Abstract
Background
Soya supplements are used in the treatment of neuropathic pain. Previous reports reveal that consumption of soy diet before nerve injury prevents the development of neuropathic pain in rats. Biochanin-A, a soy isoflavone, has a naturally occurring inhibitor of fatty acid amide hydrolase (FAAH) that metabolized endocannabinoids.
Objective
The objective was to evaluate efficacy of biochanin-A in streptozotocin (STZ) induced neuropathic pain in rat model.
Materials and methods
Diabetes mellitus was induced by an injection of STZ at a dose of 45 mg/kg, i.v. into tail vein of male albino Wistar rats. Biochanin-A was dosed at 0.1, 1 and 5 mg/kg by intraperitoneal (i.p.) administration in diabetic neuropathic rats. Mechanical hyperalgesia and allodynia was measured using Randall–Selitto analgesymeter and manual von Frey filaments of increasing weights respectively. Paw withdrawal threshold (PWT) and percent PWT was determined with respect to both hyperalgesia and allodynia.
Results
Treatment of biochanin-A at three different levels of 0.1, 1 and 5 mg/kg had not significantly altered serum glucose levels throughout the treatment period. In hyperalgesia study, acute treatment with higher dose exhibited 51.1% reversal of paw withdrawal threshold (PWT) while with chronic treatment, efficacy declined to 22.5% reversal of PWT. In allodynia study, acute treatment reversed PWT by 79.4% while with chronic treatment, efficacy was raised to 88.2% reversal of PWT.
Conclusion
Biochanin-A demonstrated better efficacy in reversing mechanical allodynia than mechanical hyperalgesia. Biochanin-A could be a good drug candidate for further studies to establish the mechanism of attenuation of neuropathic pain.
Keywords: Allodynia, Diabetic neuropathy, Hyperalgesia, Isoflavone, Streptozotocin, Hyperglycemia, Paw withdrawal threshold, von Frey filaments
1. Introduction
Diabetes mellitus is a metabolic disorder manifested by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of different organs, especially the eyes, kidneys, nerves, heart and blood vessels [1]. The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030 [2]. Recently, World Health Organization (WHO) reportedly estimated 1.5 million deaths caused by diabetes [3]. Diabetes prevalence is alarmingly increasing every year with large proportion of patients suffering from neuropathic symptoms. Diabetic neuropathic pain states are really devastating to a patient's quality of life in the long run. There is a growing importance for the treatment of diabetic neuropathic pain for which there are no appropriate treatment strategies. At present, pharmacotherapy of neuropathic pain is largely limited to mainly “off-label” use of drugs approved for other conditions, especially tricyclic anti-depressants and anti-convulsants. Hence, study was planned to address the problem of diabetic neuropathic pain in animal models.
Bioflavonoids comprise a group of phenolic secondary plant metabolites that are widespread in nature. Among bioflavonoids, isoflavones are still being extensively studied to indentify therapeutically active constituents in the area of neuropathic pain. Flavonoids like hesperidin have been demonstrated to be beneficial in experimental neuropathic pain [4]. Biochanin-A, an O-methylated isoflavone, is a natural organic compound in the class of phytochemicals known as flavonoids. Biochanin-A can be found in red clover in soy, in alfalfa sprouts, in peanuts, in chick pea (Cicer arietinum) and in other legumes [5]. Biochanin-A could provide beneficial effects for human health, including prevention of cancers, heart disease, menopausal symptoms, and osteoporosis [6]. Furthermore, soya supplements are used in the treatment of neuropathic pain [7]. Previous reports reveal that consumption of soy diet before nerve injury prevents the development of neuropathic pain in rats [8]. With remarkable beneficial evidence of soy isoflavones in neuropathic pain states, it is quite rational to explore possible efficacy of biochanin-A in animal models of neuropathic pain. Hence, our investigation was aimed to evaluate the efficacy of biochanin-A, a soy isoflavone in streptozotocin (STZ) induced neuropathic pain in rat models.
