Abstract
We recently described the induction of an efficient CD8+ T cell-mediated immune response against a tumor-associated antigen (TAA) uploaded in engineered exosomes used as an immunogen delivery tool. This immune response cleared tumor cells inoculated after immunization, and controlled the growth of tumors implanted before immunization. We looked for new protocols aimed at increasing the CD8+ T cell specific response to the antigen uploaded in engineered exosomes, assuming that an optimized CD8+ T cell immune response would correlate with a more effective depletion of tumor cells in the therapeutic setting. By considering HPV-E6 as a model of TAA, we found that the in vitro co-administration of engineered exosomes and ISCOMATRIXTM adjuvant, i.e., an adjuvant composed of purified ISCOPREPTM saponin, cholesterol, and phospholipids, led to a stronger antigen cross-presentation in both B- lymphoblastoid cell lines ( and monocyte-derived immature dendritic cells compared with that induced by the exosomes alone. Consistently, the co-inoculation in mice of ISCOMATRIXTM adjuvant and engineered exosomes induced a significant increase of TAA-specific CD8+ T cells compared to mice immunized with the exosomes alone. This result holds promise for effective usage of exosomes as well as alternative nanovesicles in anti-tumor therapeutic approaches.
Keywords: adjuvant, exosomes, Nef, HPV-E6, CD8+ T immune response
1. Introduction
Exosomes are vesicles of 50–100 nanometers released by basically all cell types. They are part of the intercellular communication network [1], and are generated by invagination of endosome membranes leading to the formation of intraluminal vesicles which then become part of multivesicular bodies [2].They can traffic to the plasma membrane, thereby releasing their vesicular contents upon membrane fusion. Exosomes have a low intrinsic immunogenic profile, their immunogenicity being related to both amounts and quality of uploaded antigens, and, in some cases, they have been tested in clinical trials [3,4,5]. Exosomes spontaneously uploading tumor antigens, mainly trans-membrane proteins like gp100,TRP-1, Her2/neu and carcinoembryonic antigen, induced activation of specific anti-tumor T cell immunity [6,7].
Despite the good tolerance of exosomes as cell-free vaccines, their therapeutic efficacy appeared quite limited, posing the need of new methods to increase their immunogenicity. Attempts to address this issue have been performed by engineering desired antigens to increase their association with the external side of exosome membranes [8,9].
The convergence of exosome and HIV biogenesis at the level of the endosomal sorting complex required for transport (ESCRT) [10] implies the possibility that viral products incorporate in exosomes, as already proven for both Gag [11] and Nef [12,13] HIV-1 proteins. HIV-1 Nef lacks enzymatic activities, yet acts as a scaffold/adaptor element [14]. We identified a Nef mutant (referred to as Nefmut) incorporating in HIV-1 virions, virus like particles (VLPs) [15], and exosomes [16] at quite high levels. This Nef mutant is defective for basically all Nef functions, and its efficiency of incorporation in nanovesicles does not change significantly when fused with large foreign proteins [16]. Manipulating Nefmut allows incorporation into exosomes of high amounts of antigens of choice which are protected from external neutralization/degradation factors.
ISCOMATRIXTM (CSL Behring LLC, King of Prussia, PA, USA) adjuvant is an immunostimulating complex formed by ISCOPREPTM (Gronberg Adv. Byra KB, Stockholm, Sweden), saponin extracted from Quillaia saponaria, phospholipids, and cholesterol [17]. Its immune adjuvanticity has already been demonstrated, in particular regarding the induction of both CD4+ and CD8+ T cell immunity directed to soluble protein antigens [18]. We have recently shown that the inoculation in the mice of exosomes engineered to upload high amounts of the TAA HPV-E7 elicits an effective CD8+ T cell response [19]. Here, we originally show that ISCOMATRIXTM adjuvant strongly improves both CD8+ T-related antigenicity and immunogenicity of proteins delivered by engineered exosomes.
2. Materials and Methods
2.1. Cell Cultures and Adjuvant
For the experiment, 293T cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) plus 10% heat-inactivated Fetal Calf Serum(FCS). The isolation of the Human leukocyte antigen (HLA)-B7 Nef-specific CD8+ T cell clone was already described [20]. It recognizes the amino acid sequence TPGPGVRYPL (aa 128–137). Mart-1-specific CD8+ T cells recognize the HLA-A.02-restricted AAGIGILTV27–35 amino acid sequence. Human monocytes were separated from peripheral blood mononuclear cell (PBMCs) of HLA-A.02 healthy donors using anti-CD14 microbeads (Miltenyi Biotec GmbH, Teterow, Germany), and differentiated to immature iDCs upon 4–5 days of culture in Roswell Park Memorial Institute (RPMI) medium supplemented with 20% FCS, 30 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF) (AbD Serotec, Bio-Rad Laboratories, s.r.l., Milan Italy), and 500 units/mL IL-4 (R&D Systems, Minneapolis, MN, US). The iDC phenotype was characterized by Fluorescence-activated cell sorting (FACS) analysis for the expression of CD11c and the absence of CD14 cell membrane markers. Both isolation and expansion of the CD8+ T cell clone specific for Mart-1 have been previously described [21]. Mouse splenocytes were cultivated in RPMI medium supplemented with 10% FCS. ISCOMATRIXTM adjuvant (CSL Behring LLC, King of Prussia, PA, USA) was prepared as previously described [17], and is composed of ISCOPREPTM saponin, cholesterol, and phospholipids. The undiluted preparation contained 115 ISCOTM Units/mL.
2.2. Production, Purification, and Quantification of Exosomes
Exosomes were produced by transiently transfecting 293T cells with vectors expressing Nefmut-based fusion proteins. The cells were seeded in the presence of exosome-deprived FCS, and supernatants harvested 48–72 h after transfection. Efficiency of transfection was routinely evaluated by intracellular Fluorescence-activated cell sorting (FACS) analysis as previously reported [22] using the anti-Nef MATG mAb kindly provided by Olivier Schwartz, Pasteur Institute, Paris, France. Exosomes were recovered by differential centrifugations as previously described [23]. The amounts of exosomes were evaluated by measuring the activity of acetylcholinesterase (AchE), i.e., a classical exosome marker [24], through the Amplex Red kit (Molecular Probes, Termo Fischer Scientific, Waltham, MA, USA). The AchE activity was measured as mU/mL, where 1 mU is defined as the amount of enzyme hydrolyzing 1 pmole of acetylcholine to choline and acetate per minute at pH 8.0 at 37 °C.
2.3. Fluorescence-Activated Cell Sorting(FACS) Analysis of Bead-Exosome Complexes
Samples were incubated with 5 µL of surfactant-free white aldehyde/sulfate latex beads (Termo Fischer Scientific, Waltham, MA, USA) overnight at room temperature (r.t.) on a rotating plate. For the assays carried out with Nefmut/Green Fluorescent protein (GFP) exosomes in the presence of ISCOMATRIXTM adjuvant, beads and exosomes were incubated in the presence of different concentrations of the adjuvant for 1 to 3 h before FACS analysis. For the characterization of different exosome preparations, bead-exosome complexes were labeled with phycoerythrin (PE)-conjugated anti-CD63 mAb (BD Biosciences Milan, Italy) for 1 h at 4 °C. Finally, beads were washed, resuspended in 1× PBS-2% v/v formaldehyde, and FACS analyzed.
