Abstract
Pinnigorgiols D (1) and E (2), two new 9,11-secosterols with a rearranged carbon skeleton, were isolated from a Taiwan gorgonian Pinnigorgia sp. The structures of these two compounds were elucidated on the basis of spectroscopic methods and were proven to possess a tricyclo[5,2,1,1]decane ring. The new secosterols 1 and 2 displayed significant inhibitory effects on the generation of superoxide anions and the release of elastase by human neutrophils.
Keywords: 9,11-secosterol; gorgonian; Pinnigorgia; anti-inflammatory; superoxide anion; elastase
1. Introduction
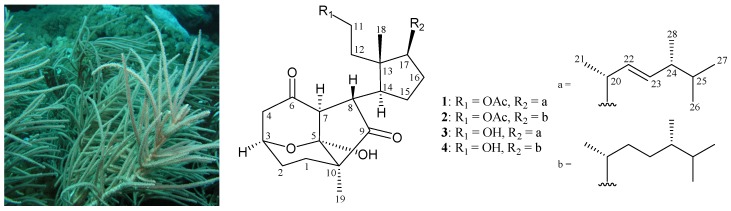
Gorgonian corals belonging to the genus Pinnigorgia (Family Gorgoniidae), have proven to be rich sources of bioactive sterols [1,2,3,4]. Previous bioassay results of these sterol analogues have demonstrated cytotoxic and anti-inflammatory activities. Following the above investigations, with the aim of discovering bioactive substances for new drug development in the future, we continue here to carry out an investigation on a Taiwan gorgonian Pinnigorgia sp., and two novel 9,11-secosterols, pinnigorgiols D (1) and E (2), with a rare carbon skeleton arrangement, were discovered (Figure 1). The ability of these two compounds to inhibit the generation of superoxide anions and the release of elastase in N-formyl methionyl leucylphenylalanine/cytochalasin B (fMLP/CB)-induced neutrophils were also evaluated.
Figure 1.
Gorgonian coral Pinnigorgia sp. and the structures of pinnigorgiols D (1), E (2), A (3), and B (4).
2. Results and Discussion
Pinnigorgiol D (1) was obtained as an oil and had the molecular formula C30H46O6 as determined by high-resolution electrospray ionization mass spectrum (HRESIMS) at m/z 525.31883 (calcd. for C30H46O6 + Na, 525.31866), requiring eight degrees of unsaturation. The IR absorptions of 1 showed the presence of hydroxy (νmax 3446 cm−1), ester (νmax 1739 cm−1), and ketonic carbonyl (νmax 1717 cm−1) groups. The 13C NMR and distortionless enhancement of polarization transfer (DEPT) data of 1 (Table 1) indicated the presence of 30 carbons, including seven methyls, seven sp3 methylenes, eight sp3 methines, a disubstituted double bond, and six quaternary carbons. The 1H NMR spectrum (Table 1) exhibited seven methyl signals at δH 2.06 (3H, s, acetate methyl), 1.12 (3H, s), 1.04 (3H, d, J = 7.2 Hz), 0.91 (3H, d, J = 6.8 Hz), 0.90 (3H, s), 0.83 (3H, d, J = 6.8 Hz), and 0.81 (3H, d, J = 6.8 Hz). The signal at δH 4.54 (1H, ddd, J = 11.6, 11.6, 4.4 Hz) and 3.86 (1H, ddd, J = 11.6, 11.6, 6.0 Hz) were assumed to be an oxymethylene group. It was found that the NMR signals of 1 were similar to those of a known 9,11-secosterol analogue, pinnigorgiol A (3) (Figure 1) [1], except that the signals corresponding to the 11-hydroxy group in 3 were replaced by signals for an acetoxy group in 1 (Table 2). The correlations from a nuclear Overhauser effect spectroscopy (NOESY) experiment of 1 also revealed that the stereochemistry of this metabolite was identical to that of 3. Thus, pinnigorgiol D (1) was found to be the 11-O-acetyl derivative of 3.