Summary
Aim
Insulin sensitivity is ~40% lower in women with polycystic ovary syndrome (PCOS) than in controls. We tested the hypothesis that 5 weeks of electroacupuncture treatment improves glucose regulation and androgen levels in overweight/obese women with PCOS.
Material and Methods
Seventeen women with PCOS, aged 18 to 38 years, with a body mass index (BMI) ≥25 kg/m2 and diagnosed with PCOS were included in this experimental and feasibility study and subjected to five weeks of electroacupuncture treatments three times/week. The primary outcome was changes in whole‐body glucose homeostasis measured by euglycemic hyperinsulinemic clamp before and after the intervention. Secondary outcome were changes in HbA1c, circulating catecholamines, adipocyte size and adipose tissue expression of sex steroids and nerve growth factor (NGF).
Results
No significant change in glucose homeostasis was observed, but HbA1c decreased by 9.5% (p = 0.004), circulating testosterone decreased by 22% (p = 0.0007) and dihydrotestosterone decreased by 12% (p = 0.007). The two vagal activity markers of plasma serotonin levels and the dopamine metabolite homovanillic acid decreased by 21% (p = 0.027) and 20% (p = 0.011), respectively. Adipose tissue concentrations of testosterone decreased by 18% (p = 0.049), and androstenedione decreased by 13% (p = 0.035), and mature NGF/proNGF ratio, a marker of sympathetic activity, increased (p = 0.04). These changes occurred without changes in anthropometrics.
Conclusion
Five weeks of electroacupuncture treatment improves HbA1c and circulating and adipose tissue androgens in women with PCOS. This effect is mediated, at least in part, via modulation of vagal activity and adipose tissue sympathetic activity. Based on these findings, we have recently initiated a randomized controlled study (NTC02647827).
Keywords: Acupuncture, adipose tissue, hyperandrogenism, insulin resistance, sympathetic nervous system
Introduction
Women with polycystic ovary syndrome (PCOS) have a three to sevenfold increased risk of developing type 2 diabetes, and this is a major health burden 1. Independent of body weight, insulin sensitivity is ~40% lower in women with PCOS than in controls, and insulin resistance has been attributed to defects in insulin signaling in adipocytes and skeletal muscle 2. Further, women with PCOS display both insulin resistance and reduced insulin responsiveness 2. Compensatory hyperinsulinemia fuels ovarian androgen production and secretion by theca cells and reduces sex hormone binding globulin (SHBG), which in turn increases free androgen levels and further exacerbates PCOS symptoms 3. Conversely, female rats that are continuously exposed to dihydrotestosterone (DHT) from puberty exhibit irregular estrous cycles, insulin resistance and obesity 4. Thus, there is a strong association between hyperinsulinemia and hyperandrogenemia that creates a vicious circle. Further, hyperandrogenism is strongly associated with high sympathetic nerve activity in women with PCOS 5, and sympathetic nerve activity increases in response to hyperinsulinemia 6. Whether sympathetic activation is a cause or a consequence of metabolic disturbances is unclear.
Overweight and obesity are closely linked to the development of PCOS. Moreover, women with PCOS have enlarged adipocytes, which might in part explain the insulin resistance in women with PCOS 7. This highlights the role of adipose tissue dysfunction as an important mechanism by which adipose tissue can negatively affect metabolic health.
Women with PCOS require long‐term individualized treatment programs. Pharmacological treatments, including the glucose‐reducing drug metformin, have limitations related to adverse effects and patient compliance 8. Therefore, there is a need for inexpensive and easily administered treatments with few negative side effects. Lifestyle management, including exercise and dietary changes, is the first line of treatment for improving whole‐body glucose homeostasis and preventing type 2 diabetes, and if successful it has the potential to improve most PCOS‐related symptoms 8. Recently, it was demonstrated that lifestyle modification with a targeted weight loss of 7% has beneficial metabolic and reproductive effects 9. For those who have difficulties in performing exercise or who are not able to follow a diet, alternative treatments such as acupuncture might be needed.