2.4. Detection of Exosome Cell Internalization
B-lymphoblastoid cell lines (BLCLs) were pre-treated with 100 nM bafilomycin A1 (Sigma-Aldrich, Milan, Italy) for 2 h in the presence or not of ISCOMATRIXTM adjuvant, and then challenged by spinoculation with fluorescent exosomes previously incubated in the presence or not of ISCOMATRIXTM adjuvant. After 2 h of incubation at either 4 or 37 °C in the presence of ISCOMATRIXTM adjuvant and/or bafilomycin A1, cells were treated for 15 min with trypsin, fixed with 2% v/v formaldehyde in 1× PBS, and FACS analyzed.
2.5. Western Blot Analysis
The equivalent of 200 µU of exosomes were lysed in PBS, 1% Triton X-100 (Sigma-Aldrich , Milan, Italy) in the presence of anti-proteolytic agents, and then separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Membranes were revealed using sheep anti-Nef antiserum ARP 444, a generous gift of Mark Harris, University of Leeds, Leeds, UK, polyclonal anti-vesicular stomatitis virus G glycoprotein (VSV-G) Abs (Immunological Consultant Laboratories, Newberg, OR, USA), and anti-intercellular adhesion molecule (ICAM)-1 mAb 15.2 (Santa Cruz Biotechnology Inc., Heidelberg, Germany).
2.6. Cross-Presentation Assay
HLA-B7 B-LCLs were challenged by spinoculation with Nefmut–based exosomes in the presence or not of different concentrations of ISCOMATRIXTM adjuvant. Five hours later, the cells were extensively washed and then co-cultivated in triplicate at 1:2 ratio with Class I major histocompatibility complex (MHC)-matched Nef-CD8+ T cells in Elispot multiwell plates pre-coated with the D1K mAb against human interferon (IFN)-γ (Mabtech, Nacka Strand Sweden) in RPMI plus 10% of AB human serum (Gibco, Termo Fischer Scientific, Waltham, MA, USA) for 16 h. Thereafter, co-cultures were removed, the Elispot assay was completed, and the spot-forming cells were analyzed and counted using an Elispot reader (Amplimedical Bioline A-EL-VIS GmbH, Turin, Italy). Cross-presentation assays using Nefmut/Mart-1 exosomes were performed basically in the same way, except that HLA-A.02 immature dendritic cells were used as antigen presenting cells (APCs), and the above described Mart-1 specific CD8+ T cell clone was used as effector cells.
2.7. Immunogenicity Assay
All studies with animals here described have been approved by the Ethical Committee of the Istituto Superiore di Sanità, Rome, Italy (protocol No. 555/SA/2012) according to Legislative Decree 116/92, which has implemented in Italy the European Directive 86/609/EEC on laboratory animal protection. Animals used in our research have been housed and treated according to the guidelines inserted in here above mentioned Legislative Decree. Eight week-old female C57 Bl/6 mice (3 per group in two independent experiments) were purchased from Charles River Laboratories Italia, (Calco, Italy) and inoculated s.c. 3 times at 2 week intervals with a total of 100 µL comprising Nefmut/E6 exosomes in the presence or not of ISCOMATRIXTM adjuvant. Two weeks after the last inoculation, mice were sacrificed, and splenocytes put in culture in the presence of 5 µg/mL of 8- or 9-mer HPV-E6 peptides already identified to efficiently bind the H-2 Kb complex of C57 Bl/6 mice [25], i.e., KLPQLCTEL (aa. 18–26) and YDFAFRDL (aa 50–57). H-2 Kb binding HPV E7-specific peptides DLYCYEQL (aa 21–28), and RAHYNIVTF (aa 49–57) [25] were used as control peptides. IFN-γ Elispot assays were performed after overnight incubation in Elispot microwells. All IFN-γ Elispot assays were performed in triplicate conditions using commercially available reagents (Mabtech AB, Nacka Strand Sweden), and spots counted using an Elispot reader.
2.8. Detection of Anti-HPV-E6 Abs
Sera from inoculated mice were pooled, and two-fold serial dilutions starting from 1:100 were assayed for the presence of anti-HPV-E6 Abs as previously reported [19] using recombinant HPV-E6 produced as described [26].
2.9. Cytotoxic T Lymphocyte (CTL) Assay
CD8+ T cells were isolated from splenocytes of exosome-inoculated mice by positive immunomagnetic selection (Miltenyi Biotec., Teterow, Germany). They were put in co-culture with EL-4 cells previously labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, Termo Fischer Scientific, Waltham, MA, USA) and treated overnight with either E6 or unrelated peptides. The co-cultures were run for 6 h in 200 µL of RPMI 20% FCS in U-bottom 96 well plates at 20:1 effector/target cell ratio. Afterwards, EL-4 cell mortality was scored by FACS analysis soon after addition of 7-AAD at a final concentration of 1 µg/mL.
2.10. Statistical Analysis
When appropriate, data are presented as mean + standard deviation (SD). In some instances, the paired Student’s t-test was used and confirmed using the non-parametric Wilcoxon rank sum test. p < 0.05 was considered significant.