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data for secosterols 1 and 2.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Multiple | δH (J in Hz) | δC, Multiple | |
| 1 | 1.38 m; 1.25 m | 26.5, CH2 | 1.38 m; 1.25 m | 26.6, CH2 |
| 2 | 2.02 m; 1.59 m | 23.8, CH2 | 2.03 m; 1.59 m | 24.1, CH2 |
| 3 | 4.65 br s | 70.2, CH | 4.65 dd (6.0, 5.2) | 70.2, CH |
| 4 | 2.91 dd (16.4, 6.8); 2.39 d (16.4) | 44.1, CH2 | 2.91 dd (16.4, 6.8); 2.39 d (16.4) | 44.0, CH2 |
| 5 | 101.4, C | 101.4, C | ||
| 6 | 207.7, C | 207.7, C | ||
| 7 | 3.04 s | 59.6, CH | 3.05 s | 59.9, CH |
| 8 | 3.18 dd (10.0, 2.0) | 48.2, CH | 3.19 dd (10.0, 2.0) | 48.0, CH |
| 9 | 216.6, C | 216.6, C | ||
| 10 | 49.4, C | 49.5, C | ||
| 11 | 4.54 ddd (11.6, 11.6, 4.4) | 61.2, CH2 | 4.52 ddd (11.6, 11.6, 4.8) | 61.3, CH2 |
| 3.86 ddd (11.6, 11.6, 6.0) | 3.87 ddd (11.6, 11.6, 6.0) | |||
| 12 | 1.89 m; 1.67 m | 36.1, CH2 | 1.89 m; 1.61 m | 36.1, CH2 |
| 13 | 46.7, C | 46.8, C | ||
| 14 | 2.04 m | 45.8, CH | 2.04 m | 46.1, CH |
| 15 | 1.94 m; 1.77 m | 27.3, CH2 | 1.92 m; 1.80 m | 27.0, CH2 |
| 16 | 1.98 m; 1.45 m | 24.0, CH2 | 2.02 m; 1.42 m | 24.2, CH2 |
| 17 | 1.43 m | 50.0, CH | 1.42 m | 49.8, CH |
| 18 | 0.90 s | 17.0, CH3 | 0.90 s | 16.8, CH3 |
| 19 | 1.12 s | 12.1, CH3 | 1.12 s | 12.1, CH3 |
| 20 | 2.27 m | 36.8, CH | 1.45 m | 33.3, CH |
| 21 | 1.04 d (7.2) | 22.6, CH3 | 1.04 d (7.2) | 20.4, CH3 |
| 22 | 5.26 dd (15.6, 7.6) | 133.7, CH | 1.45 m; 0.90 m | 32.6, CH2 |
| 23 | 5.23 dd (15.6, 7.6) | 133.3, CH | 1.37 m; 0.90 m | 31.8, CH2 |
| 24 | 1.89 m | 43.1, CH | 1.21 m | 39.0, CH |
| 25 | 1.48 m | 33.1, CH | 1.56 m | 31.5, CH |
| 26 | 0.83 d (6.8) | 20.0, CH3 | 0.78 d (6.8) | 17.6, CH3 |
| 27 | 0.81 d (6.8) | 19.7, CH3 | 0.85 d (6.8) | 20.4, CH3 |
| 28 | 0.91 d (6.8) | 17.5, CH3 | 0.77 d (6.8) | 15.5, CH3 |
| 11-OAc | 172.5, C | 172.4, C | ||
| 2.06 s | 21.2, CH3 | 2.05 s | 21.2, CH3 | |
Table 2.
NMR data for the 11-acetoxy component in pinnigorgiol D (1), and the 11-hydroxy component in pinnigorgiol A (3).
| Position | 1 | 3 a | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Multiple | δH (J in Hz) | δC, Multiple | |
| 11 | 4.54 ddd (11.6, 11.6, 4.4) | 61.2, CH2 | 3.83 m | 59.0, CH2 |
| 3.86 ddd (11.6, 11.6, 6.0) | ||||
| 12 | 1.89 m; 1.67 m | 36.1, CH2 | 1.89 dt (15.5, 5.5); 1.74 m | 39.5, CH2 |
| 13 | 46.7, C | 46.6, C | ||
| 11-OAc | 172.5, C | |||
| 2.06 s | 21.2, CH3 | |||
a Data was reported by Chang et al. [1].