2. Materials and methods
2.1. Materials
STZ and biochanin-A were purchased from the Sigma Chemical Company (St Louis, USA). Thiopentone sodium was supplied by Abbott Lab Ltd (Ankleshwar, India). Gabapentin is the generous gift sample from Sun Pharmaceuticals Ltd, Andheri, and Mumbai. All other chemicals and reagents were used of analytical grade.
2.2. Animals
Male albino Wistar rats (Mahaveera Enterprises Pvt Ltd, Hyderabad, India) weighing 200–250 g were selected. Animals were maintained under standard laboratory conditions at 25 ± 2 °C, relative humidity 50 ± 15% and normal photoperiod (12 h dark/light). Commercial pellet diet (Rayon's Biotechnology Pvt Ltd, India) and water were provided ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC approved protocol number: KVSRSCOPS/11-03-14-010) of KVSR Siddhartha College of Pharmaceutical Sciences, Siddhartha Nagar, Vijayawada and animal experiments were carried out as per Animal Regulatory Body of the Government (Regd. No. 993/a/06/CPCSEA).
2.3. Experimental design
The rats were randomly divided into five groups with six animals each. Group 1, Normal control group treated with vehicle (5% DMSO + 10% Tween-80 + 75% distilled water); Group 2, Diabetic animals treated with biochanin-A (0.1 mg/kg, i.p.); Group 3, Diabetic animals treated with biochanin-A (1 mg/kg, i.p.); Group 4, Diabetic animals treated with biochanin-A (5 mg/kg, i.p.); Group 5: diabetic animals treated with Gabapentin (100 mg/kg, i.p). Diabetes induction day was considered as Day 0. Biochanin-A acute treatment was given as a single dose on Day 21 of STZ induction and pain parameters were assessed. Chronic treatment was given for seven days following acute treatment and again on 8th day of treatment, pain was assessed.
2.4. Induction of diabetes and neuropathic pain
Diabetes was induced by a single intravenous (i.v.) injection of STZ, 45 mg/kg of body weight, dissolved in citrate buffer (pH 4.5), into the tail vein of animals [9]. Diabetes was confirmed after the third day of STZ injection by estimating serum glucose using semi-automatic analyser (Model: Erba Chem 5 plus V2; Transasia Bo-medicals Ltd) and Erba Transasia glucose kit. Animals that developed serum glucose levels of more than 250 mg/dL were considered diabetic and included into the study. Following induction of diabetes mellitus, animals were allowed for 21 days for the development of neuropathic pain. On 22nd day, animals were evaluated for the development of symptoms of mechanical hyperalgesia and mechanical allodynia. Animals were considered to be neuropathic when the same exhibited mechanical allodynia (i.e., paw withdrawal or flinching behaviour response to the application of a bending force of less than 4 g) and mechanical hyperalgesia (i.e., paw withdrawal response was observed at a paw pressure of less than or equal to 70 g).
2.5. Determination of mechanical hyperalgesia
Mechanical nociceptive threshold or PWT, an index of mechanical hyperalgesia, was assessed by previously described method [10]. The paw withdrawal threshold was quantified using the Randall–Selitto paw pressure analgesymeter (model, 37215; UGO Basile, Italy). Increasing pressure at a linear rate of 10 g/s was applied to the centre of the hind paw. Pressure at which animal withdraws its paw was recorded and expressed in mass units (g), with a cut-off of 150 g to avoid potential tissue injury. PWT was recorded for the left hind paw before and up to 4 h after treatment. Percent reversal of PWT was determined using the formula: [(Post-dose threshold − Pre-dose)/(Naive threshold − pre-dose threshold)] × 100.