3. Results
3.1. ISCOMATRIXTM Adjuvant Does Not Affect Both Structure and Cell Entry Efficiency of Exosomes
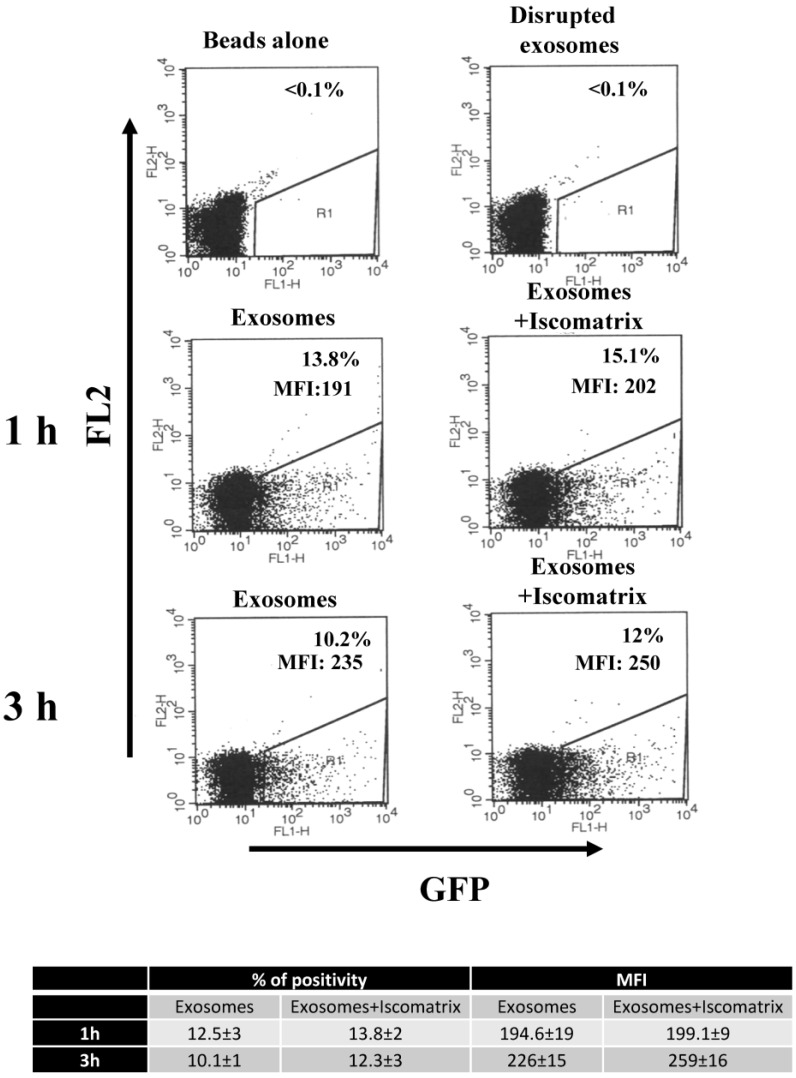
Attempting to improve the potency of the antigen-specific CTL response that we previously observed in mice inoculated with Nefmut-based exosomes, we looked for adjuvants already proven to increase the CD8+ T lymphocyte response, and whose molecular composition was expected to not impact the exosome structure. ISCOMATRIXTM adjuvant appeared as a valuable candidate. However, preliminary experiments aimed at identifying possible structural and/or functional alterations of exosomes upon interaction with ISCOMATRIXTM adjuvant were mandatory. Structural alterations were evaluated using fluorescent exosomes whose GFP contents were measured by FACS analysis after binding to aldheyde-sulphate beads. In this system, we assumed that relevant damages in the exosome structure couple with a loss of the exosome-associated fluorescence. In detail, GFP-labeled exosomes were recovered by transiently transfecting 293T cells with a vector expressing the Nefmut/GFP fusion protein as previously described [16]. A total of 1 mU of these exosomes were bound to the beads, and then incubated with either 5.75, 11.5, or 23 ISCOTM U/mL of ISCOMATRIXTM adjuvant for 1 to 3 h. As a control, the same amount of fluorescent exosomes was disrupted by heating at 95 °C for 10 min in water before the incubation with the beads. Figure 1 shows the results obtained with the highest concentration of the adjuvant. Clearly, no differences in the beads-associated fluorescence appeared between control and ISCOMATRIXTM adjuvant -treated exosomes, and similar results were obtained with lower adjuvant concentrations (not shown). This result strongly suggested that the incubation with ISCOMATRIXTM adjuvant does not induce degradation of exosomes.
Figure 1.
The treatment with ISCOMATRIXTM adjuvant does not affect the exosome-associated fluorescence. Fluorescent exosomes were incubated in the absence or presence of 23 ISCOTM U/mL of the adjuvant at 37 °C with aldehyde sulphate beads in a rotating plate. After 1 and 3 h, samples were washed, and analyzed by FACS. As a control, equivalent amounts of fluorescent exosomes were heated at 95 °C for 10 min before incubation with beads. Results representative of three independent experiments with duplicates are shown. In each plot, both percentages and, where relevant, mean fluorescence intensity (MFI) of fluorescent beads are indicated. At the bottom, mean values ± SD of both percentages and MFI of fluorescent beads from the three experiments are reported. Iscomatrix: ISCOMATRIXTM adjuvant.
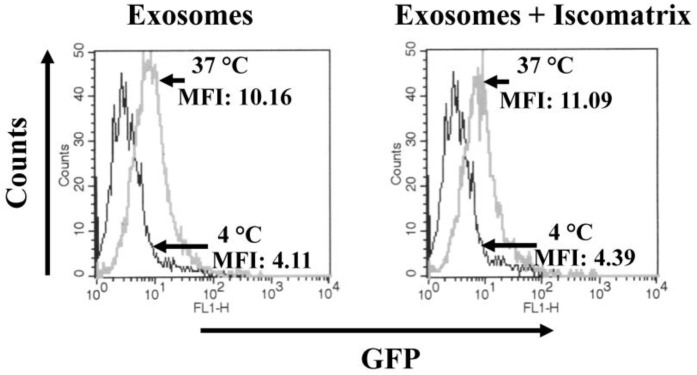
Next, we looked at possible influences of ISCOMATRIXTM adjuvant on cell entry efficiency of exosomes. To this end, fluorescent exosomes were incubated for two hours with complete medium either alone or supplemented with 23 ISCOTM U/mL in a total of 50 µL. Meanwhile, cells were pre-treated for 2 h with the adjuvant and/or bafilomycin A1, the latter required to hamper rapid intracellular degradation of incoming exosomes. Afterwards, B-LCLs were challenged with exosomes by spinoculation carried out either at 4 or 37 °C. Cells were then re-plated at the two different temperatures, and after 2 h treated with trypsin, and finally FACS analyzed. As shown in Figure 2, fluorescence levels in B-LCLs did not change significantly when exosomes were pre-treated with ISCOMATRIXTM adjuvant, indicating that the adjuvant does not affect the cell entry efficiency of exosomes.
Figure 2.
Unchanged cell internalization of exosomes in the presence of ISCOMATRIXTM adjuvant. A total of 2 × 105 B-LCLs were pre-treated for 2 h with bafilomycin A1 in the presence or not of the ISCOMATRIXTM adjuvant, and then challenged with 500 µU of fluorescent exosomes pre-incubated or not with the adjuvant. After spinoculation, the cells were incubated for an additional 2 h at either 4 or 37 °C in the presence of bafilomycin A1. Finally, cells were fixed and FACS analyzed. Fluorescence intensity of cells incubated at 4 °C was similar to that of untreated cells. Shown are the results representative of two independent experiments carried out with duplicates. Mean fluorescence intensity (MFI) of the samples are also indicated. Iscomatrix: ISCOMATRIXTM adjuvant.
From this set of experiments, we concluded that both structure and cell entry of exosomes are not influenced by the interaction with ISCOMATRIXTM adjuvant.
3.2. ISCOMATRIXTM Adjuvant Increases Cross-Presentation of Antigens Uploaded in Engineered Exosomes
For exogenous antigens, cross-presentation is on the basis of the CD8+ T cell immune adaptive response. Thus, we first tested the efficiency of the exosome-ISCOMATRIXTM adjuvant combination in an in vitro cross-presentation assay assuming that the extents of cross-presentation of antigens uploaded in engineered exosomes detectable in vitro predicts the potency of the immune response in vivo. The effects of ISCOMATRIXTM adjuvant on the cross-presentation of antigens associated with engineered exosomes were evaluated in two already established in vitro systems based on challenging B-LCLs and iDCs with exosomes uploading Nefmut alone [16] and fused with Mart-1 (i.e., a human melanoma-associated antigen also known as Melan-A) [27], respectively. We previously reported that, in this system, the antigens delivered by exosomes are cross-presented poorly unless the exosomes associate with a pH-dependent envelope protein (e.g., the G protein from vesicular stomatitis virus) [16].