Pinnigorgiol E (2) was obtained as an oil and had the molecular formula C30H48O6 as determined by HRESIMS at m/z 527.33444 (calcd. for C30H48O6 + Na, 527.33431) and by analysis of NMR spectral data, requiring seven degrees of unsaturation. Initial analyses of the 1H and 13C NMR spectral data of 2 illustrated features very similar to those of 1 (Table 1) and a known secosterol, pinnigorgiol B (4) (Figure 1) [1], except that the signals corresponding to the 11-hydroxy group in 4 were replaced by signals for an acetoxy group in 2 (Table 3). Based on the above observations, pinnigorgiol E (2) was assigned as the 11-O-acetyl derivative of pinnigorgiol B (4).
Table 3.
NMR data for the 11-acetoxy component in pinnigorgiol E (2), and the 11-hydroxy component in pinnigorgiol B (4).
| Position | 2 | 4 a | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Multiple | δH (J in Hz) | δC, Multiple | |
| 11 | 4.52 ddd (11.6, 11.6, 4.8) | 61.3, CH2 | 3.81 m | 59.1, CH2 |
| 3.87 ddd (11.6, 11.6, 6.0) | ||||
| 12 | 1.89 m; 1.61 m | 36.1, CH2 | 1.89 ddd (16.0, 6.0, 5.2); 1.69 m | 39.7, CH2 |
| 13 | 46.8, C | 46.6, C | ||
| 11-OAc | 172.4, C | |||
| 2.05 s | 21.2, CH3 | |||
a Data was reported by Chang et al. [1].
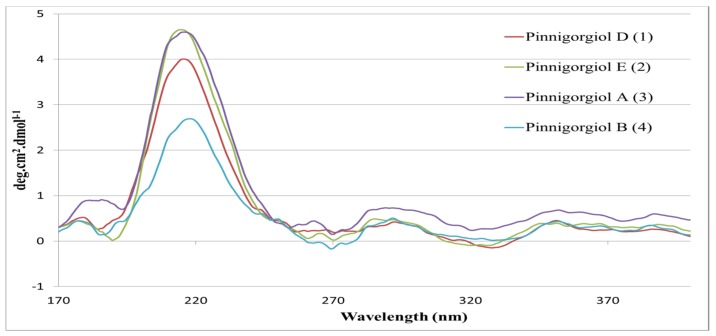
The CD spectra of pinnigoriols D (1), E (2), A (3), and B (4) in methanol displayed positive Cotton effects at 216 nm (Δε = +4.0), 214 nm (Δε = +4.6), 216 nm (Δε = +4.6), and 218 nm (Δε = +2.7), respectively, [1] (Figure 2). These highlights confirmed that secosterols 1–4 possess the same configurations.
Figure 2.
CD spectra of pinnigorgiols D (1), E (2), A (3), and B (4) [1].
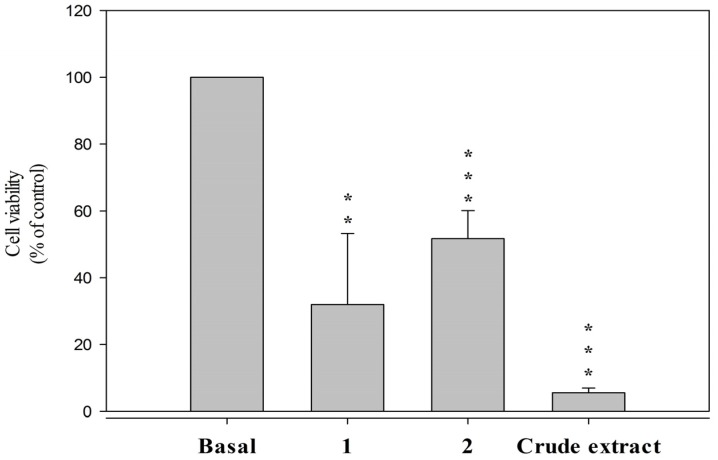
The hepatic stellate cell is the major cell type involved in liver fibrosis, which is the formation of scar tissue in response to liver damage. Secosterols 1 and 2 were tested against the HSC-T6 rat hepatic stellate cell line. Compounds 1 and 2 decreased the viability of HSC-T6 cells to 31.9% and 51.7%, respectively, at a concentration of 10 μM (Figure 3).
