Introduction
HIV infection is a known risk factor for a host of mucocutaneous conditions, most notably drug hypersensitivity reactions.1, 2 We describe an unusual case of mucosal autoimmune progesterone dermatitis (APD) in a woman with HIV disease and suggest that pathergy of HIV aphthae possibly contributed to her impressive oral involvement.
Case report
A woman in her 40s with a greater than 10-year history of HIV disease, well controlled on efavirenz/emtricitabine/tenofovir, reported a 3-year history of recurrent painful oral erosions despite chronic acyclovir therapy. Physical examination found irregularly shaped erosions of the tongue; differential diagnosis included HIV aphthae, herpes simplex virus (HSV), and erythema multiforme (EM). Acyclovir was continued, and colchicine, 0.6 mg twice a day, was prescribed. Subsequent tongue biopsy, as read by an oral pathologist, showed mucosal ulceration and parakeratosis with superficial and deep mixed inflammatory infiltrate consistent with a lichenoid hypersensitivity reaction, including both drug allergy and EM; clinicohistologic correlation supported the diagnosis of EM.
Over the next 6 months, oral erosions repeatedly flared, remitting for 2 to 3 weeks before the next outbreak. Several hospitalizations ensued for pain control and hydration. Physical examination during severe flares found diffuse erosions of the vermillion lips, tongue, buccal mucosa, and palate (Fig 1). Some episodes included anogenital erosions and violaceous papules on distal extremities. During one flare, antibodies to HSV1/2 IgM were negative while antibodies to IgG were positive. Mycoplasma IgM titer was elevated but IgG titer, culture, and respiratory polymerase chain reaction were normal, and convalescent mycoplasma titer failed to document an increase in IgG.
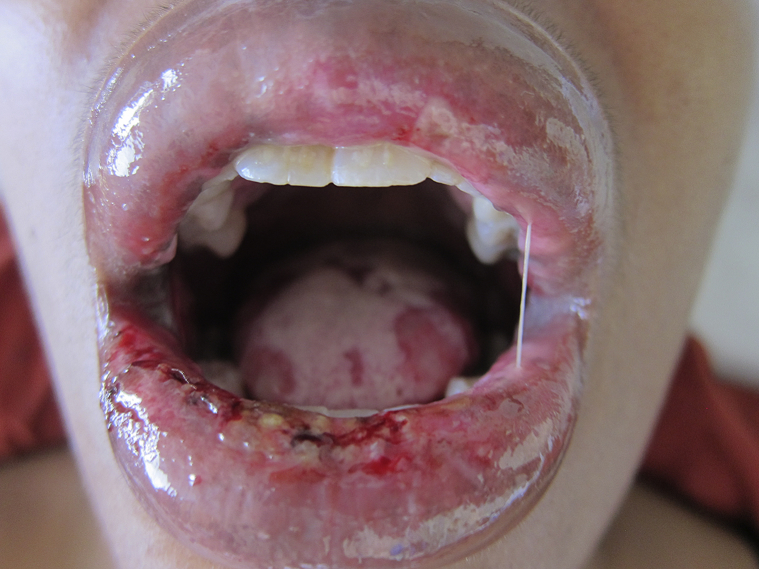
Fig 1.
APD. Mucosal erosions of the upper and lower vermillion lips and tongue.
The mucositis continued to worsen. Failed therapies included acyclovir, prednisone, dapsone, colchicine, azithromycin, niacinamide, and topical corticosteroids. It was then appreciated that monthly flares were related to her menstrual cycle, raising concern for APD. Given her age and ongoing daily tobacco use, a gynecologic consultant deemed her a poor candidate for medical hormonal therapy. Convinced of the positive relationship between her menstrual cycle and mucosal eruptions, she requested total abdominal hysterectomy–bilateral salpingo-oophorectomy (TAH-BSO). Mucosal outbreaks immediately improved postoperatively.
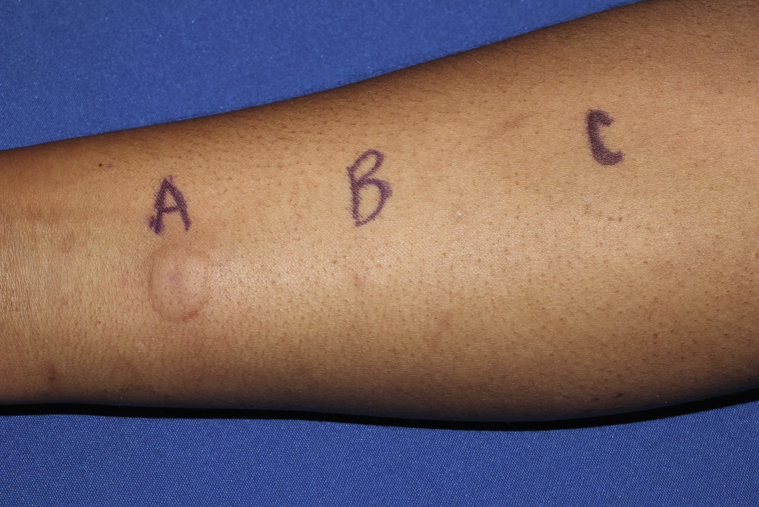
Confirming the diagnosis of APD, an intradermal progesterone test (IDP) was performed using 0.1 mL of 50 mg/mL progesterone in benzoyl alcohol with bacteriostatic and normal saline as controls. A 1.2-cm erythematous wheal immediately developed at the IDP injection site with blister formation the following day (Fig 2). Controls had no reaction.