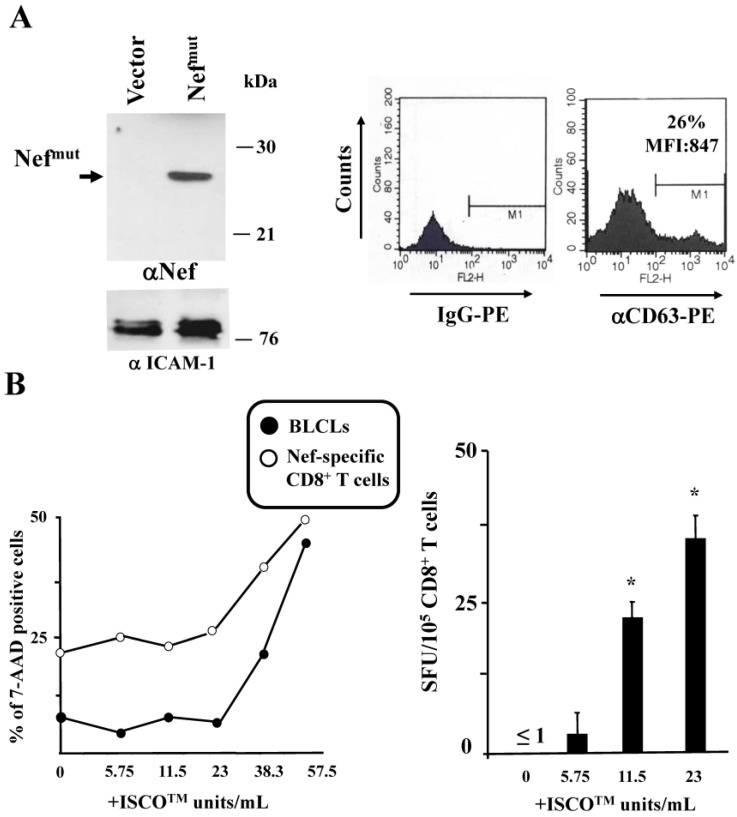
Exosomes uploading Nefmut were produced by transient transfection in 293T cells as previously described [16], and characterized for both Nefmut incorporation and CD63 membrane expression (Figure 3A). HLA-B7 B-LCLs were challenged with Nefmut-based exosomes alone or in combination with either 5.75, 11.5, or 23 ISCOTM U/mL of ISCOMATRIXTM adjuvant. Notably, no effects on growth/viability of both B-LCLs and Nef-specific CD8+ T lymphocytes were assessed after 24 h of cultivation with these doses of the adjuvant (Figure 3B, left). Exosome challenge was carried out by spinoculating cells in a total of 50 µL, followed by a 5 h incubation in the presence or not of the indicated doses of ISCOMATRIXTM adjuvant (Figure 3B, right), and thereafter co-cultivated for 24 h with HLA-matched Nef-specific CD8+ T lymphocytes in IFN-γ Elispot microwells. As shown in Figure 3B, the presence of at least 11.5 ISCOTM U/mL of the adjuvant significantly increased the cross-presentation of Nefmut delivered by exosomes.
Figure 3.
ISCOMATRIXTM adjuvant increases the cross-presentation of antigens delivered by engineered exosomes in B-lymphoblastoid cell lines (LCLs). (A) molecular characterization of exosome preparations uploading Nefmut. On the left is the Western blot analysis of 200 µU of exosomes uploading Nefmut. As a control, equivalent amounts of exosomes from cells transfected with the empty vector were loaded. Arrow signs indicate the relevant protein product. Exosome preparations were also probed for the presence of Intercellular adhesion molecule (ICAM)-1, i.e., an exosome marker. Molecular markers are given in kilodaltons (kDa). On the right, fluorescence-activated cell sorting (FACS) analysis for the presence of CD63 in exosome membrane uploading Nefmu is shown. M1 marks the range of fluorescence positivity as determined by the analysis of equivalent amounts of exosomes after incubation with isotype-specific IgGs (left histogram). Both percentages and MFI of fluorescent beads are indicated. Results shown in both panels are representative of three assays carried out on two exosome preparations; (B) on the left is cell viability of both B-LCLs and Nef-specific CD8+ T cells treated for 24 h with the indicated concentrations of ISCOMATRIXTM adjuvant as evaluated by FACS analysis after 7-AAD labeling. Shown are mean values from two independent experiments with duplicates. On the right, data from cross-presentation analysis of Nefmut delivered by exosomes in B-LCLs are presented. A total of 105 cells were challenged with 100 µU of exosomes and then cultivated for 5 h in the presence of the indicated doses of ISCOMATRIXTM adjuvant. Thereafter, the cells were put in co-culture overnight with a Nef-specific, HLA-B7 restricted CD8+ T cell line in IFN-γ Elispot microwells. Shown are the mean + SD number of SFU/105 cells calculated from five independent experiments. * p < 0.05.
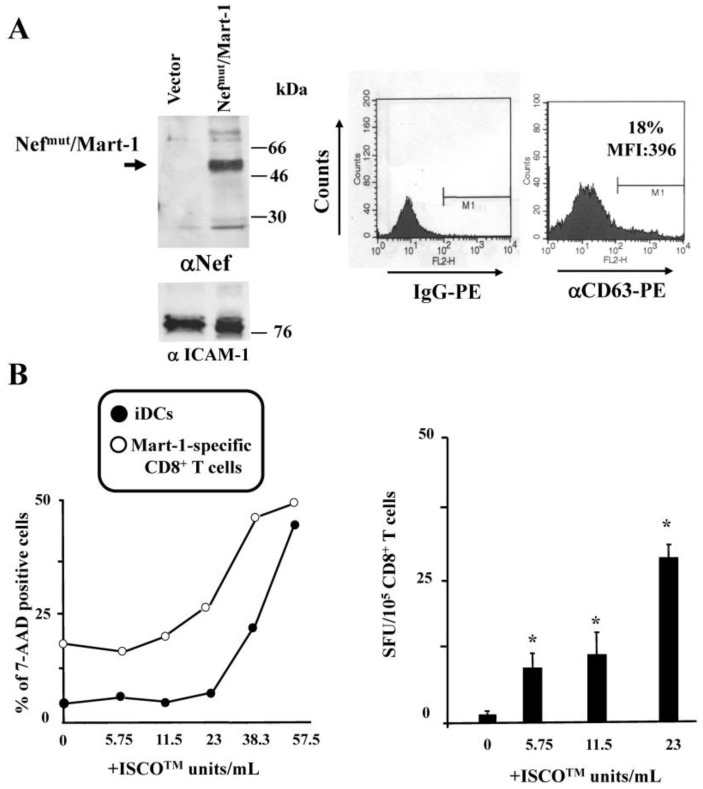
The cross-presentation assay was reproduced using monocyte-derived iDCs and exosomes uploading Nefmut/Mart-1, whose molecular characterization is shown in Figure 4A. When these exosomes were used to challenge HLA A.02 iDCs, whose viability appeared not influenced up until 23 ISCOTM U/mL (Figure 4B, left), the increase of Mart-1 cross-presentation appeared from 5.75 ISCOTM U/mL of ISCOMATRIXTM adjuvant (Figure 4B, right). According to what was already reported [28], ISCOMATRIXTM adjuvant had no detectable effects on activation/maturation of in vitro differentiated iDCs, independently from the exosome treatment (not shown).