Figure 3.
Secosterols 1 and 2 decreased viability of HSC-T6 in 10 μM for 24 h. Cells were treated with DMSO (control) and coral crude extract in 6 μg/mL. Cytotoxicity assay was monitored spectrophotometrically at 450 nm. Quantitative data are expressed as the mean ± S.E.M. (n = 3–4). ** p < 0.01, *** p < 0.001 compared to basal.
In a previous study, pinnigorgiols A (3) and B (4) were reported to significantly decrease the cell viability of HSC-T6 cells to 13.0% and 20.8%, respectively, at a concentration of 10 μM (Table 4) [1]. It seemed that the C-11 hydroxy group and the double bond between C-22/23 are critical for the cytotoxic activity of secosterols 1–4.
Table 4.
Secosterols 1–4 decreased viability of HSC-T6 in 10 μM for 24 h.
| Compound | 1 | 2 | 3 a | 4 a |
|---|---|---|---|---|
| Inhibition rate (% of basal) | 31.9 | 51.7 | 13.0 | 20.8 |
a Data was reported by Chang et al. [1].
The in vitro anti-inflammatory effects of secosterols 1 and 2 were tested. Pinnigorgiols D (1) and E (2) were found to exhibit inhibitory effects on the generation of superoxide anions (IC50 = 3.5 and 3.9 μM, respectively) and the release of elastase (IC50 = 2.1 and 1.6 μM, respectively) by human neutrophils (Table 5). Pinnigorgiols A (3) and B (4) were also found to display inhibitory effects on the generation of superoxide anions (IC50 = 4.0 and 2.5 μM, respectively) and the release of elastase (IC50 = 5.3 and 3.1 μM, respectively) [1]. Secosterol 2 show stronger activity in the inhibitory effect on the release of elastase, which indicated that an acetoxy substituent at C-11 and the absence of C-22/23 double bond would enhance the activity by comparison with the structure and anti-inflammatory data of 2 with those of 1, 3, and 4.
Table 5.
Inhibitory effects of secosterols 1–4 on the generation of superoxide anions and the release of elastase by human neutrophils in response to fMet-Leu-Phe/cytochalastin B (fMLP/CB).
| Compound | Superoxide Anion | Elastase Release |
|---|---|---|
| IC50 (μM) | IC50 (μM) | |
| 1 | 3.5 | 2.1 |
| 2 | 3.9 | 1.6 |
| 3 a | 4.0 | 5.3 |
| 4 a | 2.5 | 3.1 |
a Data was reported by Chang et al. [1].
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Jasco P-1010 digital polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). CD spectra were recorded on a Jasco J-810 circular dichroism spectropolarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). Infrared spectra were recorded on a Jasco FT/IR-4100 spectrometer (Japan Spectroscopic Corporation, Tokyo, Japan); peaks are reported in cm−1. The NMR spectra were recorded on a Varian Mercury Plus 400 spectrometer, using the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H NMR and CDCl3 (δC 77.1 ppm) for 13C NMR; coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded using a Bruker 7 Tesla solariX FTMS system (Bruker, Bremen, Germany). Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck, Darmstade, Germany); spots were visualized by spraying with 10% H2SO4 solution followed by heating. Normal-phase HPLC (NP-HPLC) was performed using a system comprised of a Hitachi L-7110 pump (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC, Rohnert Park, CA, USA). A semi-preparative normal-phase column (Supelco Ascentis Si Cat #:581515-U, 25 cm × 21.2 mm, 5 μm, Sigma-Aldrich, St. Louis, MO, USA) was used for NP-HPLC. Reversed-phase HPLC (RP-HPLC) was performed using a system comprised of a Hitachi L-2130 pump (Hitachi Ltd., Tokyo, Japan), a Hitachi L-2455 photodiode array detector (Hitachi Ltd., Tokyo, Japan), and a Rheodyne 7725 injection port (Rheodyne LLC., Rohnert Park, CA, USA). A reverse phase column (Luna® 5 μm C18(2) 100 Å, AXIA Packed, 25 cm × 21.2 mm, Phenomenex Inc., Torrance, CA, USA) was used for RP-HPLC.