2.6. Determination of mechanical allodynia
The rats were placed individually in plastic cages with a plastic mesh floor to determine withdrawal threshold. The animals were tested after acclimatization to the environment, typically 20–30 min after placement in the cage. The paw withdrawal threshold in response to mechanical stimulation was measured using the up and down method [11] by applying calibrated von Frey filaments (Aesthesio®; Ugo basile, Italy) to the hind paw from underneath the cage through openings in the mesh floor. A series of von Frey filaments (0.4, 0.7, 0.16, 0.40, 0.60, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10, 15, 26 and 60 g) were applied vertically to the plantar surface of the hind paw for 5 s while the hair was bent. Brisk withdrawal of paw or paw flinching was considered a positive response. The absence of a response in the animals at a pressure of 60 g was considered the cut off value. The stimulation with one filament was repeated five times at 10–15 s intervals, when lack of a response, the next filament with greater bending force was applied. The lowest force required to elicit a paw withdrawal response was recorded as the PWT (g). The animals that exhibited paw withdrawal response or flinching response at less than 4 g were considered to have developed the allodynia. Percent reversal of PWT was determined using the formula: [(Post-dose threshold – Pre-dose)/(Naive threshold – pre-dose threshold)] × 100.
2.7. Statistical analysis
The results were expressed as mean ± standard deviation (S.D). Differences in PWT (both in hyperalgesia and allodynia) were determined by two way analysis of variance followed by Bonferroni post hoc test. Differences at *p < 0.05, **p < 0.01, ***p < 0.001 were considered statistically significant.
3. Results
3.1. Effect of biochanin-A on serum glucose in control and treated rats
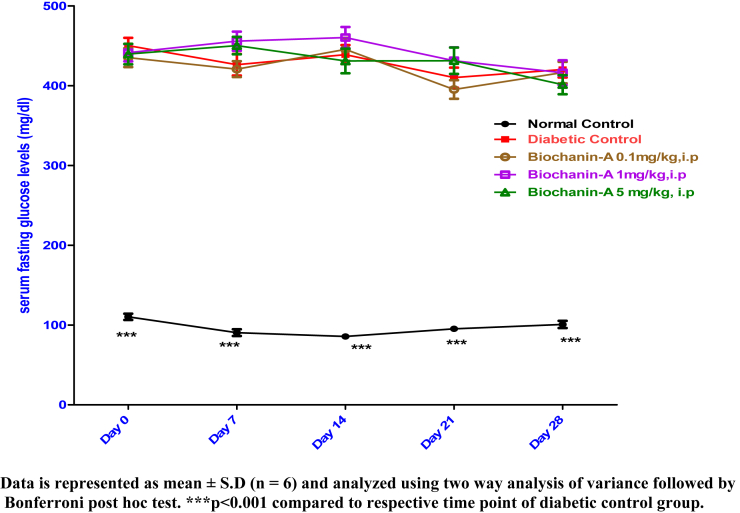
Serum fasting glucose levels were estimated on Day 0, Day 7, Day 14, Day 21 and Day 28. Fig. 1 shows serum fasting glucose levels in normal control, diabetic control and treatment groups.I.p. administration of biochanin-A at three different dose levels of 0.1, 1 and 5 mg/kg has not significantly altered serum glucose levels throughout the treatment period.
Fig. 1.
Effect of biochanin-A on serum fasting glucose levels in diabetic rats.
3.2. Effect of acute treatment of biochanin-A on mechanical hyperalgesia
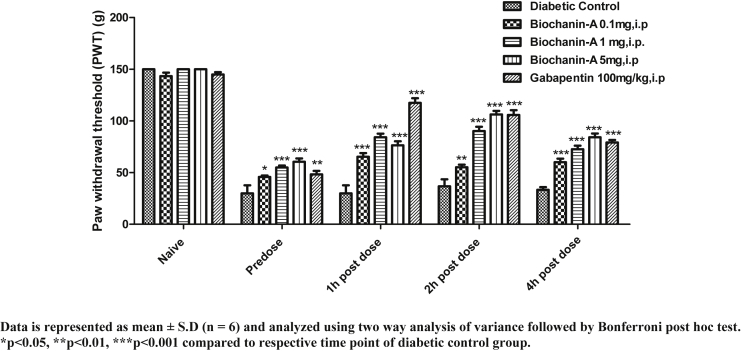
In all the animals, cut off PWT was 150 g. In normal control rats, PWT was not significantly altered. In diabetic control rats, PWT declined from 150 g (naive) to 30 g (pre-dose). In biochanin-A (0.1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 143.33 ± 8.16, 41.67 ± 7.53, 53.33 ± 9.83, 52.50 ± 7.58 and 60.00 ± 8.37 respectively. In biochanin-A (1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 143.33 ± 8.16, 42.50 ± 5.24, 60.0 ± 9.49, 54.17 ± 7.36 and 55.00 ± 10.49 respectively. In biochanin-A (5 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 143.33 ± 8.16, 43.33 ± 5.16, 73.33 ± 7.53, 65.83 ± 7.36 and 55.00 ± 5.48 respectively. The effect observed with biochanin-A in reversing mechanical hyperalgesia was almost negligible though values were statistically significant (p < 0.001) at 1 h and 2 h post-dose. The effect was neither dose dependent nor time dependent with biochanin-A whereas Gabapentin (100 mg/kg, i.p) had shown remarkable (p < 0.001) reversal of PWT at 1 h, 2 h and 4 h post-dose when compared to pre-dose PWT. Peak effect was observed at 1 h post-dose with Gabapentin (Fig. 2).
