Abstract
AIM
To evaluate the feasibility of repairing a common bile duct defect with a decellularized ureteral graft in a porcine model.
METHODS
Eighteen pigs were randomly divided into three groups. An approximately 1 cm segment of the common bile duct was excised from all the pigs. The defect was repaired using a 2 cm long decellularized ureteral graft over a T-tube (T-tube group, n = 6) or a silicone stent (stent group, n = 6). Six pigs underwent bile duct reconstruction with a graft alone (stentless group). The surviving animals were euthanized at 3 mo. Specimens of the common bile ducts were obtained for histological analysis.
RESULTS
The animals in the T-tube and stent groups survived until sacrifice. The blood test results were normal in both groups. The histology results showed a biliary epithelial layer covering the neo-bile duct. In contrast, all the animals in the stentless group died due to biliary peritonitis and cholangitis within two months post-surgery. Neither biliary epithelial cells nor accessory glands were observed at the graft sites in the stentless group.
CONCLUSION
Repair of a common bile duct defect with a decellularized ureteral graft appears to be feasible. A T-tube or intraluminal stent was necessary to reduce postoperative complications.
Keywords: Decellularization, Stent, Bile duct injury, Biliary reconstruction, Ureteral graft
Core tip: A common bile duct defect is a challenge for hepatobiliary surgeons. A decellularized ureteral graft was introduced in this experimental study to repair a common bile duct defect. If the biliary reconstruction was performed with a T-tube or stent insertion into the ureteral graft, all animals survived with normal liver function. The histology results showed a biliary epithelial layer regeneration over the graft. Thus, repair of a common bile duct defect with a decellularized ureteral graft appears to be feasible. In addition, a T-tube or stent was found to be necessary to reduce postoperative complications in this study.
INTRODUCTION
Defects of the common bile duct may result from operations to treat a congenital choledochous cyst, extrahepatic cholangiocarcinoma or Mirizzi syndrome. However, most defects generally follow iatrogenic bile duct injury during cholecystectomy[1-4]. A small defect may be successfully treated by a plastic repair to enlarge the lumen or an end-to-end anastomosis over a T-tube if there is enough normal biliary tissue to repair the defect without tension[4-7]. A long defect from extensive loss of the common bile duct is a major challenge for hepatobiliary surgeons[1-3]. The most commonly used method for repair of a long common bile duct defect is a biliary-enteric anastomosis (e.g., hepaticojejunostomy, choledochojejunostomy, choledochoduodenostomy)[1-3]. However, a biliary-enteric anastomosis may cause complications, such as retrograde biliary infection, anastomotic stricture, hepatolithiasis and biliary cirrhosis, because of the lack of the sphincter of Oddi[5-7]. An alternative method for repairing the defect is the use of autologous tubular tissue (e.g., artery, vein, appendix), which may cause fibrosis in the free graft and stricture formation at the location of the anastomosis. These issues can impair long-term success[8-11].
In recent years, several new materials with fewer foreign body reactions have been successfully introduced as substitutes for normal bile ducts for biliary defect reconstruction[12-16]. To our knowledge, decellularized ureter tissue has not yet been used as a substitute in experimental biliary defect repair. The primary aim of the present study was to evaluate the feasibility of common bile duct defect repair with a decellularized ureteral graft in a porcine model. The secondary purpose was to determine the effect of a T-tube and intraluminal stent on the outcomes of the present study.
MATERIALS AND METHODS
Laboratory animals
The study was approved by the Local Ethics Committee of West China Hospital (Sichuan, China) (Permit Number: SYXK2013-119) and was in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. A total of 18 experimental pigs (Neijiang cross) were obtained from the Chengdu Ping’an Experimental Animal Reproduction Center (Sichuan, China). Male and female pigs were used and all weighed between 25 and 35 kg. Before starting this experiment, all animals were housed and fed for one week at the Experimental Animal Center at Sichuan University to allow time for adaptation to the new environment. All procedures were carried out under anesthesia. Analgesics were administered to minimize the suffering of the animals. According to the previous published studies[12-16], the pigs were subjected to euthanasia under anesthesia 3 mo post-surgery by an intravenous injection of 10% potassium chloride. In case of an unexpected death, the animal was subjected to autopsy to find the cause of death.
Decellularized ureteral grafts
Ureters of four experimental pigs (Neijiang cross) sacrificed by another team were harvested under sterile conditions. The peripheral connective tissues surrounding the ureters were mechanically removed. Before decellularization, ureters were cut into pieces of 5.0 cm in length. First, ureters were shaken and washed in phosphate-buffered saline (PBS, pH 7.4) for 2 h. Second, ureters were placed in a 1% sodium dodecyl sulfate (SDS; Demchem, Shanghai, China) solution and stirred (500 rpm) with a magnetic stirrer for 24 h. Third, ureters were washed in PBS for 2 h. Fourth, ureters were placed in 1% Triton-100 (Amresco, OH, United States) and stirred (500 rpm) with a magnetic stirrer for 24 h. Then, the decellularized ureteral grafts were washed in PBS for 24 h. SDS, Triton-100 and PBS solutions were changed every 12 h. Finally, the decellularized ureteral grafts were sterilized by gamma radiation and stored in PBS with 1% antibiotic solution at 4 °C. These grafts were cut to 2.0 cm in length (Figure 1A). A scanning electron microscopy (SEM) photogram was obtained to evaluate the graft surface (Figure 1B).
