004;287:H1286–H1295. doi: 10.1152/ajpheart.00273.2004. [DOI] [PubMed] [Google Scholar]
- 49.Esmailidehaj M., Rasulian B., Rezvani M.E., Delfan B., Mosaddeghmehrjardi M.H., Pourkhalili K. The anti-infarct, antistunning and antiarrhythmic effects of oleuropein in isolated rat heart. EXCLI J. 2012;11:150–162. [PMC free article] [PubMed] [Google Scholar]