Fig. 2.
Effect of acute i.p. treatment of biochanin-A on mechanical hyperalgesia in diabetic neuropathic rats.
3.3. Effect of chronic treatment of biochanin-A on mechanical hyperalgesia
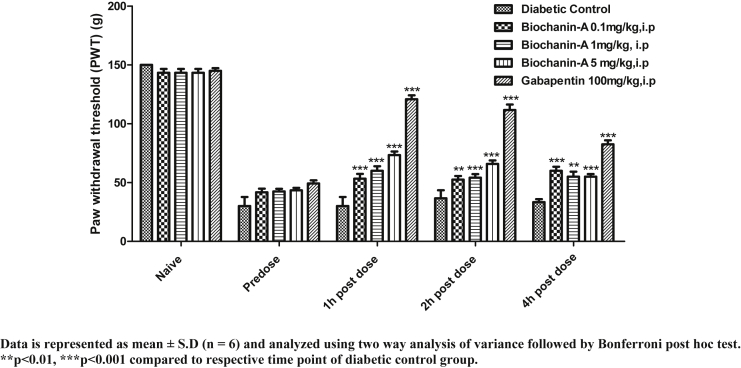
Fig. 3 shows PWT of animals in control and chronic treatment groups. In all the animals, cut off PWT was 150 g. In normal control rats, PWT was not significantly altered. In diabetic control rats, PWT declined from 150 g (naive) to 30 g (pre-dose). In biochanin-A (0.1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 143.33 ± 8.16, 45.71 ± 3.60, 65.32 ± 8.74, 55.16 ± 6.34 and 60.04 ± 8.37 respectively. In biochanin-A (1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 150.00 ± 0.00, 54.92 ± 4.70, 84.21 ± 8.40, 90.24 ± 10.20 and 72.63 ± 8.34 respectively. In biochanin-A (5 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 150.0 ± 0.00, 60.41 ± 7.98, 76.43 ± 9.34, 106.25 ± 8.32 and 84.12 ± 9.20 respectively. The effect observed with biochanin-A was statistically significant (p < 0.001) at 1 h 2 h and 4 h post-dose whereas Gabapentin (100 mg/kg, i.p) had shown remarkable (p < 0.001) reversal of PWT at 1 h, 2 h and 4 h post-dose when compared to pre-dose PWT. Peak effect was observed at 1 h post-dose with Gabapentin.
Fig. 3.
Effect of chronic i.p. treatment of biochanin-A on mechanical hyperalgesia in diabetic neuropathic rats.