Figure 1.
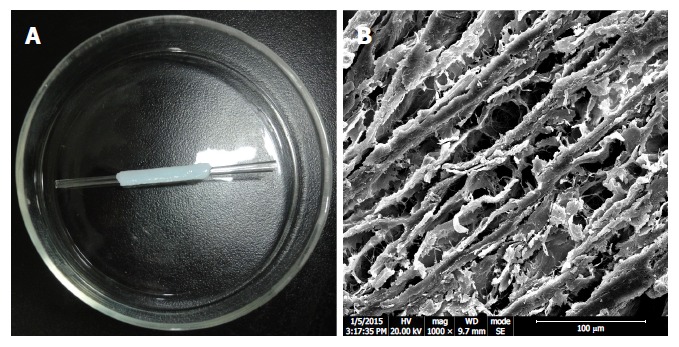
Appearance and structure of a graft used in common bile duct reconstruction. A: The decellularized ureteral graft is over a stent. B: Scanning electron microscope photogram shows porous extracellular matrix and collagen fibers. Scanning bar indicates 100 μm.
Animal model of a common bile duct defect
Preoperatively, all animals were fasted for 12 h but had free access to water. Thirty minutes before surgery, cefoxitin sodium (0.15 g/kg; Jiangsu Yangzijiang, Co., Ltd, China) was administered intravenously. Anesthesia was induced by an intravenous injection of diazepam (0.2 mg/kg) (Hubei Pharmacy, Co., Ltd, China), fentanyl (10 μg/kg) (Yichang Renhe, Co., Ltd, China), ketamine (2 mg/kg) (Zhejiang Jiuxu, Co., Ltd, China) and succinylcholine (2 mg/kg) (Jiangsu Yangzijiang, Co., Ltd, China). After endotracheal intubation, anesthesia was maintained by oxygen and isoflurane (NunanBeite, Co., Ltd, China). All operations were performed under sterile conditions. All animals were placed in the supine position. After a sterile skin preparation, a right subcostal incision was made. After gross inspection of the abdominal cavity, the hepatoduodenal ligament was dissected to identify the cystic duct and common bile duct. After mobilization of the entire common bile duct, a 1 cm long segment distal to the entrance of the cystic duct was resected to create a model of a common bile duct defect. The gallbladder was left in situ.
Study design and surgical technique
Eighteen pigs were randomly divided into three groups, namely, the T-tube (n = 6), stent (n = 6) and stentless (n = 6) groups. In the T-tube group, an 8- or 10-Fr latex T-tube was inserted through a separate incision in the proximal common bile duct approximately 0.5 cm away from the defect. The common bile duct defect was bridged by a 2 cm long decellularized ureteral graft and repaired without tension. An end-to-end anastomosis over the lower limb of the T-tube was performed in this group (Figure 2A). The anastomoses were completed using two continuous 7-0 prolene sutures (Ethicon, NY, United States). A pedicled omentum flap was brought up to wrap the interposed graft for vascularization. The abdomen was closed in two layers (peritoneum and skin) using interrupted 0# silk sutures (Ethicon, NY, United States). The T-tube was fixed on the abdomen and no abdominal drain was placed. The animal had free access to water on the day of the procedure and resumed a normal oral diet on the first postoperative day. Cefoxitin sodium (0.15 g/kg; Jiangsu Yangzijiang, Co., Ltd, China) was administered intramuscularly for the first 3 postoperative days. The T-tube was allowed to drain freely for 3 mo. In the stent group, an end-to-end anastomosis was performed with continuous 7-0 prolene sutures (Ethicon, NY, United States) over an 8-Fr silicon stent (Figure 2B). The endoluminal stent was approximately 4 cm in length and had a decellularized ureteral graft in the middle. The stent spread out 1 cm into the proximal and distal common bile duct stumps. Two transmural stitches using 7-0 prolene sutures were made to fix the position of the stent. In the stentless group, the end-to-end anastomosis was completed with continuous 7-0 prolene sutures (Ethicon, NY, United States) without any T-tube or intraluminal stent insertion (Figure 2C).
Figure 2.
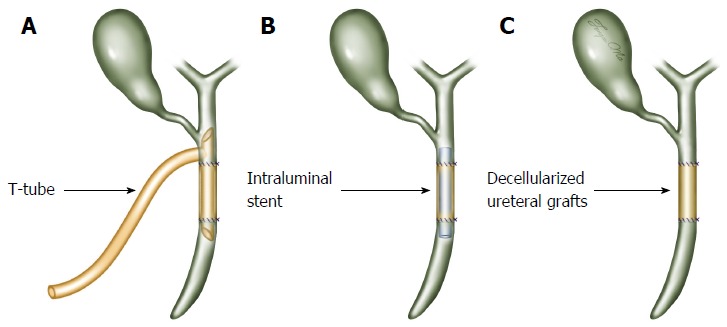
Surgical technique of common bile duct reconstruction. A: The common bile duct defect was reconstructed with a 2 cm long decellularized ureteral graft over the lower limb of the T-tube (T-tube group); B: The defect was reconstructed with a graft over an intraluminal stent (stent group); C: The defect was reconstructed with a graft alone without any T-tube or intraluminal stent insertion (stentless group).