Acupuncture needles inserted into the muscle and fat tissue and stimulated manually by rotation initiate a specific pattern of afferent activity in A‐delta fibers and C‐fibers 10. When needles are stimulated by low‐frequency (2 Hz) electrical stimulation, i.e. electroacupuncture, they cause muscle contractions that activate specific physiological pathways similar to those from voluntary muscle contractions during exercise 11. Both exercise and low‐frequency electroacupuncture have been demonstrated to increase ovulation rates and decrease circulating androgens in women with PCOS 12, 13, and this effect is at least in part mediated through a decrease in muscle sympathetic nerve activity in women with PCOS 14. The activation of sensory afferents that innervate the skin, fat and muscle by low‐frequency electroacupuncture might locally affect glucose uptake by increasing the glucose transporter 4 (GLUT4) concentration 15 as well as by increasing the microcirculation through the release of a number of neuropeptides 11. It also modulates sympathetic efferent activity at the spinal and supraspinal level 11, 16. The dosage of acupuncture is a continuous matter of discussion, and a number of parameters can affect the physiological responses to acupuncture treatment, including the number of needles used, the placement of the needles, the type of stimulation (manual rotation of the needles or electrical stimulation with different frequencies) and the frequency, duration and total number of treatments. In the present study, we decided to give acupuncture 3 times/week because lower treatment frequency (1–2 times/week) did not improve insulin sensitivity in women with PCOS 17. Comparisons between manual stimulation and electrical stimulation of the needles demonstrate that electroacupuncture improves insulin sensitivity and modulates skeletal muscle gene and protein expression more than manual stimulation of the needles in rats 18. In the present study, we combined electrical stimulation with manual stimulation of needles which is a common stimulation paradigm in the clinic. The placement of the needles is another important factor, and if placed in the same innervation area as an organ, they might directly modulate the organ's autonomic activity. In the present study, the needles were placed in the same innervation area as the pancreas and ovaries as well as at distal points in the hands and feet to enhance the effect of local needles 11. Of note, the activation of adrenergic pathways has been shown to stimulate glucose uptake, and this provides new opportunities for the treatment of type 2 diabetes 19. Further, electroacupuncture has the potential to modulate the vagal activity of the immune system, an effect that is mediated via dopamine 20. Whether or not acupuncture has such an effect in overweight or obese women with PCOS is unknown.
In this experimental and feasibility study, we tested the hypothesis that 5 weeks of low‐frequency electroacupuncture treatment given three times per week results in clinically improved glucose uptake and improved levels of HbA1c and circulating sex steroids in women with PCOS. We also hypothesized that improvements in glucose control are related to changes in adipose tissue sex steroid metabolism, to the expression of pro‐ and mature (m) nerve growth factor (NGF) (markers of sympathetic nerve activity), to changes in plasma noradrenaline and to vagal activity as reflected by dopamine turnover as measured by plasma dopamine, the dopamine metabolites 3,4‐dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and serotonin and its metabolite 5‐hydroxyinoleacetic acid (5‐HIAA).
Research design and methods
This prospective clinical study was conducted at the Sahlgrenska Academy at the University of Gothenburg, Sweden, in accordance with the Declaration of Helsinki and was approved by the Regional Ethical Review Board of the University of Gothenburg. All participants gave oral and written consent before inclusion. The study was registered at ClinicalTrials.gov (NTC01457209) and is reported according to the CONSORT and STRICTA guidelines 21.
Study participants
Overweight/obese, body mass index (BMI) (kg/m2 ≥25 to <35) women 18 to 38 years of age with PCOS were recruited by advertisements in local newspapers, in the community and at medical clinics between October 2011 and December 2013 in the Västra Götaland region, Sweden. PCOS was diagnosed as having two out of the following three Rotterdam criteria 22: polycystic ovaries verified by ultrasound, oligo/amenorrhea (>35 days or <6 menstrual bleedings in the past year) or amenorrhea (total absence of menstrual bleeding in the past 90 days), and clinical signs of hyperandrogenism defined by a self‐reported Ferriman–Gallwey (FG) score ≥8. Women were excluded if they had taken any pharmacological treatments in the previous 3 months, had received acupuncture the last 2 months or had breastfed during the last 6 months prior to the study. Other endocrine disorders, including thyroid disease and prolactin excess, were excluded as well as those with type 1 diabetes and cardiovascular disease. Further exclusion criteria were a history of daily smoking or alcohol consumption.
Study procedure
Baseline measurements started at 7:30 a.m. after an overnight fast and were performed at day 1 to 10 of a spontaneous cycle or independent of cycle stage because the majority of the participants had oligo/amenorrhea. Anthropometric measurements, including body weight and height, were taken in an upright position with light clothing and no shoes. BMI was calculated as body weight (kg) divided by body height (m) squared. Waist circumference was measured in centimeters at the midpoint between the iliac crest and lower rib margin at the end of expiration while standing and without clothing. Hip circumference was measured in centimeters at the widest point between waist and thighs and waist–hip ratio (WHR) was calculated. Body composition, including fat mass (%) and muscle mass (%), was measured with a Tanita foot‐to‐foot bioelectrical impedance device (Middlesex, UK), and blood pressure was calculated as the mean arterial blood pressure (MAP) of three measures.