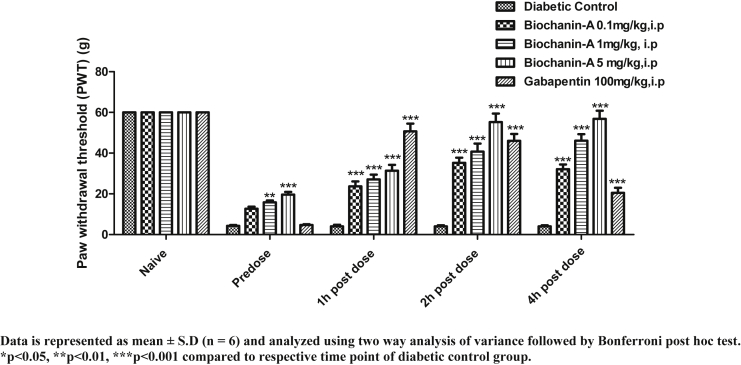
3.4. Effect of acute treatment of biochanin-A on mechanical allodynia
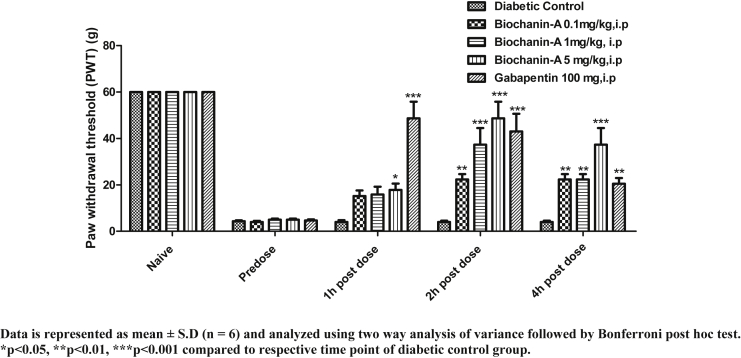
PWT of animals in allodynia test is presented in Fig. 4. In all animals, cut off PWT was 60 g. In diabetic control rats, PWT declined from 60 g (naive) to 4.3 g (pre-dose). In biochanin-A (0.1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 60.0 ± 0.0, 4.0 ± 1.6, 15.17 ± 5.85, 22.33 ± 5.68 and 22.33 ± 5.68 respectively. In biochanin-A (1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 60.0 ± 0.0, 5.0 ± 1.10, 15.83 ± 8.21, 37.33 ± 17.56 and 22.33 ± 5.68 respectively. In biochanin-A (5 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 60.0 ± 0.0, 5.0 ± 1.10, 17.83 ± 6.62, 48.67 ± 17.56 and 37.33 ± 17.56 respectively. Biochanin-A had significantly (p < 0.001) reversed PWT at 2 h and 4 h post-treatment. At all three doses, there was no significant reversal of PWT at 1 h post-treatment. The maximum reversal was observed at 2 h post-dose at all doses but effect was almost maintained at 4 h post-dose at higher dose whereas, the effect declined 4 h post-dose with biochanin-A 0.1 mg/kg and 1 mg/kg doses. Dose-dependent increase in the effect was observed with increasing dose. Biochanin-A had reversed mechanical allodynia on par with the standard drug, Gabapentin but the only difference was that effect was delayed with biochanin-A.
Fig. 4.
Effect of acute i.p. treatment of biochanin-A on mechanical allodynia in diabetic neuropathic rats.
3.5. Effect of chronic treatment of biochanin-A on mechanical allodynia
In all animals, cut off PWT was 60 g. In diabetic control rats, PWT declined from 60 g (naive) to 4.33 g (pre-dose). In biochanin-A (0.1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 60.0 ± 0.0, 12.71 ± 2.41, 23.69 ± 5.85, 35.24 ± 6.27 and 32.10 ± 5.68 respectively. In biochanin-A (1 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 60.0 ± 0.0, 15.86 ± 2.18, 27.13 ± 5.67, 40.74 ± 9.65 and 46.11 ± 7.83 respectively. In biochanin-A (5 mg/kg) treatment group, naive PWT, pre-dose PWT, 1 h, 2 h, and 4 h post-dose PWT were found to be 60.0 ± 0.0, 19.65 ± 3.24, 31.34 ± 7.16, 55.24 ± 10.28 and 56.81 ± 9.81 respectively. Biochanin-A had significantly (p < 0.001) reversed PWT at 1 h, 2 h and 4 h post-treatment at all dose levels. The maximum reversal was observed at 2 h post-dose at all doses but effect was almost maintained at 4 h post-dose at higher dose whereas, the effect was not maintained and declined 4 h post-dose with biochanin-A at 0.1 and 1 mg. Dose-dependent increase in the effect was observed with increasing dose. Our results reveal that chronic treatment exhibited superior anti-allodynic effect when compared to acute treatment (Fig. 5).