Biochemistry and cholangiography
Blood samples were drawn at postoperative week 1 and once a month. They were sent to the biochemical laboratory at West China Hospital, Sichuan University to evaluate each animal’s liver function. The tests included total bilirubin (TB), alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (GGT). At 3 mo postoperatively, cholangiography was performed for the surviving animals under general anesthesia. During the cholangiography, 20 mL of contrast media (50% iohexol injection; Zhejiang Tianrui, Co., Ltd, China) was injected into the biliary tract through either the T-tube for animals in the T-tube group or percutaneous gallbladder puncture using guided ultrasonography for animals in the stent and stentless groups. Images were obtained and evaluated by a radiologist at West China Hospital.
Histopathology
Following the cholangiography, all surviving animals underwent an exploratory laparotomy. After a general inspection of the abdominal cavity for signs of biliary leakage and abdominal abscess, all animals were sacrificed by an intravenous injection of 10% potassium chloride. The entire extrahepatic biliary tree, including grafts and native bile ducts, were harvested from the animals. Specimens of the bile ducts were fixed in either 10% neutral buffered formalin or liquid nitrogen and sent to the Pathology Department of West China Hospital for histopathology evaluation. Conventional hematoxylin/eosin (H&E) staining was performed to observe the morphology of the bile duct. An anti-cytokeratin 19 antibody (CK19, 1:200, Sigma, United States) was used for immunofluorescent staining to label the bile duct epithelium.
Statistical analysis
SPSS statistical software package (version 22.0, SPSS Inc., Chicago, IL, United States) was used for the statistical analyses. A Mann-Whitney U test or Kruskal-Wallis analysis of variance with multiple comparisons was applied to analyze various continuous variables. Data were considered statistically significant when the P value was less than 0.05.
RESULTS
Survival and complications
All 18 pigs survived the operation. The animals in the T-tube and stent groups gained weight and were in a good general condition after 3 mo at the time of sacrifice. However, the use of only a decellularized ureteral graft for the bile duct reconstruction (stentless group) led to the death of four animals in the first month and two animals in the second month. None of the animals survived until the mandatory sacrifice at 3 mo (Figure 3).
Figure 3.
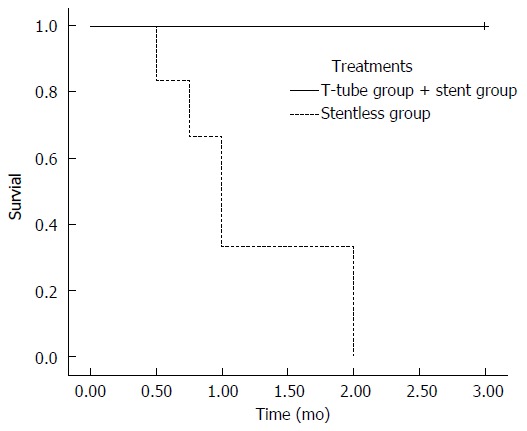
Survival rates. All animals in the T-tube group and stent group survived until their sacrifice after 3 mo. In stentless group, all of the animals died in 2 mo.
None of the animals in the T-tube group suffered from any post-operative complications. One biliary stricture occurred in the 6 pigs in the stent group. This animal appeared clinically well without a significantly decreased appetite, jaundice or weight loss at the 3-mo sacrifice. However, a mild narrowing at the proximal anastomosis and a mild intrahepatic and extrahepatic biliary dilatation were noted. It appeared that stent migration resulted in this biliary stricture because it was noted at autopsy that the stent was not present at the graft site. In the stentless group, repair of the common bile duct defect with a graft alone was not adequate. Within two weeks of the operation, 1 of the 6 pigs (16.7%) developed bile leakage and biliary stricture and subsequently died due to biliary peritonitis. The other five pigs in the stentless group suffered from severe biliary stricture and subsequent death due to acute obstructive suppurative cholangitis. Hepatolithiasis was observed in three pigs that suffered from severe biliary stricture in the stentless group (Table 1).
Table 1.
Post-operative complications in animals
| Complications |
Groups |
||
| T-tube | Stent | Stentless | |
| Bile leakage | 0/6 | 0/6 | 1/6 |
| Biliary stricture | 0/6 | 1/6 | 6/6 |
| Hepatolithiasis | 0/6 | 0/6 | 3/6 |
Biochemistry and cholangiography
The biochemical results of the animals in each group are shown in Table 2. In the T-tube and stent groups, all the biochemical parameters (TB, ALT and GGT) were within normal ranges throughout the study. There was no significant difference in any parameter at each time point between the T-tube and stent groups. In contrast, the levels of TB, ALT and GGT were significantly increased in the surviving animals in the stentless group. The mean levels of TB, ALT and GGT in the stentless group were significantly higher than those in the T-tube and stent groups at each time point.
Table 2.