Fig 2.
IDP test. Wheal formation 5 minutes after IDP injection at site A with negative bacteriostatic saline and normal saline controls at sites B and C, respectively.
At follow-up visits, the patient had punctate ulcerations of the soft palate, starkly different than that of prior presentations and consistent with HIV-associated aphthae (Fig 3). Eight months after TAH-BSO, she remained free of cyclic, debilitating EM mucositis.
Fig 3.
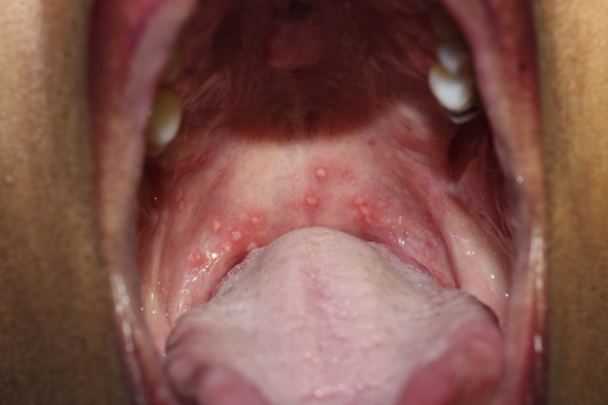
HIV aphthae. Punched out ulcerations of the soft palate consistent with HIV aphthae without evidence of EM.
Discussion
Identifying the underlying cause of EM is challenging. Although HSV is commonly touted to be the most frequent trigger, retrospective studies identified HSV infection as the cause in only 17.5-23.0% of cases.3, 4 Multiple additional triggers are documented including, as in this case, EM driven by progesterone, one morphologic variety of APD.5, 6
APD is an uncommon mucocutaneous disease characterized by cyclical alterations in skin and mucosa; variable morphologies include EM, urticaria, anaphylaxis, fixed drug, and vesiculobullous eruptions.6 Flares occur approximately 3 to 10 days before menses as serum progesterone levels increase. Although the exact pathogenesis is uncertain, APD is likely attributable to direct effects of progesterone on epithelia. The progesterone surge reportedly reduces immune and barrier functions and increases vascularity and sebum production of skin, in some way creating a trigger for dermatitis expression.7 Progesterone skin testing confirms the diagnosis of APD.
APD in the setting of HIV infection is not surprising, as HIV disease is a known risk factor for drug hypersensitivity reactions; such reactions are often more severe than in the general population.2 Because APD involving the oral mucosa is uncommon, we postulate that this patient's underlying HIV aphthae possibly set the stage for an isomorphic response or pathergy, resulting in dramatic oral involvement, which characterized her APD.
We present a noteworthy case of APD manifest as mucosal EM in the setting of HIV disease. The patient had complete resolution of EM after TAH-BSO and a dramatic reaction to IDP testing. Although HIV aphthae and EM may have been independent occurrences, the possibility of pathergy contributing to the severity of her disease is intriguing.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
This work was presented at the 61st Annual Georgia Society of Dermatology and Dermatologic Surgery Meeting, Ponte Vedra, Florida, June 3-5, 2016.
References
- 1.Coopman S.A., Johnson R.A., Platt R., Stern R.S. Cutaneous disease and drug reactions in HIV infection. N Engl J Med. 1993;328(23):1670–1674. doi: 10.1056/NEJM199306103282304. [DOI] [PubMed] [Google Scholar]
- 2.Yang C., Mosam A., Mankahla A., Dlova N., Saavedra A. HIV infection predisposes skin to toxic epidermal necrolysis via depletion of skin-directed CD4(+) T cells. J Am Acad Dermatol. 2014;70(6):1096–1102. doi: 10.1016/j.jaad.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Cretu A., Dimitriu A., Branisteanu D., Brinisteanu D.E. Erythema multiforme–etiopathogenic, clinical and therapeutic aspects. Rev Med Chir Soc Med Nat Iasi. 2015;119(1):55–61. [PubMed] [Google Scholar]
- 4.Wetter D.A., Davis M.D. Recurrent erythema multiforme: clinical characteristics, etiologic associations, and treatment in a series of 48 patients at Mayo clinic, 2000 to 2007. J Am Acad Dermatol. 2010;62(1):45–53. doi: 10.1016/j.jaad.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Nasabzadeh T.J., Stefanato C.M., Doole J.E., Radfar A., Bhawan J., Venna S. Recurrent erythema multiforme triggered by progesterone sensitivity. J Cutan Pathol. 2010;37(11):1164–1167. doi: 10.1111/j.1600-0560.2010.01607.x. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen T., Razzaque Ahmed A. Autoimmune progesterone dermatitis: Update and insights. Autoimmunity reviews. 2016;15(2):191–197. doi: 10.1016/j.autrev.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Raghunath R.S., Venables Z.C., Millington G.W. The menstrual cycle and the skin. Clin Exp Dermatol. 2015;40(2):111–115. doi: 10.1111/ced.12588. [DOI] [PubMed] [Google Scholar]