Abstract
Background
Increased oxidative stress and stress enzyme 11β hydroxysteriod dehydrogenase-1 (11β HSD-1) served as the major contributing factors for delayed wound healing in diabetes mellitus (DM). Piper betel (PB) leaves are reported to possess anti-diabetic, anti-oxidant and anti-microbial properties.
Objective
The objective was to investigate the effectiveness of topical application of PB leaves extract on oxidative stress and 11β hydroxysteriod dehydrogenase-1 (11β HSD-1) expression in diabetic wounds.
Materials and methods
A total 64 male Sprague-Dawley rats were randomly chosen. The experimental rats received a single intramuscular injection of streptozotocin (45 mg/kg). Four full thickness (6 mm) wounds were created on the dorsum of each rat. The animals were equally divided (n = 8) into four groups based on the days of treatment (i.e. days 3 and 7): Control (Ctrl), diabetic untreated (DM-Ctrl), diabetic treated with 1% silver nitrate cream (DM-SN) and diabetic treated with 50 mg/kg of P. betel leaves extract (DM-PB). The rats were sacrificed on day 3 and 7 of post wound creations.
Results
Following day 7 wound creation, topical application of PB extract showed significant increase in hydroxyproline content, superoxide dismutase (SOD) level and decreased malondialdehyde (MDA) level, 11β-HSD-1 enzyme expression in the diabetic wounds compared to untreated diabetic wounds. The results were supported by the observations based on histological and ultrastructural features of the wound tissue applied with PB extract.
Conclusion
PB leaves extract improved the delayed wound healing in diabetes mellitus by decreasing the oxidative stress markers and 11β HSD-1 expression.
Keywords: Piper betel, Diabetes mellitus, Wound, Oxidative stress, 11β-HSD-1 enzyme
1. Introduction
Diabetes mellitus (DM) is reported to be one of the most common non-communicable diseases in the world. There is reported rise in the trend of the disease both in developed and developing countries [1]. The global prevalence of DM was 285 million in 2010, and it is expected to increase up to 439 million by 2030 [1]. By the year 2030, DM is expected to be the 7th leading cause of death worldwide [2]. The disease is characterized by chronic hyperglycaemia with metabolic disorder resulting from insulin deficiency, insulin resistance, or even both [3]. Both type 1 and type 2 diabetes mellitus may lead to develop various complications such as microvascular and macrovascular insults. In addition, it causes peripheral neuropathy and autonomic dysfunction with increased risk of foot ulcers, delayed wound healing, and eventually leads to limb amputation [3]. Amputation in diabetic patients results due to delayed wound healing and causes much burden on the public sector health expenses [4].
Skin is one of the most vulnerable tissues to be affected due to oxidative stress. The oxidative damage makes the skin vulnerable to the external environment for the entry of microorganisms, ultraviolet radiation, and mechanical stimulation [5]. Delayed wound healing is considered to be one of the most important complications observed in type 1 diabetes mellitus. Increase in oxidative stress in type 1 diabetes mellitus results in increased production of reactive oxygen species (ROS) which contributes to delayed wound healing. In type 1 diabetes mellitus, there is an imbalance between oxidant and antioxidant enzymes. Antioxidant enzyme like superoxide dismutase (SOD) was reported to be reduced and malondialdehyde (MDA), a marker of lipid peroxidation was also reported to be elevated in type 1 diabetes mellitus [2], [6]. The stress enzyme 11β hydroxysteriod dehydrogenase-1 (11β HSD-1), involving in the interconversion of cortisone and cortisol, is also increased in diabetes mellitus. It is reported that this enzyme showed increased expressions in the liver, adipose tissue, and central nervous system. It also helped in tissue remodelling of the skin. Recently, it was documented that increase in this enzyme may have a negative effect on the proliferation of keratinocytes and fibroblasts in cutaneous wound healing process [7]. In addition, the process of wound healing is improved by angiogenesis and migration of fibroblasts, mast cells and epithelial cells [8]. Research studies reported less responsive fibroblasts to growth factors in DM leading to reduced fibroblast proliferation and poor wound tensile strength [9], [10]. All these facts play important role in improving the wound healing process.
Over the past few decades, herbal remedies are being studied due to their potential medicinal effects especially on the healing wounds [11]. In the present study, we mainly focused on the herb which had the potential effects of hyperglycaemia, oxidative stress disorders and wound healing process. It was reported earlier that Piper betel (PB) leaves possess multiple therapeutic properties such as anti-diabetic, anti-oxidant and anti-microbial properties [12]. It belongs to the Piperaceae family and it is commonly found in Asia. Traditionally, PB can be used to treat cough, bad mouth smell, ozoena, clears throat, vulnery, asthma and styptic [13]. Phytochemically, the leaves were found to contain alkaloids, flavonoids, carbohydrate, amino acids, tannins, terpene like bodies and steroidal components [14]. Recently, a group of researchers reported PB to exhibit wound healing effect on experimental diabetic rats by increasing the total protein content and wound contraction rate [8]. However, the detailed study related to the oxidative stress markers was not explored, to date. Hence, the present study aimed to investigate the ameliorative effect of PB extract as a topical agent in promoting the wound healing process in a diabetic rat model.
2. Materials and methods
2.1. Preparation of P. betel aqueous extract
PB leaves were procured from a local supplier and the whole plant was identified by the expert botanist with the voucher No. 39631. Then, PB leaves were dried under the sun light, grounded and mixed with water (1:1). It was boiled for 1 h and filtered with Whatmann filter paper No. 4. The filtrate was sent to Forest Research Institute Malaysia (FRIM) to yield the freeze-dried powder. The extract was stored in the dark bottles and kept under 4 °C. For the topical treatment, PB leaves powder 50 mg/kg body weight was dissolved in the 0.9% normal saline to form a paste and was topically applied to the wounds [8].
2.2. Procurement of experimental animals
Male Sprague-Dawley rats (n = 64) weighing (210–240 g) were obtained from the local animal resource unit. They were individually housed in the metabolic cages under experimental conditions of temperature 22 ± 2 °C, 12 h light/dark cycle. The animals were fed on standard pellet and water ad libitum. The rats underwent an acclimatization period of one week prior to the experiment. The experiment was conducted after receiving the ethical approval from Universiti Kebangsaan Malaysia (FP/ANAT/2013/SAHEMA/31JAN./490-FEB-.2013-FEB.-2014).
2.3. Experimental induction of diabetes
Following the acclimatization period, the animals were kept in a fasting state for 12 h prior to induce streptozotocin (STZ). Baseline fasting blood glucose level was checked in all groups of rats. STZ was diluted in 0.9% normal saline. Then, a single intramuscular dose of STZ 45 mg/kg of body weight was injected in the experimental groups of rats. Following 72 h after STZ induction, the fasting blood glucose level (FBG) was checked and the level of 8 mmol/l and above was considered to be diabetic [8], [15]. Rats from the control group received topical application of 0.9% normal saline, only. This was done to create similar stress in the animals.