3.2. Animal Material
Specimens of the gorgonian corals Pinnigorgia sp. were collected by hand via scuba off the coast of Green Island, Taiwan, in August 2012 and stored in a freezer until extraction. A voucher specimen (NMMBA-TW-GC-2012-130) was deposited in the National Museum of Marine Biology & Aquarium, Taiwan. This organism was identified by a comparison with previous descriptions [5].
3.3. Extraction and Separation
Sliced bodies of Pinnigorgia sp. (wet weight 1.98 kg; dry weight 0.86 kg) were extracted with ethyl acetate (EtOAc) at room temperature. The EtOAc extract (84.9 g) was partitioned between methanol (MeOH) and n-hexane. The MeOH layer (12.6 g) was separated on Sephadex LH-20 and eluted using a mixture of dichloromethane (DCM) and MeOH (1:1) to yield 7 subfractions A–F. Fraction F was separated by silica gel column chromatography and eluted using n-hexane/acetone (stepwise, 1:1–pure acetone) to afford eight subfractions F1–F8. Fraction F2 was purified by silica gel column chromatography and eluted using n-hexane/acetone (stepwise, 9:1–pure acetone) to yield 13 subfractions F2A–F2M. Fraction F2D was purified by NP-HPLC using a mixture of n-hexane/EtOAc (3:1) to yield 17 subfractions F2D1–F2D17. Fraction F2D15 was purified by RP-HPLC, using a mixture of MeOH/H2O (95:5) to yield 1 (6.5 mg) and 2 (8.7 mg), respectively.
Pinnigorgiol D (1): colorless oil; −6 (c 0.44, CHCl3); IR (neat) νmax 3446, 1739, 1717 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 525 [M + Na]+; HRESIMS m/z 525.31883 (calcd. for C30H46O6 + Na, 525.31866).
Pinnigorgiol E (2): colorless oil; −4 (c 0.33, CHCl3); IR (neat) νmax 3446, 1734, 1717 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 527 [M + Na]+; HRESIMS m/z 527.33444 (calcd. for C30H48O6 + Na, 527.33431).
3.4. Anti-Hepatofibric Assay
The anti-hepatofibric effects of tested secosterols 1 and 2 were assayed using a WST-1 assay method. Anti-hepatofibric assays were carried out according to procedures described previously [6].
3.5. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Measurements of superoxide anion generation and elastase release were carried out according to previously described procedures [7,8]. Briefly, superoxide anion production was assayed by monitoring the superoxide dismutase-inhibitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO–Suc–Ala–Ala–Pro–Valp–nitroanilide as the elastase substrate.
4. Conclusions
Pinnigorgiols D (1) and E (2) are rare sterols containing a tricyclo[5,2,1,1]decane ring in their structures. Prior to this study, only four compounds of this type had been isolated from sea hare Aplysia kurodai [9] and gorgonian coral Pinnigorgia sp. [1]. In an anti-inflammatory activity test, secosterol 2 displayed significantly inhibitory effects on the release of elastase by human neutrophils and may become lead compounds in future marine anti-inflammatory drug development [10,11]. The gorgonian coral Pinnigorgia sp. will be transplanted to culturing tanks located in the National Museum of Marine Biology & Aquarium, Taiwan, for the extraction of additional natural products to establish a stable supply of bioactive material.