Biochemical results of animals in each group
| Parameters | Normal range | Groups | 1 mo postoperative | 2 mo postoperative | 3 mo postoperative |
| Total bilirubin (μmol/L) | 1.7-8.5 | T-tube | 3.0 ± 0.3 | 3.2 ± 0.3 | 3.3 ± 0.4 |
| Stent | 3.2 ± 0.5 | 3.4 ± 0.5 | 3.6 ± 0.5 | ||
| Stentless | 68.2 ± 15.1ac | 106.3 ± 29.2ac | NA | ||
| Alanine aminotransferase (IU/L) | 15.0-66.0 | T-tube | 35.2 ± 12.6 | 30.1 ± 5.3 | 36.1 ± 10.3 |
| Stent | 34.3 ± 12.1 | 32.1 ± 6.2 | 33.2 ± 7.2 | ||
| Stentless | 122.5 ± 35.1ac | 209.5 ± 35.4ac | NA | ||
| Gamma-glutamyl transpeptidase (IU/L) | 10.0-88.0 | T-tube | 51.2 ± 7.2 | 50.1 ± 5.3 | 48.8 ± 6.6 |
| Stent | 47.1 ± 5.3 | 52.1 ± 6.2 | 52.0 ± 10.6 | ||
| Stentless | 233.4 ± 61.5ac | 265.8 ± 82.6ac | NA |
P < 0.05, stent group vs stentless group;
P < 0.05, T-tube group vs stentless group. NA: Not available.
The animals that survived in the T-tube and stent groups underwent cholangiography at 3 mo before sacrifice. Cholangiograms showed that the contrast passed freely from the bile duct into the duodenum. Neither biliary leakages nor filling defects were observed on any cholangiogram in the T-tube group (Figure 4A). However, 1 biliary stricture was detected among the 6 pigs in the intraluminal stent group due to stent migration. A minimal narrowing at the proximal biliary anastomosis and a mild dilation of the intrahepatic bile ducts were noted in this animal. There was no evidence of biliary stricture or proximal biliary dilatation in any other animals after 3 mo in the stent group (Figure 4B).
Figure 4.
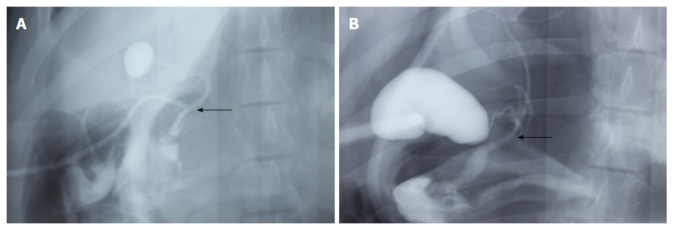
Postoperative cholangiography. A: Cholangiography of a survivor in the T-tube group performed at 3 mo; B: Cholangiography of a survivor in the stent group performed at 3 mo. Arrows indicate the area of graft interposition. There is no evidence of biliary stricture formation. There is no sign of proximal dilatation or biliary obstruction.
Histopathology
In the T-tube group, the autopsy at 3 mo revealed that the common bile ducts were patent in all the explanted specimens without any bile leakage (Figure 5A). There was no gross evidence of biliary stricture or proximal biliary dilatation. Remnants of the 7-0 prolene sutures were observed at the end-to-end anastomosis. The decellularized ureteral grafts appeared to be completely degraded. Neo-ducts were observed at 3 mo post-surgery. The lumen of each neo-bile duct was covered with a layer of smooth mucosa, which had an appearance similar to that of the native common bile duct. There was a progressive contraction of the graft area from an initial length of 2 cm to 1.1 (0.9-1.2) cm.
Figure 5.
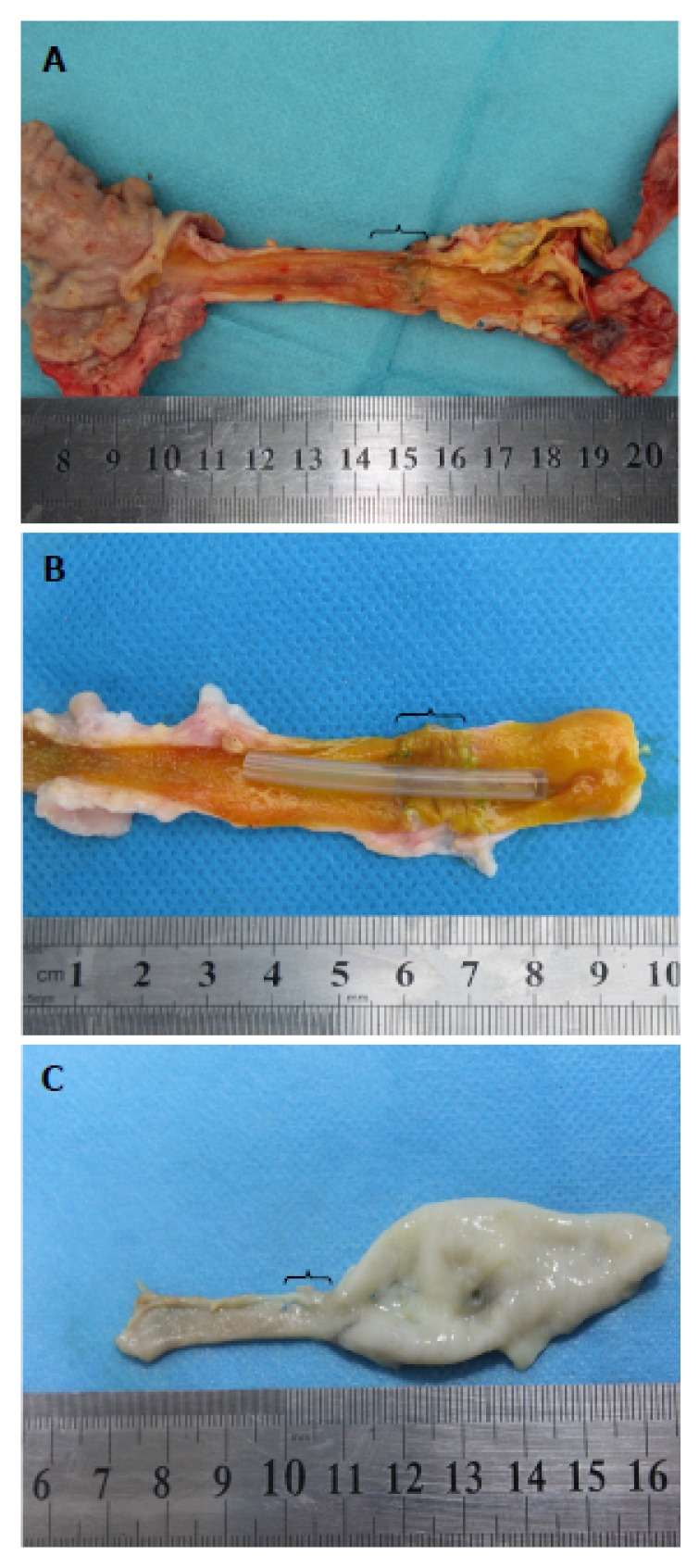
Macroscopic results. A: Explanted bile duct of a 3-mo survivor in the T-tube group; B: Explanted bile duct of a 3-mo survivor in the stent group; C: Explanted bile duct of an animal who died at 1 mo in the stentless group. Brackets indicate the area of graft interposition.