Fig. 5.
Effect of chronic i.p. treatment of biochanin-A on mechanical allodynia in diabetic neuropathic rat.
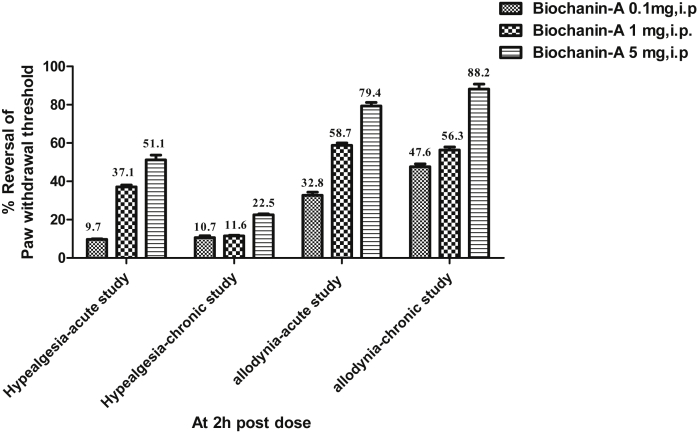
3.6. Effect of biochanin-A on percent reversal of hyperalgesia and allodynia
Percent reversal was represented at 2 h post-dose following treatment of biochanin-A. In hyperalgesia study, acute treatment with biochanin-A at three different dose levels of 0.1, 1 and 5 mg/kg had shown percent reversals of PWT as 9.7, 37.1 and 51.1 respectively while chronic treatment with biochanin-A at three different dose levels of 0.1, 1 and 5 mg/kg had shown percent reversals of PWT as 10.7, 11.6 and 22.5 respectively. In allodynia study, acute treatment with biochanin-A at three different dose levels of 0.1, 1 and 5 mg/kg had shown percent reversals of PWT as 32.8, 58.7 and 79.4 respectively while chronic treatment with biochanin-A at three different dose levels of 0.1, 1 and 5 mg/kg had shown percent reversals of PWT as 47.6, 56.3 and 88.2 respectively. The result clearly indicates that biochanin-A remarkably exhibiting higher degree of efficacy in the reversal of allodynia in comparison to moderate efficacy observed in reversing hyperalgesia (Fig. 6).
Fig. 6.
Percent reversal of PWT at 2 h post-dose in acute hyperalgesia study, chronic hyperalgesia study, acute allodynia study and chronic hyperalgesia study. Percent reversal was indicated at 2h post dose of Biochanin-A treatment.
4. Discussion
Streptozotocin is well reported for its selective pancreatic islet β-cell cytotoxicity [12] and has been widely used to induce diabetes mellitus in animals. Our results reveal that STZ injected rats exhibited significantly increased blood glucose levels as compared with control rats.
The STZ induced diabetic rat [13], [14] and mice [15] have commonly been used as model of neuropathic pain with signs of hyperalgesia and allodynia that may reflect signs observed in diabetic patients. In our study, i.v. administration of STZ (45 mg/kg body weight) significantly resulted in hyperglycemia. Altered pattern of nociception may not be due to inherent neurotoxicity of STZ [16], but STZ induced hyperalgesia clearly contributes to a wide array of pathophysiological symptoms that can lead to altered nociceptive responses tested in various animal models [15], [17], [18].
Our study results report that i.p. administration of biochanin-A at three different dose levels of 0.1, 1 and 5 mg/kg did not significantly alter serum glucose levels throughout the treatment period. Our result is in contrast to the previous finding of antihyperglycemic effect of biochanin-A in terms of reduction in glucose levels [19]. The discrepancy between these two studies could possibly be attributed to variation in experimental design, dose and route of administration. However, given that our aim was mainly to assess the mechanical hyperalgesia and mechanical allodynia, we have estimated only serum glucose levels but not focused on HbA1C and insulin.