Figure 4.
ISCOMATRIXTM adjuvant increases the cross-presentation of antigens delivered by engineered exosomes in iDCs. (A) molecular characterization of exosome preparations uploading Nefmu/Mart-1. On the left is the Western blot analysis of 200 µU of exosomes associating with Nefmut. As a control, equivalent amounts of exosomes from cells transfected with the empty vector were loaded. Arrow signs indicate the relevant protein product. Exosome preparations were also probed for the presence of ICAM-1. Molecular markers are given in kDa. On the right, the FACS analysis for presence of CD63 in exosome membrane is shown. M1 marks the range of fluorescence positivity as determined by the analysis of equivalent amounts of exosomes after incubation with isotype-specific IgGs (left histogram). Both percentages and MFI of fluorescent beads are indicated. Results shown in both panels are representative of two assays carried out on two exosome preparations; (B) on the left is cell viability of both iDCs and Mart-1-specific CD8+ T cells treated for 24 h with the indicated concentrations of ISCOMATRIXTM adjuvant as evaluated by FACS analysis after 7-AAD labeling. Mean values from two independent experiments with duplicates are shown. On the right, results from cross-presentation analysis of Mart-1delivered by Nefmut/Mart-1 exosomes in HLA-A.02 iDCs are presented. A total of 105 cells were challenged with 100 µU of the exosomes and then cultivated for 5 h in the presence of the indicated doses of ISCOMATRIXTM adjuvant. Thereafter, the cells were put in co-culture overnight with a Mart-1-specific, HLA-A.02 restricted CD8+ T cell line in IFN-γ Elispot microwells. The mean + SD number of SFU/105 cells calculated from three independent experiments are shown. * p < 0.05.
Of note, incubation of both B-LCLs and iDCs with the adjuvant in the presence of exosomes from cells transfected with empty vector did not induce activation of the CD8+ T cells (not shown).
These results indicated together that the treatment with ISCOMATRIXTM adjuvant significantly increases the cross-presentation of antigens uploaded in Nefmut-based engineered exosomes.
3.3. ISCOMATRIXTM Adjuvant and Exosome Co-Administration in Mice Increases the Pool of CD8+ T Lymphocytes Specific for the Antigen Uploaded in Engineered Exosomes
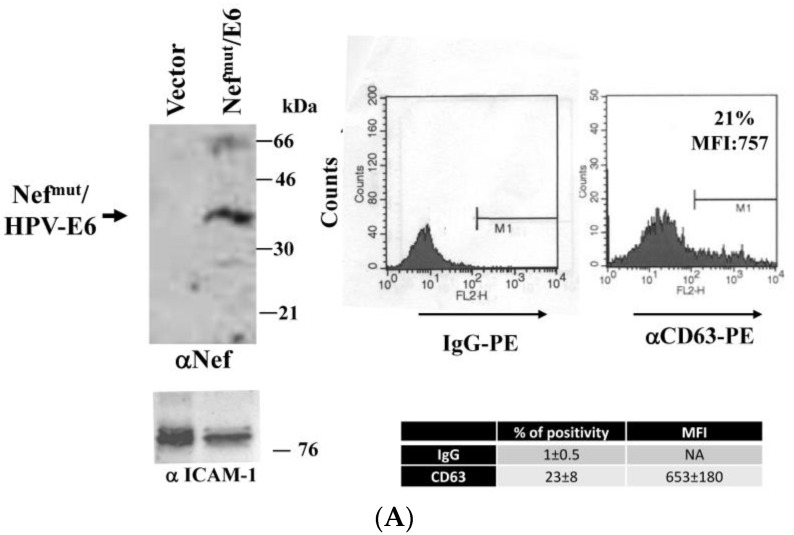
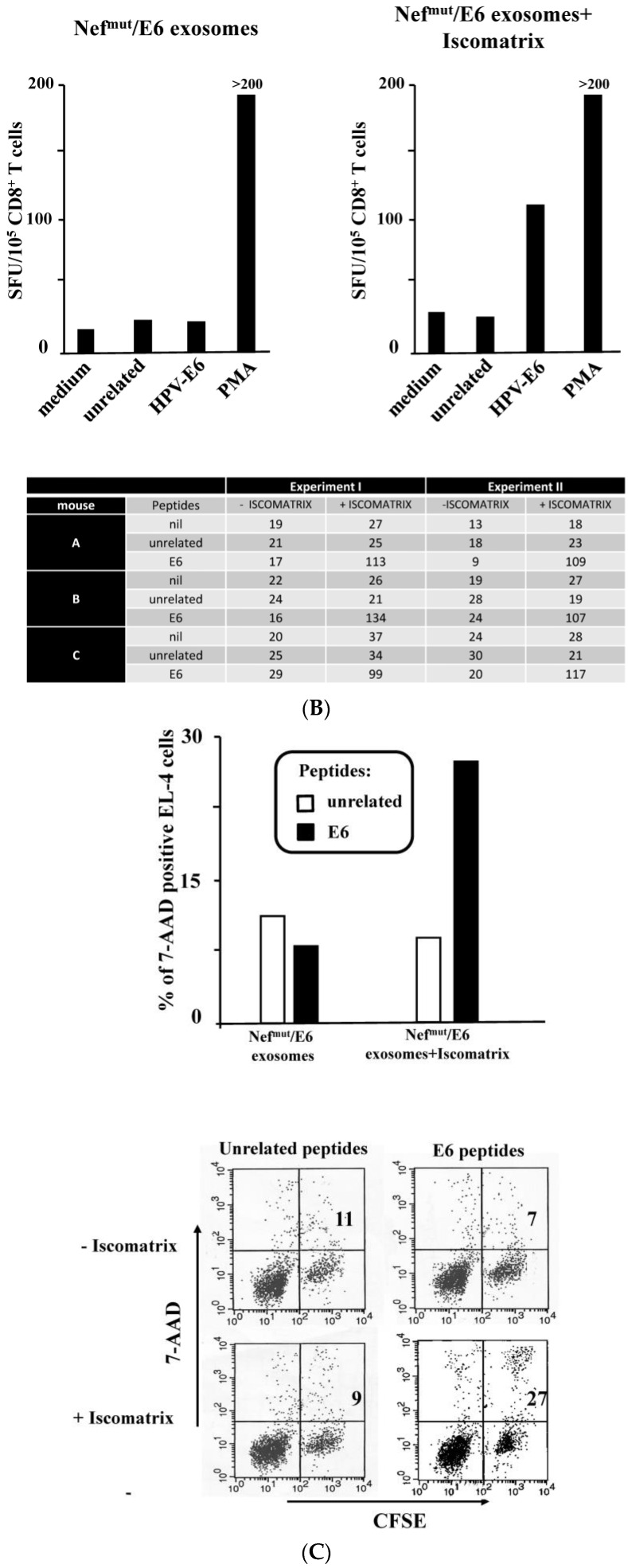
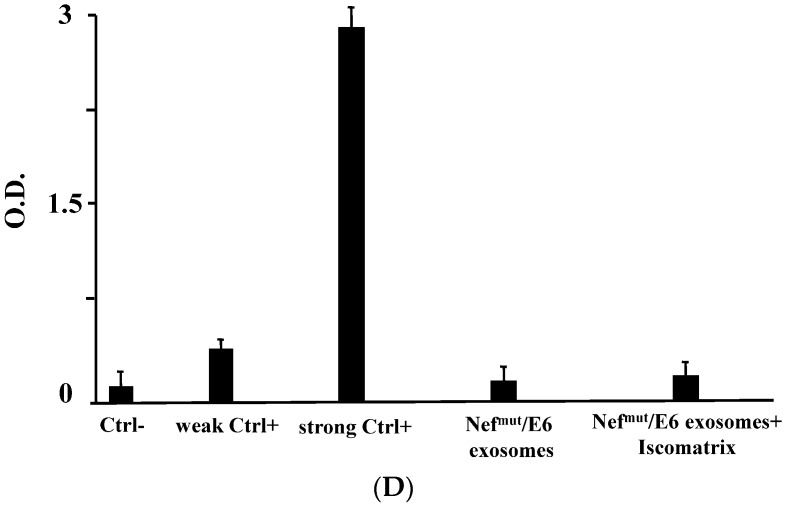
The aim of the present study was the identification of a method to improve the CD8+ T cell immunity induced by the inoculation of Nefmut-based exosomes. Hence, we next were interested in assessing whether the ISCOMATRIXTM adjuvant-dependent improvement of cross-presentation activity that we observed in vitro was associated with increased immunogenicity in animals. To this aim, exosomes engineered with the Nefmut/HPV-E6 fusion protein were purified from the supernatants of 293T transfected cells and characterized in terms of contents of both the fusion product and CD63 tetraspanin (Figure 5A). C57Bl/6 mice were inoculated subcutaneously (s.c.) three times with two week intervals with 2.1 mU of Nefmut/E6 exosomes in the presence or not of 3.8 ISCOTM Units of ISCOMATRIXTM adjuvant, i.e., the highest dose well tolerated by s.c. injected mice. Two weeks after the last immunization, mice were sacrificed, and splenocytes assayed for the presence of HPV-E6-specific CD8+ T lymphocytes. By IFN-γ Elispot assay, we detected HPV-E6-specific CD8+ T lymphocytes in splenocytes from mice inoculated with Nefmut/E6 exosomes plus ISCOMATRIXTM adjuvant, but not from mice inoculated with the exosomes alone (Figure 5B). The latter result was consistent with the previously reported evidence that the CD8+ T cell response against HPV-E7 delivered by engineered exosomes was detectable only after a 5-day culture of splenocytes in the presence of specific peptides [19]. The increased number of E6-specific CD8+ T cells within splenocytes from mice co-inoculated with ISCOMATRIXTM adjuvant correlated with the appearance of a E6-specific CTL activity, as shown by the results we obtained through a CTL assay based on the co-culture with syngeneic EL-4 cells pre-treated with E6 peptides (Figure 5C). Of note, no anti-HPV-E6 antibodies were found in sera from mice inoculated with Nefmut/E6 incorporating exosomes whatever the co-inoculation of ISCOMATRIXTM adjuvant (Figure 5D).