In the stent group, the intraluminal stents were observed in situ in all but one animal during the autopsy at 3 mo (Figure 5B). The macroscopic results of the five animals with the stent in situ were similar to those of the animals in the T-tube group (Figure 5B). However, the intraluminal stent in one animal disappeared due to stent migration. This animal developed mild biliary stricture and proximal biliary dilatation. The mean length of the graft area was 1.0 (0.8-1.1) cm. In the stentless group, marked biliary strictures and proximal biliary dilatations were observed in all the animals during the autopsy (Figure 5C). In most of the animals, the lumen of the graft site had biliary sludge and degraded decellularized ureteral graft remnants. The mean length of the graft area was 0.6 (0.4-1.0) cm.
HE staining results
At 3 mo, there was no recognizable element of the graft and the decellularized ureteral graft appeared completely degraded in the T-tube and stent groups. Cellularization and collagen remodeling were observed at the graft sites. Only slight inflammation of the graft sites was noted in both groups. The neo-bile duct showed a native biliary histological structure. A smooth mucosa layer encircled the lumen of the neo-bile duct, which was similar to that of the native bile duct (T-tube group: Figure 6A; Stent group: Figure 6B). In contrast, bile duct reconstruction using a graft alone (stentless group) led to dense fibroplasia of the graft site. No mucosa was detected (Figure 6C).
Figure 6.
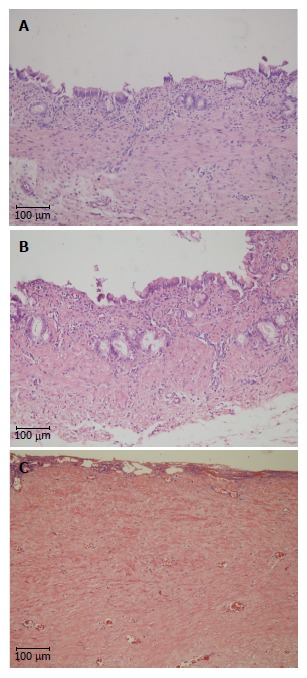
Hematoxylin/eosin staining of the graft site. A: H&E staining of a 3-mo survivor in the T-tube group; B: H&E staining of a 3-mo survivor in the stent group; C: H&E staining of an animal who died at 1 mo in the stentless group. Scale bar indicates 100 μm. H&E: Hematoxylin/eosin.
CK19 immunofluorescent staining
CK19 immunofluorescent staining was performed to investigate the biliary epithelial layer. At 3 mo, both neo-epithelial cells and accessory glands were observed at the graft sites and stained positive for CK19 in the animals in the T-tube and stent groups (T-tube group: Figure 7A; Stent group: Figure 7B). Most of the graft sites, except for some central areas, were covered by a layer of irregular biliary epithelium. However, the CK19 staining at the graft site was negative in the animals in the stentless group. Neither epithelial cells nor accessory glands were observed at the graft sites (Figure 7C).
Figure 7.
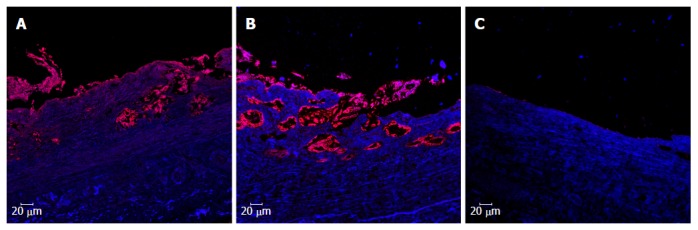
Cytokeratin 19 immunofluorescent staining of the graft site. A: CK19 staining of a 3-mo survivor in the T-tube group; B: CK19 staining of a 3-mo survivor in the stent group; C: CK19 staining of an animal who died at 1 mo in the stentless group. Scale bar indicates 20 μm. CK19: Cytokeratin 19.