Before the start of the euglycemic hyperinsulinemic clamp, blood samples were taken for later analysis of luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone binding globulin (SHBG), testosterone, DHT, dehydroepiandrosterone (DHEA), androstenedione, estrone (E1), estradiol (E2), triglycerides, total cholesterol, apolipoprotein A (ApoA) and B (ApoB), HbA1c, insulin, C‐peptide and glucose as well as noradrenaline, dopamine, the dopamine metabolites DOPAC and HVA, serotonin and the serotonin metabolite 5HIAA.
Insulin sensitivity
The clamp examination was performed as described 23. In brief, insulin (Actrapid, 100 IU/mL; Novo Nordisk, Bagsvaerd, Denmark) was infused at 500 mU/mL in isotonic saline containing 2 mL of plasma from the subject to prevent insulin loss. A 10‐min primed insulin infusion was followed by a constant infusion (40 mU · m−2 · min−1) for 120 min to reach steady state. The blood glucose levels were determined before infusion, every 10 min during the first 90 min of infusion, and every 5 min during the last 30 min of infusion. Euglycemia was maintained by infusing 20% glucose (1.11 mol/L), and the rate was adjusted to maintain a glucose level of 5.5 mmol/L. The mean glucose infusion rate (GIR) was calculated as M/Iclamp where M is mg glucose per kg body weight per minute, and Iclamp is insulin levels during the last 30 min of the clamp. The homeostasis model assessment of insulin resistance (HOMA‐IR) was calculated as fasting glucose (mmol/L) × fasting insulin (mU/L) / 22.5, and the C‐peptide index (CPI) was calculated as fasting C‐peptide (nmol/L) / fasting glucose (mmol/L) × 100.
Adipose tissue biopsy
At baseline, a needle biopsy of subcutaneous abdominal adipose tissue was obtained under local anaesthesia at two‐thirds of the distance from the iliac crest to the umbilicus. One part was snap frozen in liquid nitrogen, and one part was immediately isolated to determine adipocyte size as described 7.
All baseline measurements were repeated within 48 h after the last acupuncture treatment day 1 to 10 of a spontaneous cycle or independent of cycle stage.
Intervention
The intervention started within one week after the baseline measurements were taken. We used a Western medicine style of acupuncture, referred to as low‐frequency electroacupuncture, with a fixed protocol based on previous studies of acupuncture in women with PCOS 12, 13 and on experimental studies 15, 24, 25. Two therapists educated and experienced in Western medical acupuncture delivered the acupuncture. Treatment was given three times per week over 5 weeks, and every treatment lasted for 30 min. Two sets of needle placements were alternated for every other treatment to avoid soreness (Supplemental Table 1). Needles were inserted to a depth of 15–40 mm with the aim of reaching the muscles and structures in the hands, abdominal muscle, quadriceps muscle and lower legs with innervation corresponding to the ovaries and uterus. All acupuncture points, the number of needles and the stimulation are given in Supplemental Table 1. The needles were sterile stainless steel (Hegu Xeno, Hegu Svenska) with a length of 30 or 50 mm and a diameter of 0.30 mm. When inserted, all needles were rotated manually to evoke needle sensation (de qi). Needles placed in the abdominal and quadriceps muscles were connected to an electrical stimulator (CEFAR ACUS 4; Cefar‐Compex Scandinavia, Landsbro, Sweden) and stimulated with a low‐frequency (2 Hz) electrical signal. The intensity was adjusted to produce local muscle contractions without pain or discomfort and was adjusted every 10th minute, which was when all needles not connected to the electrical stimulator were stimulated by manual rotations to evoke de qi (Supplemental Table 1).
Biochemical analyses, adipose tissue extraction and western blot
Plasma glucose was measured with a One Touch Ultra2 (LifeScan). Insulin, HbA1c, total cholesterol, triglycerides, ApoA, ApoB, SHBG, LH and FSH were analysed by an accredited laboratory at the Department of Clinical Chemistry of Sahlgrenska University Hospital. Serum C‐peptide was measured with a human diabetes C‐peptide magnetic bead set (#171B7003M, Bio‐Rad, USA). Testosterone, DHT, DHEA, E1, E2, androstendione and progesterone in serum and adipose tissue were measured by gas chromatography‐tandem mass spectrometry (GC‐MS/MS) as described previously 26. For analyses of sex steroids in adipose tissue, biopsies were homogenized in 0.45 mL PBS buffer and frozen at −80 °C. Measurement of plasma noradrenaline, dopamine, DOPAC, HVA, serotonin and 5HIAA was performed with a split fraction HPLC‐ED system as described 27.