2.4. Wound creation
Following 3 days of STZ induction, the wound creation was performed in all groups of rats. The animals were anaesthetized with 60 mg/kg of ketamine and 10 mg/kg of xylazine through intramuscular route. The hair on the dorsum of the rats was shaved. Subsequently, the shaved area was sterilized with a 70% alcohol swab, and four full-thickness excisional wounds of standard size (6 mm) were created on the dorsum of each rat by using sterile biopsy punch needle [15]. The progress of wound healing was evaluated periodically by monitoring the physical behaviour of the rats, food and water intake and the physical appearance of the wounds.
2.5. Treatment of inflicted wounds
Subsequently, the wounds were treated. The animals were equally divided (n = 8) into four groups based on the days of treatment (i.e. days 3 and 7): non-diabetic untreated rats (Ctrl), diabetic untreated rats (DM-Ctrl), diabetic rats treated with 1% silver nitrate cream (DM-SN) and diabetic rats treated with 50 mg/kg of PB leaves extract (DM-PB). The group of rats were considered untreated as these animals received no topical supplement. Rats were remained unconscious for 2–3 h during the topical application. Rats were sacrificed on day 3 and 7 of post wound creation.
2.6. Measurement of body weight
The progressive changes in the body weight of the individual rat in each group were measured at baseline, day 3 and 7 post wound creation. In addition, behavioural changes in each group of rats were observed to ascertain the healing process.
2.7. Weight of the granulation tissue
At the end of the study, i.e., on day 3 and 7 post wounding, the granulation tissues from each group of rats (n = 8/group) that formed on the full thickness wounds, was excised and the wet weight was recorded.
2.8. Estimation of biochemical parameters
2.8.1. Hydroxyproline
On the 7th day post wound creation, the animals from each group were sacrificed using the overdose of diethyl ether inhalation. The wound tissues were collected to determine the hydroxyproline content. The wound tissue was excised and its weight was recorded. The tissue was oven-dried (70 °C) for 12 h and the weight was noted. They were then hydrolyzed in glass tubes (5N HCl for 24 h at 120 °C). The neutralized hydrolysate samples (150l) were mixed with 1 ml of 0.01M CuSO4, 1 ml of 2.0N NaOH and 1 ml of 5% H2O2 was added. The solution was thoroughly mixed and shaken occasionally for 7 min. The tubes were then incubated at 70 °C for 5min. After cooling, 5 ml of 2N H2SO4 and 2.5 ml of 5% p-dimethylaminobenzaldehyde was added. The samples were incubated at 70 °C for 20 min, and placed in a water bath at 20 °C. The absorbance was measured spectrophotometrically at 540 nm [16].
2.8.2. Superoxide dismutase (SOD) and malondialdehyde (MDA)
The collected tissues from day 7 of post wound creation were kept under −20 °C freezer. Prior to SOD assay, the tissues were homogenized in 5 ml of cold 20 mM HEPES buffer and later centrifuged at s15,000× g for 5 min at 4 °C (16). For MDA marker, the tissues were homogenized on ice in 300 μl of the MDA lysis buffer and then centrifuged. The method of SOD assay was based on the inhibition of epinephrine–adrenochrome transition by the enzyme. Then, the lipid peroxidation product, malondialdehyde (MDA) was determined by thiobarbituric acid (TBA) reaction. The determination of MDA level was done according to an earlier method described by Draper and Hadley [17].
2.8.3. 11β hydroxysteroid dehydrogenase 1 (11β-HSD-1) expression
For 11β-HSD-1 expression, day 7 wound tissues from each group (n = 2) were collected and embedded in paraffin. The tissues were deparaffinized, rehydrate and washed by PBS for endogenous peroxidase inhibition. Then, it was incubated with diluted peroxidase conjugated goat-anti rabbit IgG. Diamniobenzidinetetrahydrochloride (DBA) in PBS supplied with hydrogen peroxide was used for visualization of the reaction. A total of 6 tissues section (2 μm per section) were used from each wound. Eventually, it was counterstained with haematoxylin after immunoreaction [18]. Tissues were then dehydrated and mounted for analysis and storage. The expression of 11β-HSD-1 were observed by the double blinded fashion.
2.8.4. Light microscopic study
Wound tissues (n = 2) from the wound of the individual rat were removed immediately after sacrifice (i.e. On day 3 and 7 post wound creation, respectively). The tissues were then fixed in 10% formaldehyde, underwent a dehydration process through graded alcohol series (30–100%), cleared in xylene and embedded in paraffin wax (56 °C) the tissue-embedding centre (LEICA EG 1160®, WETZLAR, Germany). Serial sections of 5-μm thickness were cut using microtome (MICROM HM 340E®, WALLDORF, Germany) and stained with standard staining like Hematoxylin–Eosin (H&E), Masson's trichrome (MT) to observe the collagen deposit and Verhoeff's ven Geison (VvG) staining to observe the elastic fibres. The histological analysis was carried out at a magnification of ×200 for the wound tissue. The images were captured using Pix-elink colour camera (USA) which was attached to the light microscope (LEICA DM RXA2®, Germany).
2.8.5. Electron microscopic study
The healed skin tissues (n = 2) from each group of rats following day 7 post wounding were collected according to a previous protocol [19]. Tissues were sectioned into 1 mm3 size with transverse section and fixed with 2.5% glutaraldehyde for primary fixation. Post-fixation was followed in 1% osmium tetroxide for 2 h at 4 °C. Following dehydration in ethanol, embedding was carried out. Ultrathin sections were post-stained with 2% aqueous uranyl acetate and lead citrate. Then, the specimens were examined using transmission electron microscope Tecnai G2 model at the voltage of 80 kV (Philips®, Netherlands). All the electron microscopic findings were analysed by double-blinded manner. The present study mainly focused on the descriptive findings of the morphological disturbances encountered in diabetic wound tissue.
2.8.6. Data analysis
The experimental data were expressed as standard error of mean (SEM). Statistical analysis was performed by one-way ANOVA followed by Dunnett's test for multiple comparison. DM-Ctrl group was defined as a reference group.
3. Results
3.1. Body weight
Loss of body weight was observed in all the groups following post wound creations. Body weight was found to be significantly increased (P < 0.05) on day 7 of post wound creation in DM-PB group compared to DM-Ctrl group. Moreover, similar effect with regard to increase in the body weight was found in DM-SN group. However, day 3 of post wound creation groups did not show any significant increase in the body weight (Table 1).
Table 1.