DISCUSSION
This preliminary study suggests that in a porcine model, a common bile duct defect can be repaired by a decellularized ureteral graft if either a T-tube or intraluminal stent is used. The absence of either a T-tube or intraluminal stent resulted in bile leakage, biliary stricture and subsequent death. Regeneration of the biliary mucosa was observed in the surviving animals at 3 mo post-surgery.
The search for a suitable substitute to repair a long common bile duct defect caused by disease or trauma has attracted many researchers[9]. A wide variety of autografts (e.g., artery, vein, ureter, appendix, jejunum) and synthetic grafts (e.g., Vitallium, polyvinyl sponge, polytetrafluoroethylene, Teflon, Dacron) have been introduced as substitutes to repair common bile duct defects in situ[8-11,17-22]. Either a tubular graft or a patch graft was used in these experimental studies. However, the outcomes of various substitutes in large animals (e.g., dogs, goats, pigs) are controversial. Based on studies performed in the past century, most of the substitutes are not degradable and have failed mostly because of varying degrees of cholangitis, biliary stricture and biliary peritonitis[8-11,17-22]. With the development of degradable materials, success has been reported by some researchers using various degradable substitutes (e.g., small intestinal submucosa, polylactic acid and polycaprolactone, polyglycolic acid and trimethylene carbonate, collagen sponge and polypropylene) in this century[12-16]. Until recently, there were no satisfactory reliable substitutes with which to replace common bile duct defects and restore function in clinical practice. Only a few case reports have been published on repairs of the common bile duct defects using autografts[23-25].
The diameter of the ureter is similar to that of the common bile duct. Two research teams (Sedgwick et al[26] and Ulin et al[10]) evaluated the role of ureteral grafts in the repair of common bile duct defects in dogs. In general, free ureteral grafts were disappointing in both experimental studies. The entire free graft was replaced by fibrous tissue and biliary stricture occurred at the graft site due to immunological rejection and inflammatory reactions. Recently, various decellularization techniques have been introduced to remove cellular antigens and preserve the extracellular matrix (ECM)[27]. The ECM is a biomaterial composed of structural and functional proteins (e.g., collagen, elastin), glycosaminoglycans and growth factors. ECM biomaterial is biodegradable, decellularized and non-immunogenic material derived from human or animal tissue processing[12] and has been shown to have regeneration and various tissue healing capabilities[12,27]. This material has been widely used in tissue engineering[12,27]. ECM biomaterial can not only prevent immunological rejection and inflammatory reactions but also facilitate cell adhesion and differentiation to repair injured tissue and prevent scar tissue formation[12,27]. Rosen et al[12] used small intestinal submucosa (a type of ECM biomaterial) as a bile duct substitute for common bile duct reconstruction in dogs. Neither a T-tube nor intraluminal stent was used in the study[12]. At 1 mo post-surgery, the graft material was completely covered by biliary epithelium and by 5 mo, the graft material was completely replaced by a neo-bile duct. The biliary epithelium of the neo-bile duct was grossly and microscopically indistinguishable from that of a normal canine common bile duct[12]. The authors concluded that small intestinal submucosa can be used to repair bile duct defects in a canine model without requiring stenting[12]. However, Gómez et al[28] were concerned about the safety of small intestinal submucosa for common bile duct reconstruction because 1 of the 5 dogs (20%) developed a biliary stricture at 2 mo post-surgery in Rosen’s experimental study. They suggested that further studies are needed to establish the benefits and complications of small intestinal submucosa in common bile duct reconstruction[28]. In our experiment, a ureter instead of a common bile duct was chosen as the graft because the smooth muscle layer of the former is thicker than that of the latter. A simple decellularization method was adopted to remove cells and make ECM biomaterial. Regarding the ECM biomaterial for the common bile duct reconstruction, there were significant differences between our findings and Rosen’s observations. All the experimental animals in the stentless group died from various complications when a sole decellularized ureteral graft without stenting was used to repair the bile duct defect. We found at the autopsy that the decellularized ureteral graft collapsed and shrunk with severe biliary stricture formation. Possible reasons for this failure in the stentless group were that the decellularized ureteral graft had a soft texture and the pressure in the biliary system was too low to maintain the luminal structure of the graft. Consequently, it appeared that either a T-tube or intraluminal stent was necessary to improve the survival rate in this study.
T-tube drainage and biliary stenting have been introduced by some surgeons to reduce postoperative complications after bile duct reconstructions. However, the use of a T-tube and biliary stent during bile duct reconstruction is controversial. A T-tube and biliary stent may reduce the incidence of biliary stricture but may be associated with significant morbidity. T-tube-related complications include premature dislodgement, biliary leakage and biliary infection[29]. In addition, bile duct reconstruction over a T-tube requires several weeks of extra-abdominal drainage, which may cause loss of appetite and reduce quality of life. In contrast, placement of an intraluminal stent may reduce T-tube-related complications without extra-abdominal drainage. In this experiment, a silicone stent was inserted into the graft during reconstruction of the common bile duct but resulted in biliary stricture due to stent migration. In addition, the silicone stent was not biodegradable and an additional procedure (e.g., endoscopy, laparotomy) was required to remove the stent. It appeared that T-tube drainage was superior to the silicone stent during the bile duct reconstruction in this experiment. Recently, biodegradable biliary stents have been developed and some experiments have reported encouraging results with them[30-32]. Future work is needed to compare the efficacy of T-tube drainage vs a biodegradable biliary stent for common bile duct reconstruction.