For western blot, adipose tissue samples (1 mg) were homogenized by mechanical dissociation in 10 μL ice cold lysis buffer (20 mM Tris pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM EDTA pH 8.0, 20 mM NaF, 30 mM sodium pyrophosphate (Na4P7O2), and protease and phosphatase cocktail inhibitors (cat P8340, P5276 and P0044, Sigma)) without detergent using a polytron. The samples were centrifuged at 3,000 × g for 6 min at 4 °C to remove the fat cake, and the supernatant was retained as the sample. Five volumes of ice cold supplemented detergent mix (6% NP40, 0.6% SDS, 1.5% NaDOC) was added to each sample followed by incubation and shaking for 45 min on ice. The samples were then centrifuged at 13,000 rpm for 20 min at 4 °C. Protein concentration was quantified by the Bradford assay.
Adipose tissue protein concentration of mature mNGF and proNGF were detected and quantified using MAB5260Z clone 27/21 and EP1318Y antibodies (Merck Millipore), respectively, in a homemade sandwich ELISA device.
Western blot analysis for proNGF, p75NTR, phospho‐TrkA and GAPDH was performed on six tissue samples from each group. Samples (30 µg of total protein) were separated by 8% or 12% SDS‐PAGE and electrophoretically transferred to a PVDF membrane overnight. The membranes were then blocked and incubated with specific antibodies (SantaCruz Biotech, CA, USA). After washing, the membranes were incubated with horseradish peroxidase‐conjugated anti‐rabbit IgG or horseradish peroxidase‐conjugated anti‐mouse IgG as the secondary antibody (Cell Signaling Technology, MA, USA) at room temperature. The blots were developed with an ECL assay (Millipore, MA, USA). The GAPDH bands were used as a control for equal protein loading. The ImageJ software (http://rsb.info.nih.gov/ij/) was used for gel densitometry and protein quantification.
Sample size calculation
The sample size for detecting changes in glucose uptake as measured by the euglycemic hyperinsulinemic clamp was determined based on a previous study with a similar design but with exercise instead of acupuncture 28. We expected a ΔM/Iclamp change of 1.5 with a standard deviation (SD) of 2 from baseline after 5 weeks of the intervention. With a target power of 80% and the significance level set to 5%, we required a minimum of 16 subjects to detect a 15% change in M/Iclamp.
Statistical analyses
Data are presented as the mean and SD or standard error of the mean (SEM) for western blot densitometries. Fisher's test for pair‐wise comparisons was used to analyse changes between measurements at baseline and at follow‐up after 5 weeks of acupuncture per‐protocol. A p‐value < 0.05 was considered significant.
Results
Clinical characteristics, treatment compliance and side‐effects
In total, 21 women with PCOS were included and underwent all baseline measurements and started treatment (Supplemental Figure 1). Four dropped out after one to six treatments and did not return for follow‐up, leaving 17 women who were included in the analysis. The reasons for dropout were time constraint (n = 3) and starting a pharmacological treatment (n = 1). The number of treatments varied from 11 to 19. Few side effects of the acupuncture treatment were reported, and the most common were temporary pain and bruises. Nine women fulfilled all three diagnostic criteria of PCOS, one presented with hyperandrogenism and oligomenorrhea, two presented with hyperandrogenism and PCO morphology, and six presented with oligomenorrhea and PCO morphology. The low number of participants did not allow sub‐group analyses. Baseline characteristics of the study participants are presented in Table 1.
Table 1.