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology and Aquarium; the National Dong Hwa University; the National Sun Yat-sen University; the National Research Program for Biopharmaceuticals, Ministry of Science and Technology (Grant Nos. MOST 105-2325-B-291-001, 105-2811-B-291-003, 104-2320-B-291-001-MY3 and 104-2325-B-291-001); the National Health Research Institutes (NHRI-EX103-10241BI); and in part by a grant from the Chinese Medicine Research Center, China Medical University (Ministry of Education, Aim for the Top University Plan), Taiwan, awarded to Jyh-Horng Sheu, Yang-Chang Wu, and Ping-Jyun Sung.
Author Contributions
Jyh-Horng Sheu, Yang-Chang Wu and Ping-Jyun Sung designed the whole experiment and contributed to manuscript preparation. Yu-Chia Chang researched data. Tsong-Long Hwang analyzed the data and performed data acquisition.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Chang Y.-C., Kuo L.-M., Su J.-H., Hwang T.-L., Kuo Y.-H., Lin C.-S., Wu Y.-C., Sheu J.-H., Sung P.-J. Pinnigorgiols A–C, 9,11-secosterols with a rare ring arrangement from a gorgonian coral Pinnigorgia sp. Tetrahedron. 2016;72:999–1004. doi: 10.1016/j.tet.2015.12.072. [DOI] [Google Scholar]
- 2.Chang Y.-C., Kuo L.-M., Hwang T.-L., Yeh J., Wen Z.-H., Fang L.-S., Wu Y.-C., Lin C.-S., Sheu J.-H., Sung P.-J. Pinnisterols A–C, new 9,11-secosterols from a gorgonian Pinnigorgia sp. Mar. Drugs. 2016;14:12. doi: 10.3390/md14010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Y.-D., Cheng C.-H., Wen Z.-H., Wu Y.-C., Sung P.-J. New anti-inflammatory sterols from a gorgonian Pinnigorgia sp. Bioorg. Med. Chem. Lett. 2016;26:3060–3063. doi: 10.1016/j.bmcl.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y.-C., Chen N.-F., Hwang T.-L., Tseng C.-C., Wu T.-Y., Peng B.-R., Wen Z.-H., Fang L.-S., Wu Y.-C., Sheu J.-H., et al. New marine sterols from an algal-bearing gorgonian coral Pinnigorgia sp. Steroids. 2016;115:123–129. doi: 10.1016/j.steroids.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Fabricius K., Alderslade P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. 1st ed. Australian Institute of Marine Science; Townsville, Australia: 2001. pp. 218–219. [Google Scholar]
- 6.Kuo L.-M., Kuo C.-Y., Lin C.-Y., Hung M.-F., Shen J.-J., Hwang T.-L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules. 2014;19:3327–3344. doi: 10.3390/molecules19033327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H.-P., Yang S.-C., Chung P.-J., Ho C.-M., Kuo C.-Y., Hung M.-F., Huang Y.-T., Chang W.-Y., Chang Y.-W., et al. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013;190:6511–6519. doi: 10.4049/jimmunol.1202215. [DOI] [PubMed] [Google Scholar]
- 8.Yu H.-P., Hsieh P.-W., Chang Y.-J., Chung P.-J., Kuo L.-M., Hwang T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011;50:1737–1748. doi: 10.1016/j.freeradbiomed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura A., Kita M., Kigoshi H. Aplysiasecosterol A: A 9,11-secosteroid with an unprecedented tricyclic γ-diketone structure from the sea hare Aplysia kurodai. Angew. Chem. Int. Ed. 2015;54:7073–7076. doi: 10.1002/anie.201501749. [DOI] [PubMed] [Google Scholar]
- 10.Wei W.-C., Sung P.-J., Duh C.-Y., Chen B.-W., Sheu J.-H., Yang N.-S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs. 2013;11:4083–4126. doi: 10.3390/md11104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senthilkumar K., Kim S.-K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid.-Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/572859. [DOI] [PMC free article] [PubMed] [Google Scholar]