Biochanin-A exhibited moderate effect in reversing mechanical hyperalgesia while it elicited high degree of efficacy in reversing mechanical allodynia upon both acute and chronic treatment. Previous in-vivo study reported that biochanin-A is a naturally occurring inhibitor of FAAH, which metabolites endocannabinoids [20]. However, the efficacy of biochanin-A is higher at 2 h post-dose in both hyperalgesia and allodynia. Our result is consistent with earlier pharmacokinetic studies that demonstrate maximum concentrations (Tmax) of biochanin-A was observed in the range of 2–4 h. However, re-entry peaks of biochanin-A in the plasma were observed, that are likely due to enterohepatic recirculation, occurred at 4–5 h after oral administration of the 5 and 50 mg/kg doses of biochanin-A [21], [22]. In our study, biochanin-A was found to be effective even at 4 h post-treatment. This indicates the results are possibly correlating with the earlier pharmacokinetic studies [21], [22].
Similar study also demonstrated that kaempferol, isoflavone, and related naturally occurring flavonoids inhibit FAAH [23]. Another in-vitro study implied that inhibition of the cellular uptake of anandamide by genistein and its analogue daidzein in cells with different levels of FAAH-driven uptake was observed. This clearly indicates that isoflavones have the ability to enhance the endocannabinoids that are known to attenuate neuropathic pain states. In 2004, McFarland et al. reported that the soy isoflavone genistein could interfere with the cellular uptake of anandamide (AEA) [24]. Subsequent studies showed that property was shared by daidzein and was due to the ability of the compounds to inhibit FAAH [25], [26].
In Asian countries, where soy consumption is higher than in Western countries, serum concentrations of genistein and daidzein can attain as high as 2–4 mM [27], implied that the concentrations necessary for inhibition of FAAH are reachable in-vivo. Biochanin-A was a mixed-type inhibitor of the rat FAAH and showed similar low micromolar potencies towards rat, mouse and recombinant human FAAH [20].
In another study, biochanin-A was tested at doses of 30, 100 and 300 mg and was found to be effective in formalin induced ERK phosphorylation in a manner antagonized by AM251. Thus, biochanin-A behaved like URB597 after local administration to the paw [20]. Thus, biochanin-A could be suitable drug candidate for neuropathic pain patients in alleviating majorly mechanical allodynia and moderately mechanical hyperalgesia.
5. Conclusion
Biochanin-A demonstrates better efficacy in reversing mechanical allodynia than in mechanical hyperalgesia. Biochanin-A could be a deserved candidate for further studies to establish the mechanisms in the area of neuropathic pain.
Funding
The research work was funded by University Grants Commission (UGC), New Delhi Government of India.
Declaration of interests
Authors declare that there are no competing interests for each other.