Changes from baseline to after 5 weeks of electroacupuncture (EA) treatment (three treatments/week) in women with PCOS
| Variable | Baseline (n = 17) | After EA (n = 17) | ∆ (After − Baseline) | p * |
|---|---|---|---|---|
| Body composition | ||||
| Body mass index (kg/m2) | 30.8 ± 4.20 | 30.8 ± 3.84 | −0.012 ± 0.77 | 0.952 |
| Weight (kg) | 85.1 ± 14.6 | 83.2 ± 17.3 | −1.84 ± 6.59 | 0.314 |
| Waist circumference (cm) | 95.5 ± 10.1 | 95.4 ± 9.23 | −0.12 ± 2.39 | 0.921 |
| Hip circumference (cm) | 110.8 ± 8.34 | 110.3 ± 7.71 | −0.47 ± 3.06 | 0.586 |
| Waist‐to‐hip ratio | 0.86 ± 0.074 | 0.87 ± 0.08 | 0.003 ± 0.02 | 0.751 |
| Fat mass (%) | 34.6 ± 9.74 | 34.4 ± 9.00 | −0.19 ± 1.53 | 0.638 |
| Lean mass (%) | 50.5 ± 5.41 | 50.6 ± 5.21 | 0.10 ± 1.15 | 0.723 |
| Adipocyte volume (µm3) | 117.28 ± 9.07 | 112.34 ± 12.48 | −4.93 ± 11.52 | 0.098 |
| Ferriman Gallwey score | 10.4 ± 7.17 | 11.1 ± 8.34 | 0.50 ± 3.69 | 0.492 |
| Metabolic variables | ||||
| ApoA1 (g/L) | 1.40 ± 0.24 | 1.39 ± 0.22 | −0.01 ± 0.09 | 0.516 |
| ApoB (g/L) | 0.88 ± 0.26 | 0.83 ± 0.22 | −0.04 ± 0.16 | 0.272 |
| ApoA1/ApoB ratio | 0.65 ± 0.18 | 0.64 ± 0.20 | −0.006 ± 0.17 | 0.877 |
| Cholesterol (mmol/L) | 4.69 ± 1.36 | 4.56 ± 1.21 | −0.13 ± 0.70 | 0.434 |
| Triglycerides (mmol/L) | 1.15 ± 0.51 | 1.10 ± 0.56 | −0.05 ± 0.34 | 0.556 |
| Glucose (mmol/L) | 4.91 ± 0.33 | 4.92 ± 0.30 | 0.00 ± 0.23 | 0.811 |
| Insulin (mU/L) | 12.4 ± 6.89 | 11. 2 ± 5.57 | −0.18 ± 5.03 | 0.408 |
| HOMA‐IR | 3.08 ± 1.92 | 2.46 ± 1.61 | −0.62 ± 1.21 | 0.051 |
| C‐peptide (ng/mL) | 1.28 ± 0.52 | 1.15 ± 0.45 | −0.13 ± 0.34 | 0.137 |
| C‐peptide index | 8.91 ± 4.05 | 7.65 ± 3.20 | −1.26 ± 2.35 | 0.051 |
| Endocrine variables | ||||
| LH (IU/L) | 6.81 ± 5.36 | 7.79 ± 9.25 | 0.94 ± 8.79 | 0.798 |
| FSH (IU/L) | 3.96 ± 1.39 | 3.96 ± 1.87 | −0.001 ± 2.70 | 0.981 |
| LH/FSH ratio | 1.75 ± 1.25 | 1.66 ± 1.43 | −0.08 ± 1.11 | 0.831 |
| SHBG (nmol/L) | 38.2 ± 14.9 | 36.9 ± 15.3 | −1.23 ± 7.31 | 0.506 |
| Progesterone (pg/mL) | 1436 ± 2877 | 2486 ± 3776 | 1049 ± 5376 | 0.432 |
| DHEA (pg/mL) | 6732 ± 3101 | 8087 ± 5417 | 1354 ± 4068 | 0.191 |
| E1 (pg/mL) | 66.3 ± 29.0 | 65.8 ± 29.6 | −0.49 ± 28.1 | 0.943 |
| E2 (pg/mL) | 76.8 ± 37.7 | 71.1 ± 47.6 | −5.64 ± 55.5 | 0.680 |
| Adipose tissue sex steroids | ||||
| Progesterone (pg/g) | 12,382 ± 24,960 | 27,152 ± 38,813 | 14,769 ± 52,528 | 0.246 |
| DHEA (pg/g) | 23,439 ± 10,646 | 24,252 ± 10,841 | 813 ± 7080 | 0.943 |
| E1 (pg/g) | 522 ± 273 | 570 ± 280 | 47.8 ± 229 | 0.407 |
| E2 (pg/g) | 162 ± 116 | 707 ± 1960 | 561 ± 1963 | 0.084 |
All values are means ± SD.
: Fisher's test for pair‐wise comparisons (baseline vs. after 5 weeks of low‐frequency EA).
ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; DHEA, dehydroepiandrostenedione; E1, estrone; E2, estradiol; FSH, follicle stimulating hormone; HOMA‐IR, homeostasis model assessment of insulin resistance; LH, luteinizing hormone; SHBG, sex hormone binding globuline.
Changes after 5 weeks of electroacupuncture
Five weeks of electroacupuncture did not significantly affect whole‐body glucose homeostasis as measured by M/Iclamp (Figure 1A). HbA1c decreased by 9.5% (p = 0.004) (Figure 1B), and the fasting‐derived markers of insulin resistance (HOMA‐IR and C‐peptide index) tended to decrease (p = 0.051 for both) after 5 weeks of treatment (Table 1).
Figure 1.