Effect of P. betel extract application on body weight (gm)
| Group | Days following wound creation |
||
|---|---|---|---|
| 0 |
3 |
7 |
|
| Body weights (gm) | |||
| Ctrl | 228 ± 5.32 | 219 ± 2.32 | 231 ± 1.94 |
| DM-Ctrl | 236 ± 4.91 | 212 ± 4.58 | 201 ± 5.79 |
| DM-SN | 232 ± 3.24 | 218 ± 3.22 | 226 ± 2.31 |
| DM-PB | 230 ± 2.95 | 216 ± 3.67 | 223 ± 3.46*† |
Values were expressed as mean ± SEM. Significant changes * (P < 0.05) observed in body weight in DM-PB group compared to DM-Ctrl group. † Indicates no significant difference in DM-PB group compared to DM-SN group (P > 0.01).
3.2. Weight of granulation tissue
There were significant changes (P < 0.05) in the wet weight of granulation tissue between the untreated diabetic wound and diabetic wound which received the treatment on various days of post wound creation (i.e. day 3 and 7). The weight of granulation tissue for both DM-SN and DM-PB groups was significantly increased (P < 0.05) compared to DM-Ctrl group on day 7 post wound creation (Table 2).
Table 2.
Effect of P. betel extract application on the wet weight of granulation tissue
| Group | Days following wound creation |
|
|---|---|---|
| 3 |
7 |
|
| Wet weight of granulation tissue (mg) | ||
| Ctrl | 2.86 ± 0.13 | 3.85 ± 0.13 |
| DM-Ctrl | 1.51 ± 0.85 | 2.04 ± 0.19 |
| DM-SN | 2.66 ± 0.56 | 3.72 ± 0.14 |
| DM-PB | 1.98 ± 0.63 | 3.19 ± 0.15*† |
Wet weight of granulation tissues on various days following wound creation. Data were expressed as mean ± SEM (n = 6) in each group. Ctrl: normal control group; DM-Ctrl: untreated diabetic group; DM-SN: 1% silver nitrate cream treated diabetic group; DM-PB: 50 mg/kg of PB extract treated diabetic group. *Statistically significant compared to DM-Ctrl group (P < 0.05). † Indicates no significant difference in DM-PB group compared to DM-SN group (P > 0.01).
3.3. Hydroxyproline content
Hydroxyproline is a major component in protein collagen, which plays a key role in collagen stability. From the present findings, it was noted that DM-PB rats showed significant increase (P < 0.05) in the hydroxyproline content on day 7 post wound creation. No significant difference of hydroxyproline level was observed in DM-PB group compared to DM-SN group (P > 0.01) (Table 3). However, day 3 wounds of DM-SN and DM-PB groups did not show any significant increase in the hydroxyproline content compared to control group.
Table 3.
Levels of hydroxyproline, SOD and MDA in wound tissues
| Groups | Ctrl | DM-Ctrl | DM-SN | DM-PB |
|---|---|---|---|---|
| Hydroxyproline (μg/mg) | 0.0073 ± 0.0013 | 0.0186 ± 0.0017 | 0.0087 ± 0.0013 | 0.0092 ± 0.0016*† |
| SOD (U/mL) | 3.12 ± 0.12 | 2.15 ± 0.48 | 4.57 ± 0.36∗ | 6.82 ± 0.75*† |
| MDA (nmol/mg) | 0.08 ± 0.67 | 0.17 ± 0.58 | 0.15 ± 0.79 | 0.11 ± 0.63*† |
Each value was expressed as mean ± SEM (n = 6). ∗P < 0.05, DM-PB group versus DM-Ctrl group; †P > 0.01, DM-PB group versus DM-SN group. SOD: superoxide dismutase activity; MDA: Malondialdehyde activity.
3.4. Determination of superoxide dismutase (SOD) level in wound tissues
The level of wound tissue SOD on the day 7 was shown in Table 3. Significant difference of SOD level was observed in the diabetic groups compared to the Ctrl group. The activity of antioxidant enzyme SOD was found to be significantly increased (P < 0.05) in the wound tissues of PB treated diabetic group (DM-PB). However, the diabetic untreated group (DM-Ctrl) did not show any increase in the SOD level in wound tissues. Similar finding was observed in wound tissues of DM-SN group. A significant increase in SOD level was observed in DM-PB group compared to DM-SN group (P < 0.01).
3.5. Determination of malondialdehyde (MDA) level in wound tissues
In Table 3, the level of lipid peroxidation (MDA) in wound tissue following 7th day of wound creation was shown. As the wound healing proceeded, the lipid peroxidation was found to be increased in all the groups. On the day 7, level of MDA was improved in control and PB treated diabetic group of rats (DM-PB). However, in diabetic untreated group (DM-Ctrl), the MDA level remained high due to impaired wound healing. A significant difference in MDA level was observed between DM-PB (P < 0.05) and DM-Ctrl groups. A significant decrease in MDA level was observed in DM-PB group compared to DM-SN group (P < 0.01).
3.6. 11β-HSD-1 enzyme expression in wound tissues
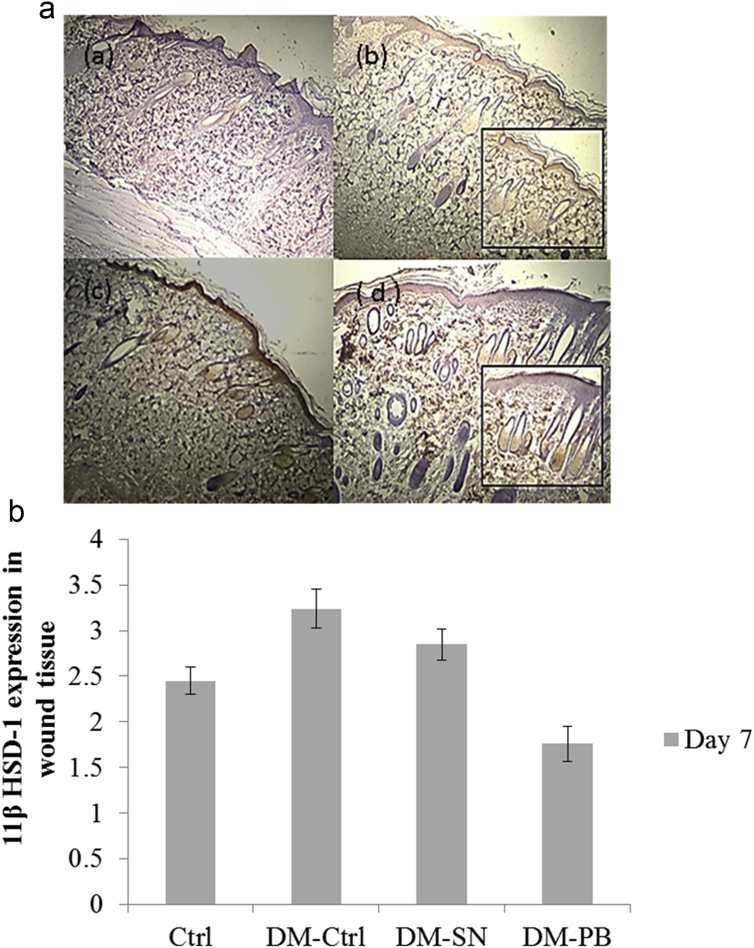
Immunolocalization of 11β-HSD-1 in the wound tissues was observed primarily in the epidermal epithelial cells (keratinocytes), fibroblasts and the outer root sheath of hair follicles (Fig. 1). Greater intensity (brown) staining was observed in the epidermal lining in the wounded skin of DM-Ctrl and DM-SN groups (Fig. 1b and c) compared to the Ctrl and DM-PB groups (Fig. 1a and d). Staining of outer root sheath of hair follicles was seen in all groups. However, the control group did not show any expression of 11β-HSD-1 in wounded tissues.