Figure 5.
The co-inoculation in mice of ISCOMATRIXTM adjuvant and engineered exosomes increases the number of HPV-E6 specific CD8+ T lymphocytes. (A) molecular characterization of exosome preparations uploading Nefmu/HPV-E6. On the left is the Western blot analysis of 200 µU of exosomes associating Nefmut/E6. As a control, equivalent amounts of exosomes from cells transfected with the empty vector were loaded. Arrow signs indicate the relevant protein product. Exosome preparations were also probed for the presence of ICAM-1. Molecular markers are given in kDa. On the right, the FACS analysis for the presence of CD63 in exosome membrane is shown. M1 marks the range of fluorescence positivity as determined by the analysis of equivalent amounts of exosomes after incubation with isotype-specific IgGs (left histogram). Results shown in both panels are representative of five assays carried out on three exosome preparations. At the bottom right, mean values ±SD of both percentages of positivity and MFI from the five assays are also reported; (B) CD8+ T cell immune response in mice inoculated with Nefmut/E6 exosomes in the presence or not of ISCOMATRIXTM adjuvant. C57 Bl/6 mice (three per group) were inoculated s.c. at the lower right flank three times with exosomes uploading Nefmut/E6 in the presence or not of the adjuvant. Two weeks after the last inoculation, splenocytes were isolated and 105 cells were incubated overnight with or without 5 µg/mL of either unrelated or HPV-E6-specific peptides in IFN-γ Elispot microwells in triplicate conditions. As a control, untreated cells were incubated with 10 ng/mL of phorbol-12-myristate-13-acetate (PMA) and 500 ng/mL of ionomycin. Afterwards, cell activation extents were evaluated by IFN-γ Elispot assay carried out in triplicate with 105 cells/well. Cultures of splenocytes from each inoculated mouse were carried out separately. The mean of SFU/105 cells calculated on the basis of data reported at the bottom which were obtained in two independent immunization experiments are shown. Iscomatrix: ISCOMATRIXTM adjuvant; (C) Cytotoxic T lymphocyte (CTL) assay carried out with CD8+ T cells isolated from splenocytes of mice inoculated with exosomes uploading Nefmut/E6 in the presence or not of ISCOMATRIXTM adjuvant. CD8+ T lymphocytes from pooled splenocyte cultures were co-cultivated for 6 h at 20:1 effector/target cell ratio with EL (European lymphoblast)-4 cells previously labeled with Carboxyfluorescein succinimidyl ester (CFSE), and treated with either unrelated or E6 peptides for 16 h. Finally, the EL-4 cell mortality levels were scored by FACS analysis upon 7-AAD labeling. At the top, are the results obtained using pooled splenocytes from a representative of two independent immunization experiments. At the bottom, the dot-plot FACS analysis of the co-cultures is reported. Cells were gated on the basis of their apparently unaffected morphology, and the percentages of double-positive over the total of CFSE-labelled cells are reported; (D) anti-E6 antibody detection in plasma from mice inoculated with the Nefmut/E6 exosomes in the presence or not of ISCOMATRIXTM adjuvant. Plasma were tested in an in-house Elisa assay upon 1:10 dilution. As internal standards, 1:10 diluted plasma from mock-inoculated mice was used as negative control (Ctrl−), whereas both 1:1000 (weak Ctrl+) and 1:100 (strong Ctrl+) dilutions of plasma from mice injected with 10 µg of recombinant E6 protein plus adjuvant were used as positive controls. Shown are the mean absorbance values +SD of triplicates of plasma samples from each inoculated mouse.
