Abstract
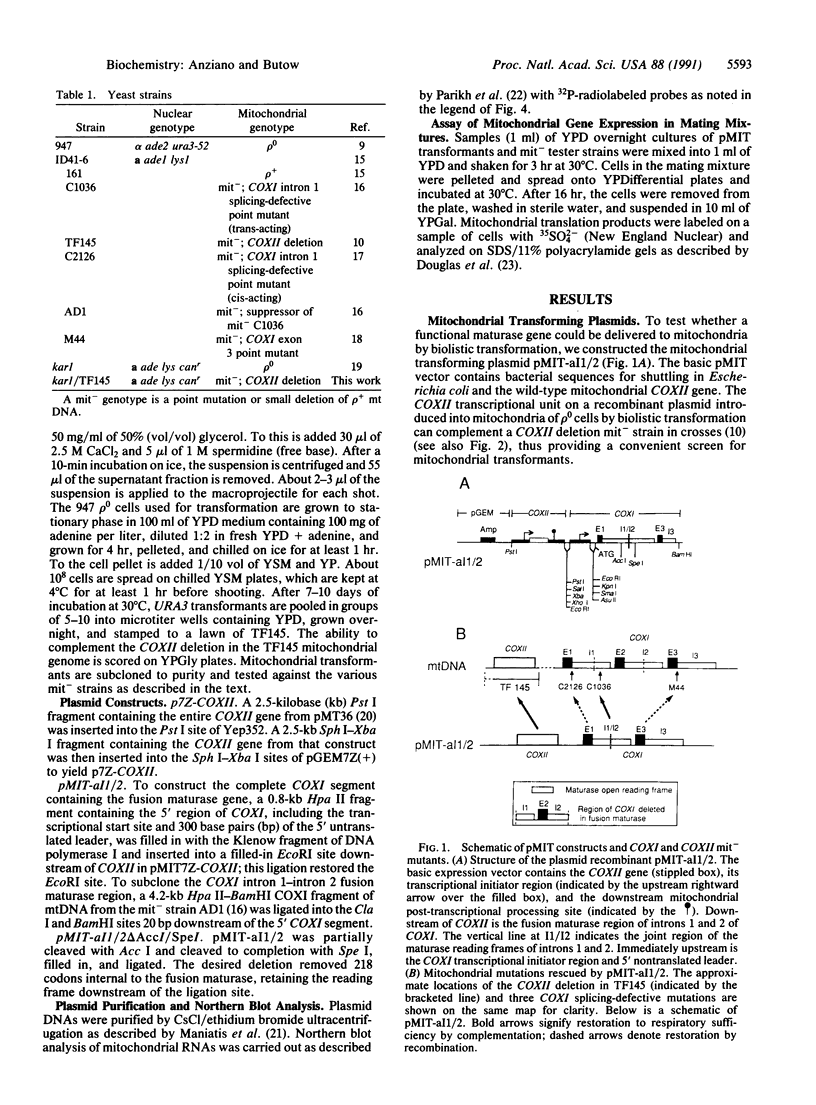
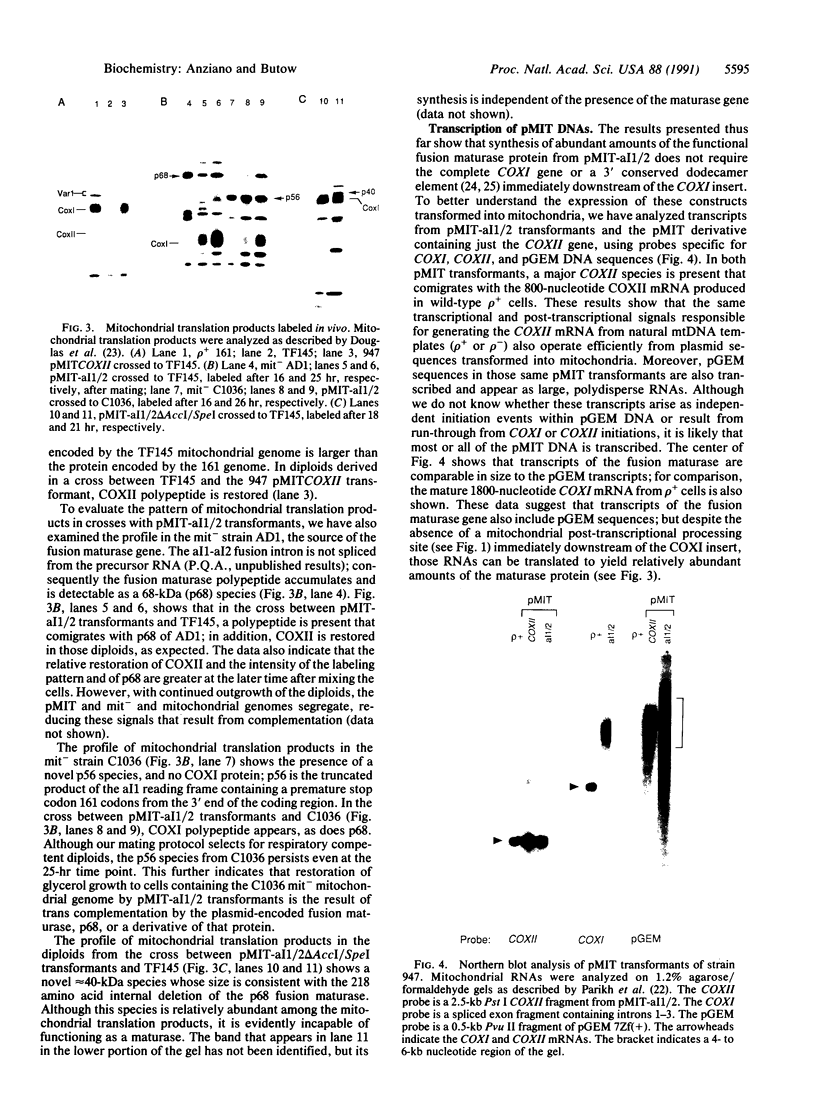
We have developed a recombinant vector, termed pMIT, for transient expression of genes delivered to yeast mitochondria by biolistic transformation. Using that vector, we introduced a hybrid RNA maturase (splicing) gene into mitochondria of rho 0 petite cells and showed the gene to be functional in crosses. The hybrid maturase is an in-frame fusion between the N-terminal half of the maturase encoded by intron 1 of the COXI (cytochrome oxidase) gene and the C-terminal half of a similar maturase encoded by COXI intron 2. pMIT transformants can provide a functional maturase in crosses to restore respiration and COXI polypeptide synthesis to a respiratory-deficient strain defective in the synthesis of a maturase encoded by COXI intron 1; the transformant will also restore respiration to two splicing-defective cis mutants of COXI introns 1 and 3. We detect a 68-kDa polypeptide comparable in abundance to other major mitochondrial translation products as a likely product of the hybrid maturase gene. Transformants containing an internal 218-amino acid deletion mutation of the hybrid maturase gene no longer express a functional maturase in crosses and produce the expected shortened polypeptide of approximately 40 kDa; however, those transformants still restore respiration to the COXI cis mutants. These studies show the utility of the pMIT transformation system for the expression and reverse genetic analysis of yeast mitochondrial genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. J., Vincent R. D., Perlman P. S., Miller D. H., Hanson D. K., Mahler H. R. Regulatory interactions between mitochondrial genes. I. Genetic and biochemical characterization of some mutant types affecting apocytochrome b and cytochrome oxidase. J Biol Chem. 1979 Apr 10;254(7):2471–2479. [PubMed] [Google Scholar]
- Anziano P. Q., Hanson D. K., Mahler H. R., Perlman P. S. Functional domains in introns: trans-acting and cis-acting regions of intron 4 of the cob gene. Cell. 1982 Oct;30(3):925–932. doi: 10.1016/0092-8674(82)90297-5. [DOI] [PubMed] [Google Scholar]