Fig. 1.
Expression of 11β-HSD-1 by immunohistochemistry in wounded skin of diabetic rat model. (a) Ctrl (b) DM-Ctrl (c) DM-SN, (d) DM-PB groups. Inserts: higher magnification of epidermal keratinocytes layer. Decreased in expression was observed in the epidermal layer of the wounded skin of DM-PB group (d) compared to DM-Ctrl and DM-SN groups (b,c).
3.7. Light microscopic study
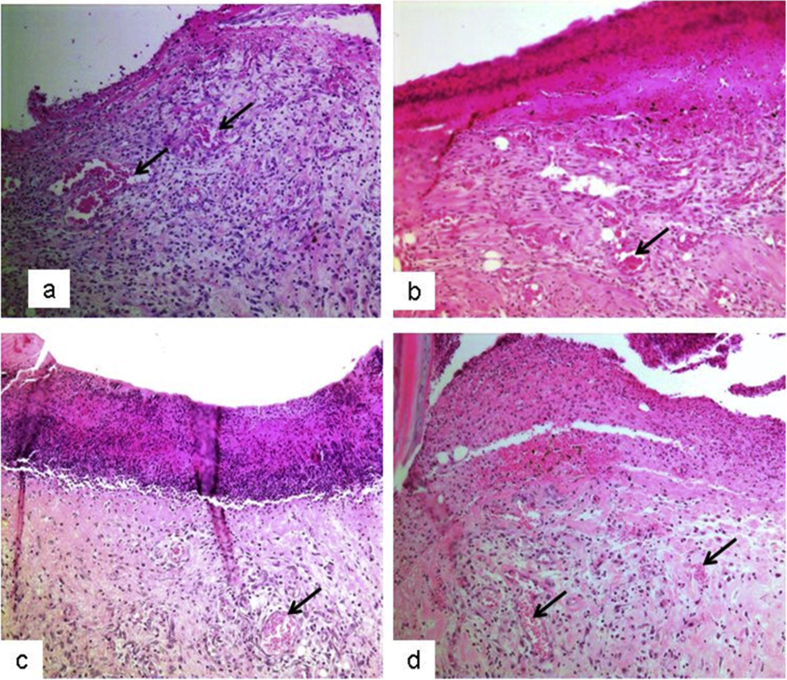
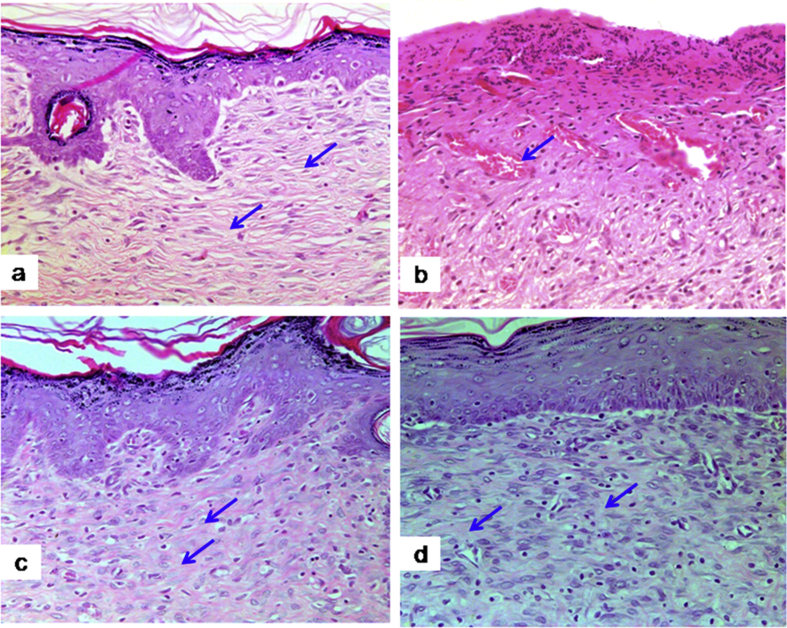
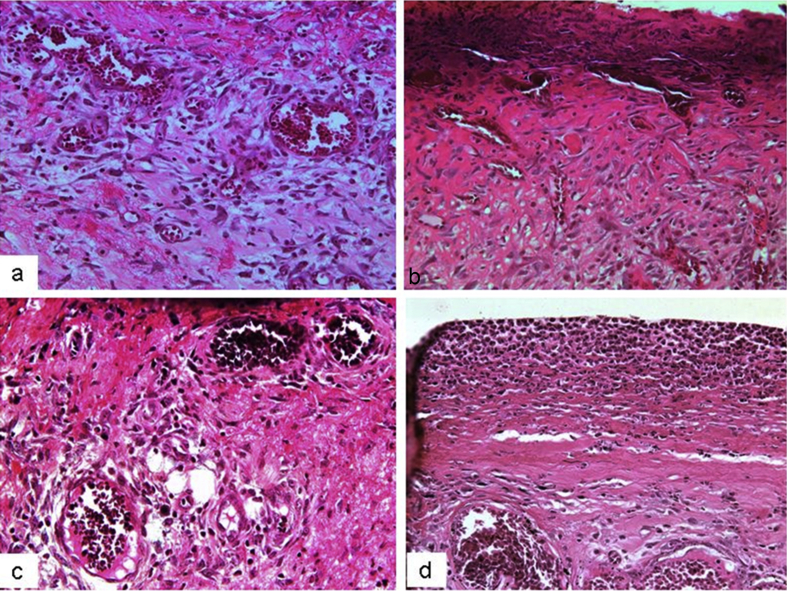
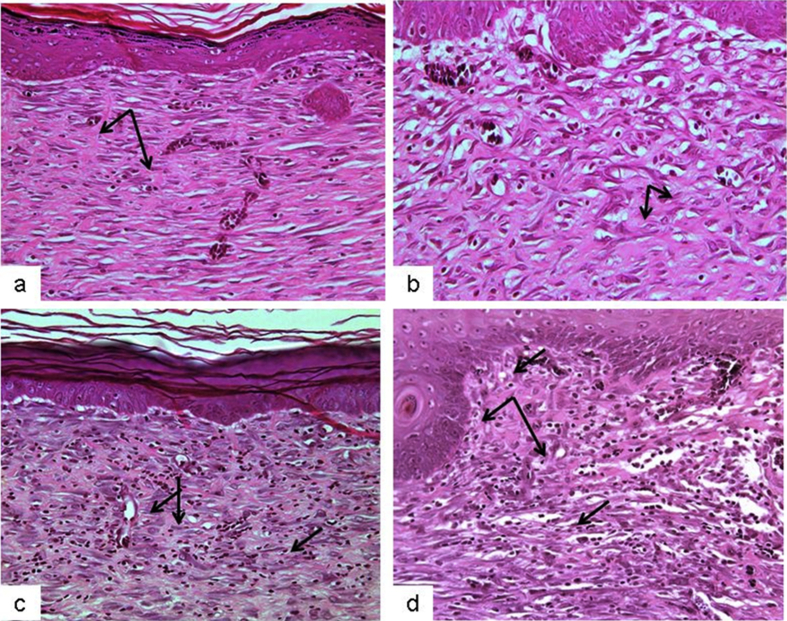
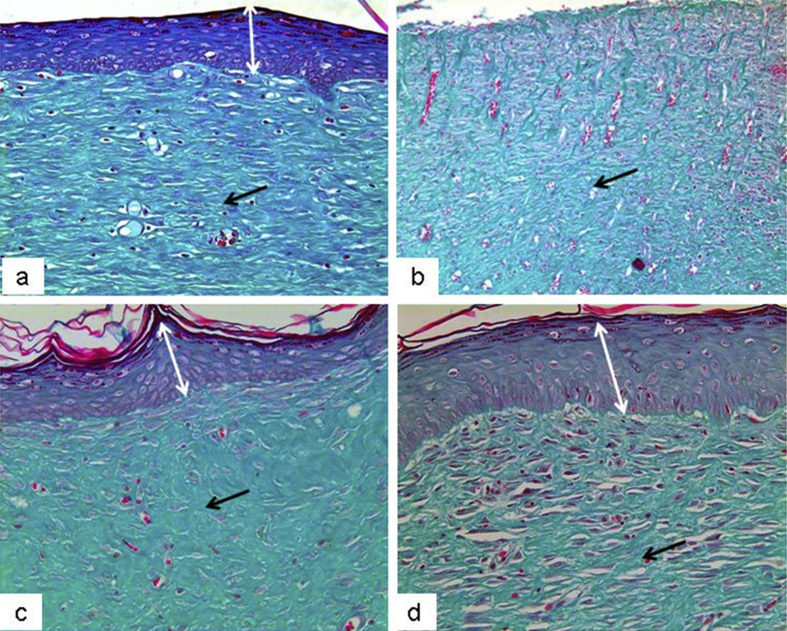
The present study investigated the histological changes of the wound tissues of each rat on day 3 and 7 post wound creations. Under H & E stain, Fig. 2 showed increased blood vessel congestion with fibroblast infiltration in the granulation tissue of DM-PB diabetic rats on day 3. The deposition of keratin with increased epidermal thickening was well noticed in DM-PB group compared to DM-Ctrl group. However, the morphological findings of the wound tissues in DM-PB group revealed no obvious difference with DM-Ctrl on day 3 (Fig. 2). Interestingly, on day 7 post wound creation, the invagination of the epidermis with complete re-epithelialization was observed in wound tissues of the DM-PB group compared to DM-Ctrl group (Fig. 3). Similar findings were observed in DM-SN group. Under VvG stain, day 3 wound tissues did not show any prominent changes (Fig. 4). However, day 7 wound tissues of DM-PB group revealed more fibroblasts and few macrophages in comparison to DM-Ctrl group (Fig. 5). With MT staining, day 3 wound tissue in DM-PB group of rats showed the congestion of blood vessels (Fig. 6). On day 7 wound tissues, the increased deposition of regularly arranged collagen fibres was observed in the wound tissues of DM-PB group compared to DM-Ctrl group. Similar morphological architectures were observed in wound tissues of DM-SN group. However, there was decreased deposition of collagen with irregular arrays of collagen bundles was noted in DM-Ctrl group (Fig. 7).
Fig. 2.
Histomicrograph showing day 3 wound tissues under H&E staining. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. arrows = congestion,×200 magnification.