From these data, we concluded that ISCOMATRIXTM adjuvant is instrumental in increasing the CD8+ T cell immune response against exosome-associated antigens.
4. Discussion
Nefmut-based exosomes represent an original tool for the induction of a Class I MHC-unrestricted CTL immune response against antigens of choice. The basically unvaried efficiency of exosome incorporation of Nefmut when fused at its C-terminus with an heterologous antigen guarantees the great flexibility of this immunogen platform. Considering that the inoculation of Nefmut-based exosomes does not induce specific Abs, leading exclusively to antigen-specific CD8+ T lymphocyte immunity, adjuvants already characterized for their ability to increase this arm of the adaptive immunity could represent useful tools to strengthen the immunogenicity of Nefmut-based exosomes.
Exosomes engineered to upload HPV-E7 fused with the Nefmut exosome-anchoring protein have been recently shown to efficiently elicit a CD8+ T-specific adaptive immune response against E7 [19].This immune response blocked the growth of tumor cells implanted after immunization, appearing however only partly efficient in the therapeutic setting. Here, we present a simple method to increase the immunogenicity of the Nefmut-based exosomes useful for possible therapeutic applications. The increase of antigen-specific CTL immunity that we documented in splenocytes from ISCOMATRIX™ adjuvant-co-inoculated mice is expected to be associated with increased survival of animals challenged with HPV E6-expressing tumor cells, as we already demonstrated for HPV E7- engineered exosomes [19]. To the best of our knowledge, for the first time, the activity of a saponin-based adjuvant favoring the immunogenicity of a nanovesicle-delivered antigen has been demonstrated.
Cross-presentation in DCs relies on two non-mutually exclusive mechanisms [29]. In the first one, referred to as “cytosolic”, the antigen transits from the endosomal compartment to the cytosol. In the case of endocytosed vesicles, this passage can be greatly favored by pH-dependent envelope fusion proteins. In cytosol, the antigen is degraded by proteasome, and resulting peptides are loaded on Class I MHC upon Transporter associated with antigen processing (TAP)-mediated translocation into endoplasmatic reticulum. The second mechanism, defined as “vacuolar”, is based on the action of endo-lysosomal proteases degrading both vesicles and associated proteins, whose resulting peptides are loaded into Class I MHC recycling at vesicular levels. We assumed that the CD8+ T-related immunogenicity that we observed using exosomes produced in the absence of foreign envelope proteins was a consequence of the vacuolar cross-presentation activity in APCs ingesting the exosomes.
We selected an ISCOMATRIX™ adjuvant mainly based on our in-depth understanding of its mechanism of action. ISCOMATRIX™ adjuvant induces integrated responses including antibody and cellular immune responses to various types of soluble antigens [17]. The particulate nature of ISCOMATRIX™ adjuvant (40–50 nm) contributes to some of its properties. ISCOMATRIX™ adjuvant is efficiently endocytosed by APCs where it exerts its immunomodulatory activities. Generation of high frequency antigen-specific CD8+ T cell responses is a reproducible feature of ISCOMATRIX™ adjuvant vaccines in both animal models and in humans [28,30,31]. We have already demonstrated that ISCOMATRIX™ adjuvant induces prolonged antigen cross-presentation persisting in vivo up to seven days after priming, which, together with efficient Ag delivery, provides a mechanistic explanation for the strong CD8+ T cell responses induced by ISCOMATRIX™ adjuvant vaccines [32]. In the same study, it was also demonstrated that ISCOMATRIX™ adjuvant vaccines are more efficient at inducing CTL responses in vivo than other adjuvants such as aluminum hydroxide, incomplete Freund’s adjuvant, CpG, lipopolysaccharides (LPS) or Pam3Cys. Wilson and colleagues further reported that cells (at both draining lymph nodes and injection site) that directly encounter/take up ISCOMATRIX™ adjuvant likely undergo metabolic cell stress, which triggers multiple “danger” signaling pathways [33]. All of these effects could represent an advantage compared to using other adjuvants which either trigger only one particular pathway, or having undefined mechanism of action.
It was already shown that ISCOMATRIXTM adjuvant significantly increases the cross-presentation of soluble antigens [17]. Although the exact underlying mechanisms remains to be fully elucidated, it has been reported that in vivo it: (i) induces DC activation; (ii) facilitates antigen cross-presentation in a specific subset of DCs, i.e., the CD8α+; and (iii) generates a pro-inflammatory milieu in the inoculation site, with increased production of both IL-1β and IL-6 [29]. An inflammatory milieu leading to activation of professional APCs is expected to favor adaptive immune responses. However, whether and how inflammatory factors are relevant for cross-presentation of exosome-associated antigens remains to be established.
ISCOMATRIXTM adjuvant has also been proven to boost the antibody response against soluble antigens [17]. In our hands, consistently with what previously reported for HPV E7-uploaded engineered exosomes [19], no antibody response against HPV-E6 has been detected in mice challenged with exosomes incorporating Nefmut/E6 also in the presence of ISCOMATRIXTM adjuvant. This evidence was strongly suggestive of a basically exclusive presentation of HPV-E6 peptides in Class I MHC also in the presence of the adjuvant.
Anti-tumor experimental vaccine strategies described so far are based on the use of either peptides, recombinant proteins, DNA, viral vectors, or VLPs. Some intrinsic limitations reduce the possibility of effective transfer to clinics for many of these approaches. The use of the engineered exosomes in anti-tumor vaccine strategies described here presents a number of advantages including: (i) a quite low basal immunogenicity; (ii) the incorporation of the whole tumor-associated antigen, which implies a Class I MHC-unrestricted use; (iii) the lack of genetic material, and a minimal presence of non-human antigens; (iv) a manufacturing simpler than that required for viral vectors and VLPs. The promising results that we obtained justify further investigations on both immunogenicity and efficacy of exosomes engineered with additional antigens of therapeutic significance.
5. Conclusions
Overall, our results indicate that the molecular composition of ISCOMATRIXTM adjuvant is perfectly compatible with the structural integrity of exosomal nanovesicles. ISCOMATRIXTM adjuvant increases cross-presentation of exosome-associated antigens in vitro, and consistently improves the CD8+ T cell response against the foreign antigen incorporated in engineered exosomes. Hence, besides soluble antigens, ISCOMATRIXTM adjuvant is also useful for increasing the immunogenicity of antigens incorporated in nanovesicles. This result could be of utility for both current and future immunotherapies based on nanovesicle-associated antigens.
Acknowledgments
This work was supported by a grant from the “Ricerca Finalizzata” project RF-2010-2308334 from the Ministry of Health, Rome Italy. We thank Pietro Arciero and Stefania De Menna, Istituto Superiore di Sanità, for their excellent technical support.