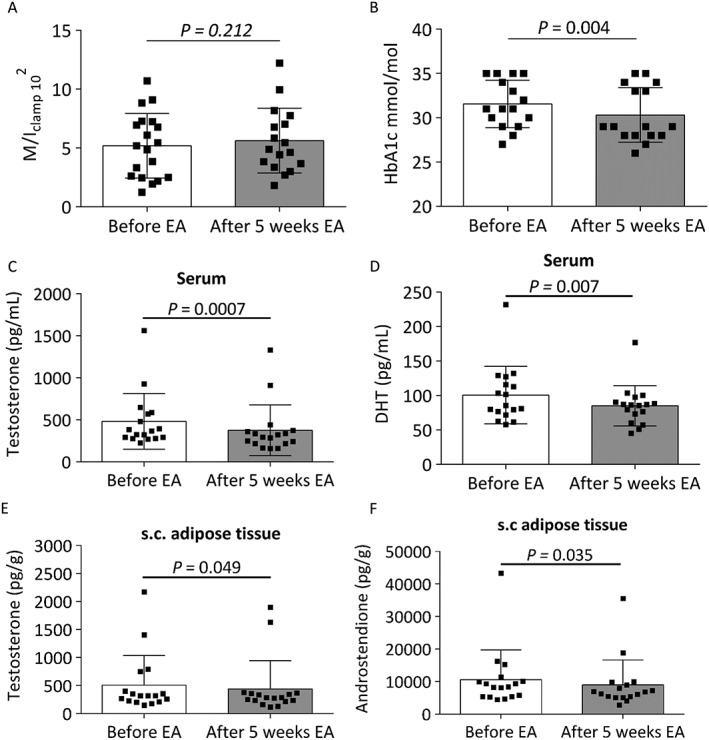
Changes in A) euglycemic hyperinsulinemic clamp (M/Iclamp); B) HbA1c; C) circulating testosterone; D) circulating dihydrotestosterone (DHT); E) subcutaneous adipose tissue concentrations of testosterone and F) subcutaneous adipose tissue concentrations of androstenedione after 5 weeks of electroacupuncture (EA).
Circulating testosterone decreased by 22% and DHT by 12% after 5 weeks of electroacupuncture treatment (p = 0.0007 and p = 0.007, respectively) (Figure 1C–D), with no changes in androstenedione, DHEA, E1, E2 or progesterone (Table 1). Because electroacupuncture has been shown to decrease circulating sex steroids in several studies 12, 13, we used the highly sensitive and specific GC‐MS/MS method to analyse adipose tissue concentrations (for the first time) of the same panel of sex steroids as in circulation. Adipose tissue concentrations of testosterone decreased by 18% and androstenedione by 13% after 5 weeks of treatment (p = 0.049 and p = 0.035, respectively) (Figure 1E–F), with no changes in DHT, DHEA, E1, E2 or progesterone (Table 1). Although not significant, adipocyte size tended to decrease after 5 weeks of treatment (p = 0.092) (Table 1). All of these changes occurred without affecting weight or any other anthropometrics.
Dopamine turnover was measured because the effect of electroacupuncture, at least in part, has been shown to be mediated via changes in the autonomic nervous system. Serotonin decreased by 21% and HVA by 20% after 5 weeks of electroacupuncture (p = 0.027 and p = 0.011, respectively) (Figure 2A–B). The plasma levels of dopamine, noradrenalin and DOPAC were below the level of detection of the HPLC system.
Figure 2.
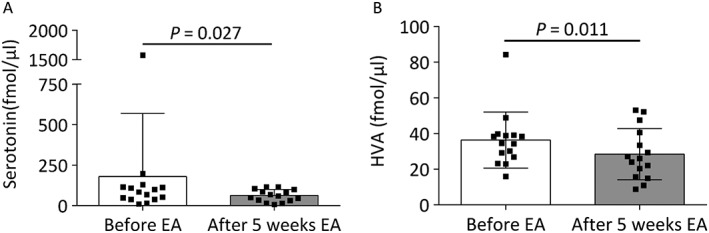
Changes in plasma levels of A) serotonin and B) homovanillic acid (HVA) after 5 weeks of electroacupuncture (EA).
Further, NGF, a marker of sympathetic activity, was analysed in adipose tissue and we found an increase in the ratio of mNGF to pro‐NGF protein after 5 weeks of treatment (Figure 3C). The most expressed forms of pro‐NGF were the 50 kDa form (Figure 3D), which represents a major glycosylation state, and the 34 kDa form, which corresponds to the pro‐NGF‐A splicing variant, and neither of these changed after 5 weeks of electroacupuncture (Figure 3E–G). The expression of phosphorylated TrkA, which is the receptor of pro‐NGF and mNGF, was significantly increased by electroacupuncture indicating the activation of TrkA downstream signaling pathways (Figure 3H–I), but there was no change in the protein expression of the p75NTR receptor (Figure 3H, J).