In summary, the use of decellularized ureteral grafts appears to be feasible for the repair of common bile duct defects. Either a T-tube or intraluminal stent is necessary to improve the survival rate and prevent bile leakage and biliary stricture formation. Additional studies are planned to investigate the optimal time for T-tube or intraluminal stent removal and the optimal method to promote regeneration of the biliary tract.
ACKNOWLEDGMENTS
We thank Jie Zhang and Yi-Xin Lin for assistance with the development of the article.
COMMENTS
Background
A common bile duct defect generally follows iatrogenic bile duct injury during cholecystectomy, a challenge for hepatobiliary surgeons.
Research frontiers
In recent years, several new materials with fewer foreign body reactions have been successfully introduced as substitutes to normal bile ducts for biliary defect reconstruction.
Innovations and breakthroughs
A decellularized ureteral graft was introduced in this experimental study to repair a common bile duct defect. If the biliary reconstruction was performed with a T-tube or stent insertion into the ureteral graft, all animals survived with normal liver function. The histology results showed a biliary epithelial layer regeneration over the graft.
Applications
Repair of a common bile duct defect with a decellularized ureteral graft appears to be feasible. In addition, a T-tube or stent was found to be necessary to reduce postoperative complications.
Terminology
Extracellular matrix is a biomaterial composed of structural and functional proteins (e.g., collagen, elastin), carbohydrate polymers and growth factors. Decellularization is the process to remove cellular components from a tissue.
Peer-review
This is a novel and well designed experiment that provides a new approach to a complex and common surgical problem. The authors have provided excellent evidence that the decellularized ureteral graft might be an adequate replacement for a damaged common bile duct. Long term results with the new method need to be addressed in future.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Local Ethics Committee of West China Hospital (Sichuan, China).
Institutional animal care and use committee statement: All of the procedures were performed strictly according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Appropriate measures were taken to minimize the animals’ pain and discomfort.
Conflict-of-interest statement: We declare that we have no financial or personal relationships with other people or organizations that could inappropriately influence our work.
Data sharing statement: The technical appendix, statistical code and dataset are available from the corresponding author at nanshengcheng@gmail.com.
Peer-review started: August 29, 2016
First decision: September 20, 2016
Article in press: November 22, 2016
P- Reviewer: Leitman M, Shimizu Y S- Editor: Gong ZM L- Editor: Roemmele A E- Editor: Liu WX
References
- 1.Melton GB, Lillemoe KD, Cameron JL, Sauter PA, Coleman J, Yeo CJ. Major bile duct injuries associated with laparoscopic cholecystectomy: effect of surgical repair on quality of life. Ann Surg. 2002;235:888–895. doi: 10.1097/00000658-200206000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg. 2006;93:158–168. doi: 10.1002/bjs.5266. [DOI] [PubMed] [Google Scholar]
- 3.de Reuver PR, Busch OR, Rauws EA, Lameris JS, van Gulik TM, Gouma DJ. Long-term results of a primary end-to-end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg. 2007;11:296–302. doi: 10.1007/s11605-007-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, Talamini MA, Pitt HA, Coleman J, Sauter PA, et al. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786–792; discussion 793-795. doi: 10.1097/01.sla.0000161029.27410.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nealon WH, Urrutia F. Long-term follow-up after bilioenteric anastomosis for benign bile duct stricture. Ann Surg. 1996;223:639–645 discussion 645-648. doi: 10.1097/00000658-199606000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moraca RJ, Lee FT, Ryan JA, Traverso LW. Long-term biliary function after reconstruction of major bile duct injuries with hepaticoduodenostomy or hepaticojejunostomy. Arch Surg. 2002;137:889–893; discussion 893-894. doi: 10.1001/archsurg.137.8.889. [DOI] [PubMed] [Google Scholar]
- 7.Walsh RM, Henderson JM, Vogt DP, Brown N. Long-term outcome of biliary reconstruction for bile duct injuries from laparoscopic cholecystectomies. Surgery. 2007;142:450–456; discussion 456-457. doi: 10.1016/j.surg.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Belzer FO, Watts JM, Ross HB, Dunphy JE. Auto-reconstruction of the common bile duct after venous patch graft. Ann Surg. 1965;162:346–355. doi: 10.1097/00000658-196509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers RT, Meredith JH, Rhodes J, Gilbert JW. The fate of free grafts in the common bile duct. Ann Surg. 1960;151:776–782. [PMC free article] [PubMed] [Google Scholar]
- 10.Ulin AW, Van Ess L, Entine J, Pearce AE, Martin WL. Further experiences with the experimental reconstruction of the common bile duct: use of autogenous and homologous, fresh and preserved grafts of blood vessel, ureter and common duct. Am Surg. 1953;19:867–873. [PubMed] [Google Scholar]