Fig. 3.
Histomicrograph showing day 7 wound tissues under H&E staining. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. arrows = collagen bundle, ×200 magnification.
Fig. 4.
Histomicrograph showing day 3 wound tissues under VVG staining. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups, ×200 magnification.
Fig. 5.
Histomicrograph showing day 7 wound tissues under VVG staining. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. Black arrows = fibroblasts, ×200 magnification.
Fig. 6.
Histomicrograph showing day 3 wound tissues under MT staining. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. Black arrows = collagen deposits, ×200 magnification.
Fig. 7.
Histomicrograph showing day 7 wound tissues under MT staining. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. Black arrows = collagen deposits, ×200 magnification.
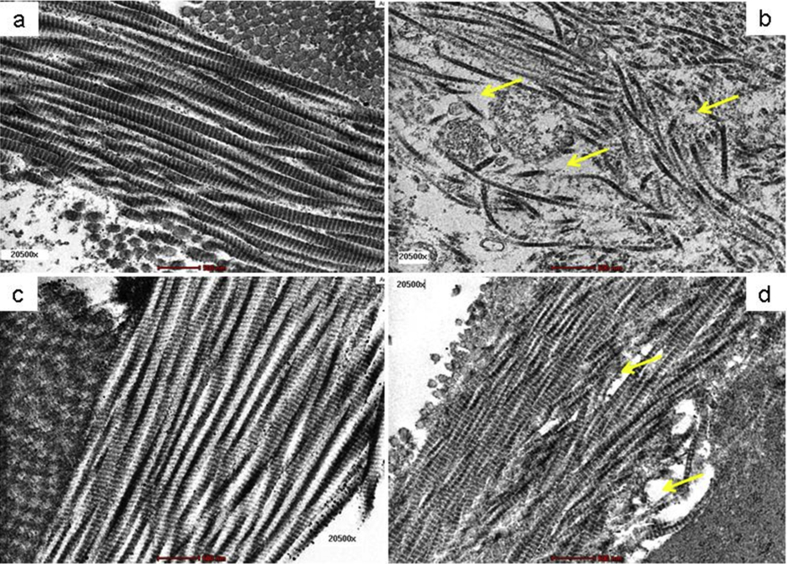
3.8. Transmission electron microscopic study
3.8.1. Collagen
Under TEM examination, day 7 wound tissue of Ctrl group showed regularly arranged and intact collagen fibres (Fig. 8a). However, the arrangement in the collagen fibres was found to be disturbed in wound tissue of the DM-Ctrl group. The deterioration and misalignments in the collagen strands which lead to develop wider cytoplasmic spaces was noted in wound tissue of the DM-Ctrl group (Fig. 8b). The collagen fibres in the wound tissues were found to be interrupted in the DM-Ctrl group. An improved subcellular architecture of collagen fibres was observed in DM-SN group (Fig. 8c). Similarly, all the ultrastructural changes of wound tissue were reverted to normal in DM-PB group. The long chain of collagen fibres with less cytoplasmic spaces were observed in DM-PB group (Fig. 8d). The ultrastructural findings showed that the wound healing process was disturbed in diabetic untreated rats. It was observed that topical application with PB extract improved the healing process by repairing the degenerative changes and restoring the ultrastructural damages in diabetic wound tissue following day 7 of post topical application.