Author Contributions
F.M prepared and characterized exosomes; P.d.B. carried out experiments in mice; B.R. performed in vitro Elispot assays; S.A. carried out exosome-bead binding experiments; C.A. carried out exosome internalization experiments; C.C. performed ex vivo Elispot assays, A.B.M. provided the adjuvant and designed the experiments; and M.F. supervised the experiments and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Milane L., Singh A., Mattheolabakis G., Suresh M., Amiji M.M. Exosome mediated communication within the tumor microenvironment. J. Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 2.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015;6 doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., Valente N., Shreeniwas R., Sutton M.A., Delcayre A., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005 doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudier B., Dorval T., Chaput N., Andre F., Caby M.P., Novault S., Flament C., Leboulaire C., Borg C., Amigorena S., et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J. Transl. Med. 2005 doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H., Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan A., De La Pena H., Seifalian A.M. The application of exosomes as a nanoscale cancer vaccine. Int. J. Nanomed. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput N., Thery C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 8.Hartman Z.C., Wei J.P., Glass O.K., Guo H.T., Lei G.J., Yang X.Y., Osada T., Hobeika A., Delcayre A., Le Pecq J.B., et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361–9367. doi: 10.1016/j.vaccine.2011.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiu F.M., Cai Z.J., Yang Y.S., Wang X.J., Wang J.L., Cao X.T. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J. Mol. Med. 2007;85:511–521. doi: 10.1007/s00109-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 10.Usami Y., Popov S., Popova E., Inoue M., Weissenhorn W., Gottlinger H.G. The ESCRT pathway and HIV-1 budding. Biochem. Soc. Trans. 2009;37:181–184. doi: 10.1042/BST0370181. [DOI] [PubMed] [Google Scholar]
- 11.Booth A.M., Fang Y., Fallon J.K., Yang J.M., Hildreth J.E.K., Gould S.J. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenassi M., Cagney G., Liao M.F., Vaupotic T., Bartholomeeusen K., Cheng Y.F., Krogan N.J., Plemenitas A., Peterlin B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4(+) T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muratori C., Cavallin L.E., Kratzel K., Tinari A., De Milito A., Fais S., D’Aloja P., Federico M., Vullo V., Fomina A., et al. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6:218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Basmaciogullari S., Pizzato M. The activity of Nef on HIV-1 infectivity. Front. Microbiol. 2014 doi: 10.3389/fmicb.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peretti S., Schiavoni I., Pugliese K., Federico M. Cell death induced by the herpes simplex virus-1 thymidine kinase delivered by human immunodeficiency virus-1-based virus-like particles. Mol. Ther. 2005;12:1185–11396. doi: 10.1016/j.ymthe.2005.06.474. [DOI] [PubMed] [Google Scholar]
- 16.Lattanzi L., Federico M. A strategy of antigen incorporation into exosomes: Comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine. 2012;30:7229–7237. doi: 10.1016/j.vaccine.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Drane D., Gittleson C., Boyle J., Moraskovsky E. ISCOMATRIX (TM) adjuvant for prophylactic and therapeutic vaccines. Expert Rev. Vaccines. 2007;6:761–6772. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]
- 18.Maraskovsky E., Schnurr M., Wilson N.S., Robson N.C., Boyle J., Drane D. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol. Cell Biol. 2009;87:371–376. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- 19.Di Bonito P., Ridolfi B., Columba-Cabezas S., Giovannelli A., Chiozzini C., Manfredi F., Anticoli S., Arenaccio C., Federico M. HPV-E7 Delivered by engineered exosomes elicits a protective CD8+ T cell-mediated immune response. Viruses. 2015;7:1079–1099. doi: 10.3390/v7031079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Bonito P., Grasso F., Mochi S., Petrone L., Fanales-Belasio E., Mei A., Cesolini A., Laconi G., Conrad H., Bernhard H., et al. Anti-tumor CD8(+) T cell immunity elicited by HIV-1-based virus-like particles incorporating HPV-16 E7 protein. Virology. 2009;395:45–55. doi: 10.1016/j.virol.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Rivoltini L., Kawakami Y., Sakaguchi K., Southwood S., Sette A., Robbins P.F., Marincola F.M., Salgaller M.L., Yannelli J.R., Appella E., et al. Induction of tumor-reactive CTL from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J. Immunol. 1995;154:2257–2265. [PubMed] [Google Scholar]
- 22.Arenaccio C., Anticoli S., Manfredi F., Chiozzini C., Olivetta E., Federico M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology. 2015 doi: 10.1186/s12977-015-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thery C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 24.Rieu S., Geminard C., Rabesandratana H., Sainte-Marie J., Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha 4 beta 1. Eur. J. Biochem. 2000;267:583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S., Heeg K., Wagner H., Lipford G.B. Identification of H-2Kb binding and immunogenic peptides from human papilloma virus tumor antigens E6 and E7. Scand. J. Immunol. 1995;42:317–323. doi: 10.1111/j.1365-3083.1995.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 26.Di Bonito P., Grasso F., Mochi S., Accardi L., Dona M.G., Branca M., Costa S., Mariani L., Agarossi A., Ciotti M., et al. Serum antibody response to Human papillomavirus (HPV) infections detected by a novel ELISA technique based on denatured recombinant HPV16 L1, L2, E4, E6 and E7 proteins. Infect. Agent Cancer. 2006 doi: 10.1186/1750-9378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busam K.J., Jungbluth A.A. Melan-A, a new melanocytic differentiation marker. Adv. Anat. Pathol. 1999;6:12–18. doi: 10.1097/00125480-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Wilson N.S., Yang B., Baz Morelli A., Koernig S., Yang A., Loeser S., Airey D., Provan L., Hass P., Braley H., et al. ISCOMATRIX vaccines mediate CD8(+) T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol. Cell Biol. 2012;90:540–552. doi: 10.1038/icb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joffre O.P., Segura E., Savina A., Amigorena S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 30.Davis I.D., Chen W., Jackson H., Parente P., Shackleton M., Hopkins W., Chen Q., Dinopoulos N., Luke T., Murphy R., et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated Ab and CD4+ and CD8+ T cell responses in humans. Proc. Natl. Acad. Sci. USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drane D., Maraskovsky E., Gibson R., Mitchell S., Barnden M., Moskwa A., Shaw D., Gervase B., Coates S., Houghton M., et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: A phase I study in healthy volunteers. Hum. Vaccines. 2009;5:151–157. doi: 10.4161/hv.5.3.6614. [DOI] [PubMed] [Google Scholar]
- 32.Duewell P., Kisser U., Heckelsmiller K., Hoves S., Stoitzner P., Koernig S., Morelli A.B., Clausen B.E., Dauer M., Eigler A., et al. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J. Immunol. 2011;187:55–63. doi: 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson N.S., Duewell P., Yang B., Marsters S., Koenig S., Latz E., Maraskovsky E., Baz Morelli A., Schurr M., Ashkenazi A. Inflammatory dependent and-independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J. Immunol. 2014;92:3259–3268. doi: 10.4049/jimmunol.1302011. [DOI] [PubMed] [Google Scholar]