Acknowledgements
Authors wish to thank University Grants Commission (UGC), New Delhi for funding the project. We would also like to thank Siddhartha Academy of General and Technical Education (SAGTE) for providing research facilities. The authors are grateful to Sun Pharmaceuticals Ltd for the generous gifts of Gabapentin.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; Geneva: 2014. Global health estimates: deaths by cause, age, sex and country, 2000-2012. [Google Scholar]
- 4.Visnagri A., Kandhare A.D., Chakravarty S., Ghosh P., Bodhankar S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014;52:814–828. doi: 10.3109/13880209.2013.870584. [DOI] [PubMed] [Google Scholar]
- 5.Tan J.W., Tham C.L., Israf D.A., Lee S.H., Kim M.K. Neuroprotective effects of biochanin-A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem Res. 2013;38:512–518. doi: 10.1007/s11064-012-0943-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen H.Q., Jin Z.Y., Li G.H. Biochanin-A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci Lett. 2007;417:112–117. doi: 10.1016/j.neulet.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Bermejo P.E. Soya supplements in the treatment of neuropathic pain. Rev Neurol. 2007;45:479–481. [PubMed] [Google Scholar]
- 8.Shir Y., Raja S.N., Weissman C.S., Campbell J.N., Seltzer Z. Consumption of soy diet before nerve injury preempts the development of neuropathic pain in rats. Anesthesiology. 2001;95:1238–1244. doi: 10.1097/00000542-200111000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Annapurna A., Reddy C.S., Akondi R.B., Rao S.R. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2009;61:1365–1374. doi: 10.1211/jpp/61.10.0014. [DOI] [PubMed] [Google Scholar]
- 10.Randall L.O., Selitto J. A method for measurement of analgesic activity of inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:209–219. [PubMed] [Google Scholar]
- 11.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Papaccio G., Pisanti F.A., Latronico M.V., Ammendola E., Galdieri M. Multiple low-dose and single high-dose treatments with streptozotocin do not generate nitric oxide. J Cell Biochem. 2000;77:82–91. [PubMed] [Google Scholar]
- 13.Mert T., Oksuz H., Tugtag B., Kilinc M., Sahin E., Altun I. Anti-hypernociceptive and anti-oxidative effects of locally treated dobutamine in diabetic rats. Pharmacol Rep. 2015;67:1016–1023. doi: 10.1016/j.pharep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Fischer T.Z., Tan A.M., Waxman S.G. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res. 2009;1268:154–161. doi: 10.1016/j.brainres.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 15.Calcutt N.A., Chaplan S.R. Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol. 1997;122:1478–1482. doi: 10.1038/sj.bjp.0701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamei J., Zushida K., Morita K., Sasaki M., Tanaka S. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol. 2001;422:83–86. doi: 10.1016/s0014-2999(01)01059-7. [DOI] [PubMed] [Google Scholar]
- 17.Courteix C., Eschalier A., Lavaraenne J. STZ-induced diabetic rats: behavioral evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- 18.Hounsom L., Tomlinson D.R. Does neuropathy develop in animal models? Clin Neurosci. 1997;4:380–389. [PubMed] [Google Scholar]
- 19.Harini R., Ezhumalai M., Pugalendi K.V. Antihyperglycemic effect of biochanin-A, a soy isoflavone, on streptozotocin-diabetic rats. Eur J Pharmacol. 2012;676:89–94. doi: 10.1016/j.ejphar.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Thors L., Burston J.J., Alter B.J., McKinney M.K., Cravatt B.F., Ross R.A. Biochanin-A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br J Pharmacol. 2010;160:549–560. doi: 10.1111/j.1476-5381.2010.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon Y.J., Sagawa K., Frederick K., Zhang S., Morris M.E. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006;8(3):E433–E442. doi: 10.1208/aapsj080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachdeva C., Mishra N., Sharma S. Development and characterization of enteric-coated microparticles of biochanin A for their beneficial pharmacological potential in estrogen deficient-hypertension. Drug Deliv. 2016;23(6):2044–2057. doi: 10.3109/10717544.2015.1114046. [DOI] [PubMed] [Google Scholar]
- 23.Thors L., Belghiti M., Fowler C.J. Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br J Pharmacol. 2008;155:244–252. doi: 10.1038/bjp.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarland M.J., Porter A.C., Rakhshan F.R., Rawat D.S., Gibbs R.A., Barker E.L. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem. 2004;279:41991–41997. doi: 10.1074/jbc.M407250200. [DOI] [PubMed] [Google Scholar]
- 25.Thors L., Alajakku K., Fowler C.J. The ‘specific’ tyrosine kinase inhibitor genistein inhibits the enzymic hydrolysis of anandamide: implications for anandamide uptake. Br J Pharmacol. 2007;150:951–960. doi: 10.1038/sj.bjp.0707172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thors L., Eriksson J., Fowler C.J. Inhibition of the cellular uptake of anandamide by genistein and its analogue daidzein in cells with different levels of fatty acid amide hydrolase-driven uptake. Br J Pharmacol. 2007;152:744–750. doi: 10.1038/sj.bjp.0707401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton M.S., Arisaka O., Miyake N., Morgan L.D., Evans B.A. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]