Fig. 8.
Photomicrograph showing organization of collagen in day 7 wound tissues. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. Yellow arrows = disruption in collagen arrangement, Transmission electron micrograph, ×20,500 magnification.
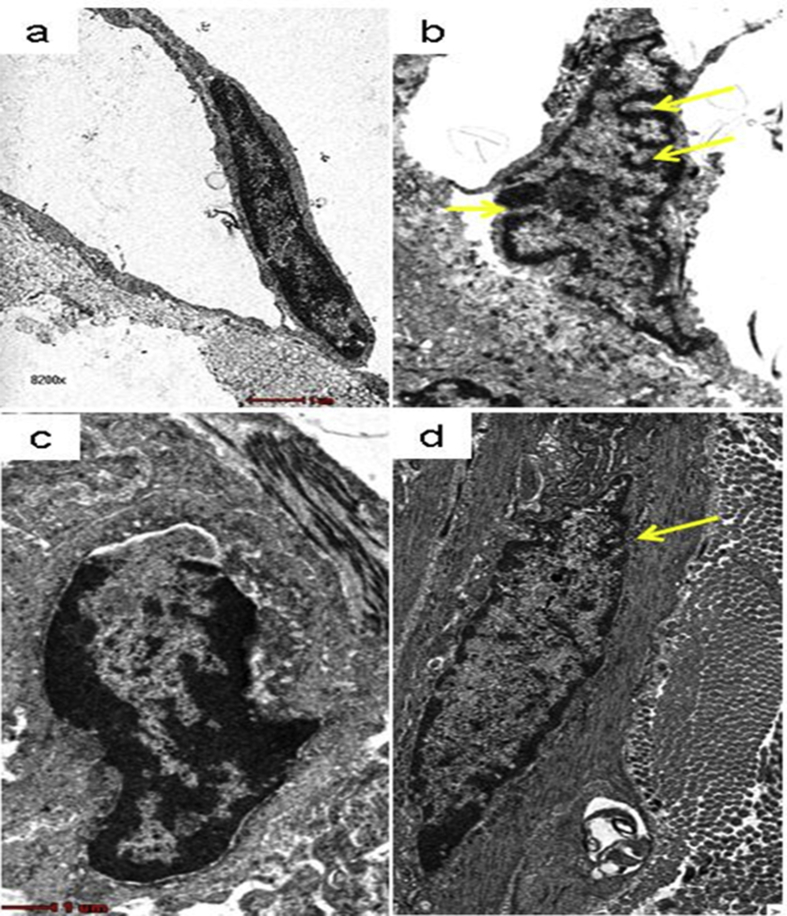
3.8.2. Fibroblasts
The ultrastructural findings of fibroblasts in the wound tissue of Ctrl group rats showed normal morphological features with regard to its shape and size (Fig. 9a). However, features of fibroblasts in wound tissue of DM-Ctrl group revealed invaginations in the cellular outline of fibroblasts. In addition, the shape of fibroblasts was deformed with presence of loose cytoplasmic matrix in DM-Ctrl group (Fig. 9b). It was observed that fibroblasts in the wound tissue obtained from DM-SN group showed similar features with the Ctrl group (Fig. 9c). The wound tissue from DM-PB group was also found to possess the invaginations in the fibroblasts. However, these invaginations were found to be less in DM-PB group compared to DM-Ctrl group. The shape of the fibroblasts also reverted to normal in DM-PB group (Fig. 9d). On day 7 post topical application PB extract showed the promising effect on diabetic wound tissue under electron microscope.
Fig. 9.
Photomicrograph showing features of fibroblasts in day 7 wound tissues. (a) Ctrl (b) DM-Ctrl (c) DM-SN (d) DM-PB groups. Yellow arrows = invaginations, Transmission electron micrograph, ×8200 magnification.
4. Discussion
PB is enriched with anti-diabetic, anti-oxidant and anti-microbial properties [8], [12], [13]. Delayed wound healing is one of the common complications that diabetic patients are severely facing till date. Topical application with PB extract showed positive impact on diabetic wounds. It was reported that wounds trigger a hypermetabolic and net catabolic state that may lead to weight loss [16]. This was observed in day 3 of post wound creation that all the groups of rats experienced decrease in body weight (Table 1). However, with optimal wound care, body weight was gradually restored on day 7 post wound creation following topical PB treatment. The similar effect was also observed on day 7 wound tissues of DM-SN rats. Similarly, an increase in the wet weight of granulation tissue was noted in DM-PB group (Table 2) compared to DM-Ctrl group. This may be due to the potent active compounds present in the extract which act as a free radical scavenger in oxidative stress disorder. It was reported that the active compounds, flavonoids, present in PB leaves have a potential effect towards cutaneous wound healing [20].
Hydroxyproline being a major component of protein collagen is often used as an indicator for collagen turnover [21]. It is synthesized during the proliferative phase of the healing process and the strength of the healing wound is determined by the presence of collagen deposition. Therefore, significantly increased hydroxyproline content (P < 0.05) in day 7 diabetic wound tissues of DM-PB group showed an increase in collagen synthesis and improved the healing process compared with DM-Ctrl group. The finding in DM-PB group was compared with DM-SN group. There was a significant difference in hydroxyproline content between the DM-PB and DM-Ctrl (Table 3).