- 11.Grosfeld JL, Weinberger M, Clatworthy HW. Vascularized appendiceal transplants in biliary and urinary tract replacement. J Pediatr Surg. 1971;6:630–638. doi: 10.1016/0022-3468(71)90389-7. [DOI] [PubMed] [Google Scholar]
- 12.Rosen M, Ponsky J, Petras R, Fanning A, Brody F, Duperier F. Small intestinal submucosa as a bioscaffold for biliary tract regeneration. Surgery. 2002;132:480–486. doi: 10.1067/msy.2002.126505. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa M, Torii T, Toshimitsu Y, Okada K, Koyama I, Ikada Y. A tissue-engineered artificial bile duct grown to resemble the native bile duct. Am J Transplant. 2005;5:1541–1547. doi: 10.1111/j.1600-6143.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Nau P, Liu J, Ellison EC, Hazey JW, Henn M, Muscarella P, Narula VK, Melvin WS. Novel reconstruction of the extrahepatic biliary tree with a biosynthetic absorbable graft. HPB (Oxford) 2011;13:573–578. doi: 10.1111/j.1477-2574.2011.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Tao L, Chen B, Ren H, Hou X, Zhou S, Zhou J, Sun X, Dai J, Ding Y. Extrahepatic bile duct regeneration in pigs using collagen scaffolds loaded with human collagen-binding bFGF. Biomaterials. 2012;33:4298–4308. doi: 10.1016/j.biomaterials.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Pérez Alonso AJ, Del Olmo Rivas C, Romero IM, Cañizares Garcia FJ, Poyatos PT. Tissue-engineering repair of extrahepatic bile ducts. J Surg Res. 2013;179:18–21. doi: 10.1016/j.jss.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Trentini EA, Crema E, Llanos JC, Lerco MM, Magna LA, Leonardi LS. Biliary tract reconstruction using jejunal tube: an experimental study in dogs. Hepatobiliary Pancreat Dis Int. 2009;8:179–185. [PubMed] [Google Scholar]
- 18.Pearse HE. Vitallium tubes in biliary surgery. Ann Surg. 1942;115:1031–1042. doi: 10.1097/00000658-194206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergan JJ, Anderson MC, Lounsbury BF. Vascularized polyvinyl sponge prosthesis. Its use to replace the canine common bile duct. Arch Surg. 1962;84:301–305. doi: 10.1001/archsurg.1962.01300210035007. [DOI] [PubMed] [Google Scholar]
- 20.Mendelowitz DS, Beal JM. Expanded polytetrafluoroethylene in reconstruction of the canine biliary system. Am J Surg. 1982;143:221–224. doi: 10.1016/0002-9610(82)90073-3. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JP, Metropol HJ, Myers RT. Teflon patch graft for reconstruction of the extrahepatic bile ducts. Ann Surg. 1964;160:967–970. doi: 10.1097/00000658-196412000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens FO, Dunphy JE. Grafts of the common bile duct. An historical review. Aust N Z J Surg. 1963;33:53–57. doi: 10.1111/j.1445-2197.1963.tb03291.x. [DOI] [PubMed] [Google Scholar]
- 23.Helmy AA, Hamad MA, Aly AM, Sherif T, Hashem M, El-Sers DA, Semieka M. Novel technique for biliary reconstruction using an isolated gastric tube with a vascularized pedicle: a live animal experimental study and the first clinical case. Ann Surg Innov Res. 2011;5:8. doi: 10.1186/1750-1164-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M, Yamazaki K, Tsuchiya M, Otsuka Y, Tamura A, Shimokawa K, Kaneko H, Teramoto T. Use of an opened umbilical vein patch for the reconstruction of the injured biliary tract. J Hepatobiliary Pancreat Surg. 2007;14:270–275. doi: 10.1007/s00534-006-1183-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaushik R, Sharma R. Jejunal serosal onlay flap for repair of large common bile duct defect in Mirizzi’s syndrome. Trop Gastroenterol. 2001;22:169–170. [PubMed] [Google Scholar]
- 26.Sedgwick CE. Reconstruction of the common bile duct with a free ureteral graft; an experimental study. Surg Gynecol Obstet. 1951;92:571–573. [PubMed] [Google Scholar]
- 27.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Gómez NA, Zapatier JA, Vargas PE. Re: “Small intestinal submucosa as a bioscaffold for biliary tract regeneration”. Surgery. 2004;135:460. doi: 10.1016/j.surg.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Wills VL, Gibson K, Karihaloot C, Jorgensen JO. Complications of biliary T-tubes after choledochotomy. ANZ J Surg. 2002;72:177–180. doi: 10.1046/j.1445-2197.2002.02308.x. [DOI] [PubMed] [Google Scholar]
- 30.Grolich T, Crha M, Novotný L, Kala Z, Hep A, Nečas A, Hlavsa J, Mitáš L, Misík J. Self-expandable biodegradable biliary stents in porcine model. J Surg Res. 2015;193:606–612. doi: 10.1016/j.jss.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Liang Y, Wang W, Jin R, Cai X. Management of electrothermal injury of common bile duct with a degradable biliary stent: an experimental study in a porcine model. J Gastrointest Surg. 2013;17:1760–1765. doi: 10.1007/s11605-013-2316-0. [DOI] [PubMed] [Google Scholar]
- 32.Tashiro H, Ogawa T, Itamoto T, Ushitora Y, Tanimoto Y, Oshita A, Amano H, Asahara T. Synthetic bioabsorbable stent material for duct-to-duct biliary reconstruction. J Surg Res. 2009;151:85–88. doi: 10.1016/j.jss.2008.02.056. [DOI] [PubMed] [Google Scholar]