Figure 3.
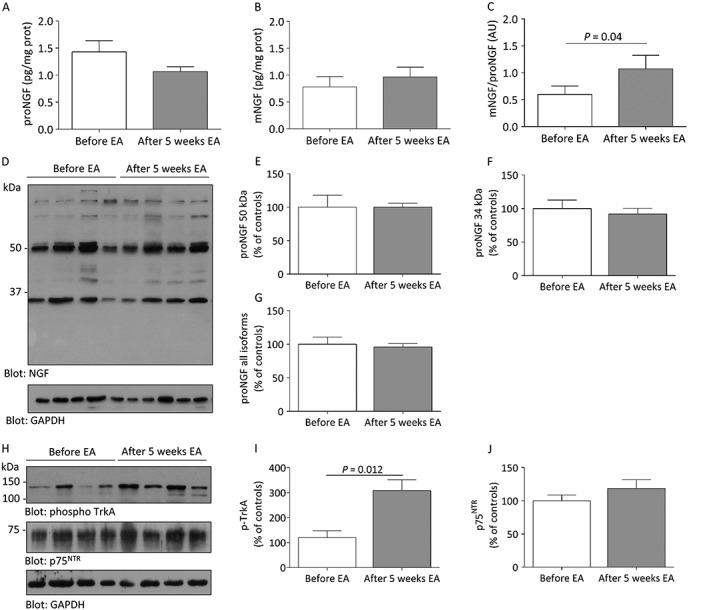
Changes in adipose tissue expression of A) pro nerve growth factor (proNGF), B) mature NGF (mNGF) and C) the mNGF/proNGF ratio after 5 weeks of electroacupuncture (EA) measured by ELISA. D) Representative blot of each proNGF splicing variant from before and after 5 weeks of EA measured by western blot and corresponding densitometry analyses for E) proNGF 50 kDa; F) proNGF 34 kDa and G) all proNGF variants detectable in adipose tissue, and H) representative western blots and corresponding gel densitometry analyses for adipose tissue expression of the mNGF/proNGF receptors I) phosphorylated‐TrkA and J) p75NTR.
Discussion
Persons with impaired glucose tolerance and compensatory hyperinsulinemia are at increased risk of developing type 2 diabetes and should be referred to lifestyle management programs, including physical exercise and dietary advice, to improve their insulin sensitivity 29, 30. The increasing referrals to different complementary and alternative treatments indicate a shift towards non‐drug‐based therapies to complement more conventional approaches for improving health 31. To increase our knowledge of the effect and mechanism of action of one such treatment, we investigated the effect of low‐frequency electroacupuncture as an alternative to lifestyle management. We focused on overweight and obese women with PCOS and found that 5 weeks of electroacupuncture improved HbA1c even though there was no change in peripheral insulin sensitivity (M/Iclamp value). Improved glucose regulation is supported by the non‐significant decrease in HOMA‐IR and CPI, two markers believed to primarily reflect hepatic insulin sensitivity 32, and suggests that electroacupuncture improves hepatic insulin sensitivity. These changes occurred together with a decrease in circulating and adipose tissue concentrations of androgens. The decrease in HbA1c is equivalent to un‐supervised exercise in persons with impaired glucose tolerance 33. Remarkably, the changes observed in our study occurred with no changes in body weight or waist circumference.
It is well known that physical exercise with muscle contraction is an effective strategy to prevent and treat type 2 diabetes 34. Exercise in insulin‐resistant and overweight/obese women with PCOS improves insulin sensitivity 28, 35 and reduces fat content 35, and this effect does not appear to be related to changes in mitochondrial variables 36.
This study is the first to translate the experimental findings in the DHT‐induced rat PCOS model 25 into the clinical situation by demonstrating that electroacupuncture has the potential to improve HbA1c in overweight and obese women with PCOS. The decrease in HbA1c by electroacupuncture is in agreement with the decrease observed with short bouts of moderate to vigorous‐intensity physical activity in persons at risk for type 2 diabetes 37 and is aligned with physical activity guidelines for at‐risk persons 38.