Increased oxidative stress results in delayed wound healing which can be reversed by balancing the antioxidant system [22]. In the present study, level of antioxidant enzyme, SOD, was significantly increased in DM-PB group compared to DM-Ctrl (P < 0.05) and DM-SN groups (P < 0.01) in day 7 diabetic wound tissues (Table 3). This can be explained by the antioxidant activity of phenols present in PB extract [23]. Furthermore, the tissue MDA level of day 7 diabetic wounds was significantly increased in DM-Ctrl rats (Table 3). It reflects the increase in production of ROS or decrease in antioxidant defence mechanisms or both. Topical application of PB extract significantly (P < 0.05) decreased the level of MDA in day 7 diabetic wounds of DM-PB rats compared to DM-Ctrl and DM-SN groups confirming the role of PB as an effective antioxidant agent. It is due to the presence of flavonoids and polyphenols compounds such as catecol and allylpyrocatecol in betel leaf extract which provides antioxidant activity and inhibits the lipid peroxidation process, effectively [24], [25], [26].
In addition, 11β-HSD-1 enzyme modulates the fibroblasts and keratinocyte proliferation in the skin [27], [28]. However, increase in expression of 11β-HSD-1 inhibits the wound healing process by altering the process of cellular proliferation. Increased 11β-HSD-1 expression was observed in normal wound tissues in an experimental study [25]. Our present finding was synchronized with previous study in which the increase (brown) staining of 11β-HSD-1 was found in the epidermal lining of day 7 wound tissues of all groups of rats. However, in the diabetic untreated group (DM-Ctrl), 11β-HSD-1 expression was found to be remarkably increase in staining in the epidermal lining which was shown with high intensity of the staining (Fig. 1b). It might be due to the presence of oxidative stress enzyme which is most likely to be superimposed with DM and cutaneous wound creation [22]. Surprisingly, in the diabetic treated PB extract group (DM-PB), the expression was found to be decreased by showing a decreased intensity of the staining in the epidermal lining. PB served as an antioxidant agent resulted in decreased 11β-HSD-1 expression (Fig. 1d). A modern treatment with 1% silver nitrate cream provides an effective mechanism in promoting wound healing. However, some of the active antioxidant compounds are lacking in this type of agent. This fact is supported by showing the decreased 11β-HSD-1 expression in wound tissues of the diabetic group treated with 1% silver nitrate cream (Fig. 1c). The remaining expression of 11β-HSD-1 in wound tissue found in DM-PB was due to the gradual effect of PB extract. Both the antioxidant and antidiabetic properties of PB leaves attributed to enhance tissue repair in diabetic wounds. Thus, the expression of 11β-HSD-1 was significantly reduced.
The results of light microscopic study revealed that there was re-epithelialization of the wound tissues, increase in keratin deposition, increase in fibroblasts and macrophages and regular arrangement of collagen bundles found in the DM-PB group compared to the DM-Ctrl group. However, these findings were more pronounced in day 7 post wound creation. Previously, it was mentioned that the improper controlled DM promote free radical production, which contributes to the widespread damage of the vessel walls [15]. Diabetic untreated rats showed less congestion of blood, a reduced amount of deposition of fibroblast and macrophages on day 7 post wound creation under VvG staining. The analysis of MT stained section shown the irregular arrangement of collagen bundles in the same group. It was reported that fibroblasts are responsible for synthesizing the collagen in skin injury. Less fibroblast cells indicate the alteration in cellular integrity and extracellular environment [29].
It is noteworthy to mention that the potency of restoring the histological integrity of diabetic wound tissue treated with PB extract showed compatibility with 1% silver nitrate cream group (DM-SN group). Increase in angiogenesis was observed in H&E-stained (Fig. 2, Fig. 3), deposition of new fibroblasts and macrophages were observed under VvG-stained (Fig. 4, Fig. 5) and regular arrangement of collagen bundles was highlighted with MT-stained wound tissue sections (Fig. 6, Fig. 7). It may be suggested that PB can repair the wound tissue in hyperglycaemic state. Several researches were conducted on PB leaves with regards to its antioxidant and antidiabetic properties [26], [27], [30]. Phytochemical constituents present in PB leaves have free radical scavenging activity and increase the anti-oxidant activity. Furthermore, the flavonoid compounds present in PB leaves possess antimicrobial property which promotes the wound repair process by accelerating the healing process and increasing the wound tissue re-epithelialization [31].
In order to confirm the findings achieved from light microscope, the transmission electron microscopic analysis of wound tissues was performed. On day 7 post wound tissues, the interruption of collagen fibres with wider cytoplasmic spaces were observed in the wound tissue of DM-Ctrl group (Fig. 8b). The ultrastructural features of fibroblasts with loss of normal contour and deformation in the shape were identified in untreated diabetic wounds (Fig. 9b). It can be explained that in the uncontrolled glycaemic state, there is the distorted cellular outline due to poor nutrition into the cell. However, the basic ultrastructural features of the collagen fibres (Fig. 8d) and fibroblasts (Fig. 9d) were observed prominently in PB treated diabetic wounds. Previous study highlighted the interruption in the collagen fibres and deformed fibroblast in wound tissues of diabetic rats [30]. Our study showed that treatment with PB extract repaired ultrastructural damages of diabetic wounds.
In the present study, we studied the gradual onset of the wound healing, especially on day 3 and 7. However, the less pronounced effect in day 3 wound tissues with PB treatment might be probably due to the shorter treatment duration. The specific time period is mandatory for proper healing of the wounds. It can also be agreed upon that the herbal extract showed their potential effect gradually compared to the synthetic ones [32]. Following the positive findings observed in DM-PB group, it was believed that PB leaves enhanced the wound healing process on day 7 post wound creation. We admit to have few limitations in the study; we did not aim to observe the changes in the DM-SN group as silver nitrate cream is already in commercial use. Hence, no comparison with regard to statistical findings were done. It was expected that present findings will draw the attention of the future researchers in determining the details actions of PB towards diabetic wounds by measuring the maximum tensile strength, indentation stiffness and orientation of collagen fibres with recently developed technology such as optical coherence tomography (OCT) [33].
5. Conclusion
PB leaves increased hydroxyproline content in the diabetic wound tissue. This enhanced wound healing process is most probably due to the increase in SOD level, decrease in MDA level and decrease in 11β-HSD-1 expressions in the wound tissues of experimentally-induced diabetic rat. The results were supported by the evidence from light and electron studies of diabetic wound tissues applied with PB extract. The data from the present study might be beneficial in understanding the delayed wound healing process in type 1 diabetes mellitus and designing future novel therapeutic agents. Further studies are needed to corroborate such facts.
Conflict of interest
The authors have declared that no competing interests exist.