- Anziano P. Q., Moran J. V., Gerber D., Perlman P. S. Novel hybrid maturases in unstable pseudorevertants of maturaseless mutants of yeast mitochondrial DNA. Nucleic Acids Res. 1990 Jun 11;18(11):3233–3239. doi: 10.1093/nar/18.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banroques J., Delahodde A., Jacq C. A mitochondrial RNA maturase gene transferred to the yeast nucleus can control mitochondrial mRNA splicing. Cell. 1986 Sep 12;46(6):837–844. doi: 10.1016/0092-8674(86)90065-6. [DOI] [PubMed] [Google Scholar]
- Butow R. A., Fox T. D. Organelle transformation: shoot first, ask questions later. Trends Biochem Sci. 1990 Dec;15(12):465–468. doi: 10.1016/0968-0004(90)90299-q. [DOI] [PubMed] [Google Scholar]
- Butow R. A., Zhu H., Perlman P., Conrad-Webb H. The role of a conserved dodecamer sequence in yeast mitochondrial gene expression. Genome. 1989;31(2):757–760. doi: 10.1139/g89-134. [DOI] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad-Webb H., Perlman P. S., Zhu H., Butow R. A. The nuclear SUV3-1 mutation affects a variety of post-transcriptional processes in yeast mitochondria. Nucleic Acids Res. 1990 Mar 25;18(6):1369–1376. doi: 10.1093/nar/18.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahodde A., Goguel V., Becam A. M., Creusot F., Perea J., Banroques J., Jacq C. Site-specific DNA endonuclease and RNA maturase activities of two homologous intron-encoded proteins from yeast mitochondria. Cell. 1989 Feb 10;56(3):431–441. doi: 10.1016/0092-8674(89)90246-8. [DOI] [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Sanford J. C., McMullin T. W. Plasmids can stably transform yeast mitochondria lacking endogenous mtDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7288–7292. doi: 10.1073/pnas.85.19.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. A., Anziano P. Q., Shark K., Sanford J. C., Butow R. A. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science. 1988 Jun 10;240(4858):1538–1541. doi: 10.1126/science.2836954. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Perlman P. S. Involvement of aminoacyl-tRNA synthetases and other proteins in group I and group II intron splicing. Trends Biochem Sci. 1990 Nov;15(11):440–444. doi: 10.1016/0968-0004(90)90283-h. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Lopez I. C., Farrelly F., Butow R. A. Trans action and the var1 determinant region on yeast mitochondrial DNA. Specific labeling of mitochondrial translation products in zygotes. J Biol Chem. 1981 Jun 25;256(12):6496–6501. [PubMed] [Google Scholar]
- Osinga K. A., De Vries E., Van der Horst G., Tabak H. F. Processing of yeast mitochondrial messenger RNAs at a conserved dodecamer sequence. EMBO J. 1984 Apr;3(4):829–834. doi: 10.1002/j.1460-2075.1984.tb01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V. S., Conrad-Webb H., Docherty R., Butow R. A. Interaction between the yeast mitochondrial and nuclear genomes influences the abundance of novel transcripts derived from the spacer region of the nuclear ribosomal DNA repeat. Mol Cell Biol. 1989 May;9(5):1897–1907. doi: 10.1128/mcb.9.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S. Genetic analysis of RNA splicing in yeast mitochondria. Methods Enzymol. 1990;181:539–558. doi: 10.1016/0076-6879(90)81150-s. [DOI] [PubMed] [Google Scholar]
- Poutre C. G., Fox T. D. PET111, a Saccharomyces cerevisiae nuclear gene required for translation of the mitochondrial mRNA encoding cytochrome c oxidase subunit II. Genetics. 1987 Apr;115(4):637–647. doi: 10.1093/genetics/115.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Thorsness P. E., Fox T. D. Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature. 1990 Jul 26;346(6282):376–379. doi: 10.1038/346376a0. [DOI] [PubMed] [Google Scholar]
- Weiss-Brummer B., Rödel G., Schweyen R. J., Kaudewitz F. Expression of the split gene cob in yeast: evidence for a precursor of a "maturase" protein translated from intron 4 and preceding exons. Cell. 1982 Jun;29(2):527–536. doi: 10.1016/0092-8674(82)90169-6. [DOI] [PubMed] [Google Scholar]