Acknowledgement
The authors are grateful to the Faculty of Medicine, Universiti Kebangsaan Malaysia for providing financial assistance (FF-2014-013). The authors also acknowledge the kind help received from the staff members in the Department of Anatomy.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Amos A.F., McCarty D.J., Zimmet P. The rising global burden of diabetes and its complications. Diabet Med. 1997;14(5):7–85. [PubMed] [Google Scholar]
- 2.Armstrong D.G., Lavery L.A., Wrobel J.S., Vileikyte L. Quality of life in healing diabetic wounds: does the end justify the means? J Foot Ankle Surg. 2008;47(4):278–282. doi: 10.1053/j.jfas.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty D., Shah B. Antimicrobial anti-oxidative and anti-hemolytic activity of Piper betel leaf extracts. Int J Pharm Pharm Sci. 2011;3:192–199. [Google Scholar]
- 5.Singla R., Ganguli A., Ghosh M., Sohal S. Evaluation of sanitizing efficacy of acetic acid on Piper betle leaves and its effect on antioxidant properties. Int J Food Sci Nutri. 2009;60(7):297–307. doi: 10.1080/09637480903114110. [DOI] [PubMed] [Google Scholar]
- 6.Hillman G. The role of phytotherapeutics in wound management. Review. Ostomy Wound Manag. 2001;47(5):28–33. [PubMed] [Google Scholar]
- 7.Rasik A.M., Shukla A. Antioxidant status in delayed healing type of wounds. Int J Exp Pathol. 2000;l8(2):257–263. doi: 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keat E.C., Razak S.S., Fadil N.M., Yusof F.M., Chan L.H., Chyi F.K. The effect of P.betel extract on wound healing process in experimental induced diabetic rat. Clin Ter. 2010;161(2):117–120. [PubMed] [Google Scholar]
- 9.Demling R.H., DeSanti L. Involuntary weight loss and the nonhealing wound: the role of anabolic agents. Adv Wound Care. 1999;12(1):1–14. [PubMed] [Google Scholar]
- 10.Itoi S., Terao M., Murota H., Katayama I. 11β-Hydroxysteroid dehydrogenase 1 contributes to the pro-inflammatory response of keratinocytes. Biochem Biophys Res Commun. 2013;440(2):265–270. doi: 10.1016/j.bbrc.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 11.Shaw J.E., Sicree R.A., Zimmet P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Prac. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar B., Ray Chaudhuri S.G., Ray A. Effect of ethanol extract of Piper betle Linn leaf on healing of NSAID-induced experimental ulcer–a novel role of free radical scavenging action. Ind J Exp Biol. 2003;41:311–315. [PubMed] [Google Scholar]
- 13.Nalina T., Rahim Z.H.A. The crude aqueous extract of Piper betleL. and its antibacterial effect towards Streptococcus mutans. Am J Biotech Biochem. 2007;3:10–15. [Google Scholar]
- 14.Shila G., Natasa S.B. Wound healing properties of Carica papayalatex : in vivo evaluation in mice burn model. J Ethnopharmacol. 2009;121:338–341. doi: 10.1016/j.jep.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Teoh S.L., Latiff A.A., Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol. 2009;34(7):815–822. doi: 10.1111/j.1365-2230.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 16.Dissemond J., Goos M., Wagner S.N. The role of oxidative stress in the pathogenesis and therapy of chronic wounds. Hautarzt. 2002;53:718–723. doi: 10.1007/s00105-001-0325-5. [DOI] [PubMed] [Google Scholar]
- 17.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 18.Tiganescu A., Walker E.A., Hardy R.S., Mayes A.E., Stewart P.M. Localization age- and site-dependent expression and regulation of 11β-hydroxysteroid dehydrogenase type 1 in skin. J Investig Dermatol. 2011;131(1):30–36. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 19.Misra H.P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3178. [PubMed] [Google Scholar]
- 20.Wang B.J., Guo Y.L., Guo H.R., Chang H.Y. Piper betle L. inflorescence causes allergic contact dermatitis of the hands during betel quid assembly. Contact Dermat. 2008;58(6):368–370. doi: 10.1111/j.1600-0536.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 21.Piccoli G.B., Clari R., Ghiotto S., Castelluccia N., Colombi N., Mauro G. Type 1 diabetes diabetic nephropathy and pregnancy: a systematic review and meta-study. Rev Diabet Stud Spring. 2013;10(1):6–26. doi: 10.1900/RDS.2013.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallou G., Ruelland A., Legras B., Maugendre D., Allannic H., Cloarec L. Plasma malondialdehyde in type 1 and type 2 diabetic patients. Clin Chim Acta. 1993;214(2):227–234. doi: 10.1016/0009-8981(93)90114-j. [DOI] [PubMed] [Google Scholar]
- 23.Nayak B.S., Pinto Pereira L.M. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement Altern Med. 2006;58(6):41–46. doi: 10.1186/1472-6882-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasekarana N.S., Nithyac M., Rosec C. The effect of finger millet feeding on the early responses during the process of wound healing in diabetic rats. Biochim Biophys Acta. 2004;1689(3):190–201. doi: 10.1016/j.bbadis.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Parillon F., Edward H. Universiti Putra Malaysia; 2006. Antimicrobial activity of Psidium guajava and Piper betle extracts on selected foodborne bacteria; pp. 28–31. [Google Scholar]
- 26.Santhakumari P., Prakasam A., Pugalendi K.V. Antihyperglycemic activity of Piper betle leaf on streptozotocin-induced diabetic rats. J Med Food. 2006;9(1):108–112. doi: 10.1089/jmf.2006.9.108. [DOI] [PubMed] [Google Scholar]
- 27.Nabasree D., Bratati D. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004;88:219–224. [Google Scholar]
- 28.Terao M., Murota H., Kimura A., Kato A., Ishikawa A., Igawa K. 11β-Hydroxysteroid dehydrogenase-1 is a novel regulator of skin homeostasis and a candidate target for promoting tissue repair. PLoS One. 2011;6(9):e25039. doi: 10.1371/journal.pone.0025039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velnar T., Bailey T., Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 30.Santhakumari P., Prakasam A., Pugalendi K.V. Modulation of oxidative stress parameters by treatment with Piper betle leaf in streptozotocin induced diabetic rats. Ind J Pharmacol. 2003;35:373–378. [Google Scholar]
- 31.Tsuchiya H., Sato M., Miyazaki T., Fujiwara S., Tanigaki S., Ohyama M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50(1):27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 32.Shenoy R.R., Sudheendra A.T., Nayak P.G., Paul P., Kutty N.G., Rao C.M. Normal and delayed wound healing is improved by sesamol an active constituent of Sesamum indicum (L.) in albino rats. J Ethnopharmacol. 2011;133:608–612. doi: 10.1016/j.jep.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 33.Choi M.C., Cheung K.K., Ng G.Y., Zheng Y.P., Cheing G.L. Measurement of diabetic wounds with optical coherence tomography-based air-jet indentation system and a material testing system. J Wound Care. 2015;24(11):519. doi: 10.12968/jowc.2015.24.11.519. 522–4, 526-8. [DOI] [PubMed] [Google Scholar]