Circulating testosterone and DHT levels were decreased by 5 weeks of electroacupuncture. This is in line with our previous observations in two independent randomized controlled trials in which menstrual frequency and ovulation frequency were also improved by electroacupuncture 12, 13. Together with the marked decrease in circulating testosterone and DHT, we also found a tendency for a decline in adipocyte size. The novelty of the present study is that we measured subcutaneous adipose tissue sex steroid concentrations with the highly sensitive and specific GC‐MS/MS method, and we found that androstenedione and testosterone concentrations were significantly lower after 5 weeks of treatment, mimicking the decrease in circulating androgens. The reduction of both circulating and adipose tissue concentrations of androgens by electroacupuncture might indicate decreased production of ovarian testosterone, which in turn decreases subcutaneous adipose tissue concentrations and reflects the modulation of sex steroid‐inactivating enzymes in adipose tissue. These assumptions require further investigation. The adipose tissue is known to play a central role in determining whole‐body insulin sensitivity, and it has been shown that testosterone induces selective insulin resistance in female subcutaneous adipose tissue 39. Thus, decreased circulating and subcutaneous adipose tissue concentrations might directly modulate glucose regulation as reflected by a decrease in HbA1c and a tendency for decreased HOMA‐IR and CPI.
Because the effect of electroacupuncture is at least in part mediated via modulation of sympathetic nerve activity 14, 16 and vagal activity 20, we analysed the adipose tissue protein expression of NGF. It has been shown that mNGF is a major regulator and activator of sympathetic drive towards peripheral organs and that it is involved in adipocyte metabolism in vitro 40, and that proNGF is able to activate both TrkA and p75NTR‐related signaling pathways 41. In the present study, the adipose tissue mNGF/proNGF ratio was increased after 5 weeks of electroacupuncture, indicating that mNGF activity was stimulated. Consistent with this, the phosphorylation of TrkA, which is the high‐affinity receptor for mNGF, was also increased by 5 weeks of electroacupuncture, indicating that the activation of a mNGF/TrkA‐mediated downstream signalling pathway might contribute to the electroacupuncture‐mediated improvement of glucose regulation and the circulating and adipose tissue concentrations of androgens. Serotonin affects glucose homeostasis and insulin resistance by acting via vagal afferent serotonergic neurons and receptors in peripheral tissue and is increased in type 2 diabetes patients 42, and HVA is responsive to changes in blood glucose levels 43. Thus, it is important to note that both of these decreased indicating that electroacupuncture was able to modulate the vagal system.
Considering that only a single group was studied and the exploratory/experimental nature of this study, the effect of the treatment might not be an actual effect but a statistical artifact. This underlies the importance of performing controlled studies. Thus, the limitations of this study are the lack of a comparison group and its small sample size. Based on these findings, we have now been able to perform a formal sample size estimation and have initiated a randomized controlled study (Clinicaltrial.gov: NTC02647827).
In conclusion, this study has shown that 5 weeks of electroacupuncture has a significant effect on improving glucose regulation, including HbA1c and circulating and adipose tissue androgen concentrations, an effect that, at least in part, is mediated via activation of adipose tissue sympathetic activity and modulation of the vagal system.
Funding
This work was supported by the Swedish Medical Research Council (Project No. 2014‐2775); the Jane and Dan Ohlsson Foundation; Wilhelm and Martina Lundgrens's Science Fund; the Hjalmar Svensson Foundation; the Adlerbert Research Foundation; the Novo Nordisk Foundation (NNF15OC00151902); Strategic Research Programme in Diabetes at Karolinska Institutet; the Swedish federal government under the LUA/ALF agreement ALFGBG‐429501 and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (all ESV) and by FONDECYT 11130250 (MM). MS and VP were supported by CNR Short Term Mobility 2015 Fellowships. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors have nothing to disclose.
Author contributions
E.S‐V. and A.B. designed the study, acquired and analysed the data, and wrote the manuscript. M.M., M.K. M.S., V.P. and L.M. acquired the data and contributed to the discussion and reviewed/edited the manuscript. E.J‐H and CO acquired the data and reviewed/edited the manuscript. A.Z. screened all of the study subjects and reviewed/edited the manuscript. C‐J.B. and K.H. contributed to study design, acquired the data and reviewed/edited the manuscript. M.L. reviewed/edited the manuscript.
Supporting information
Supplemental Figure 1: Study flow chart.
Supplemental Table 1: Acupuncture points, stimulation, localization, tissue in which needles were inserted and innervation areas. The two sets were alternated every other treatment
Supporting info item
Supporting info item
Acknowledgements
We thank Carola Gustafsson at the Research Unit for Cardiology at Sahlgrenska University Hospital, Gothenburg for performing all euglycemic hyperinsulinemic clamp as well as fat and muscle biopsies, and Professor Anders Odén, Department of Mathematical Sciences, Chalmers University of Technology, Sweden.
Stener‐Victorin, E. , Maliqueo, M. , Soligo, M. , Protto, V. , Manni, L. , Jerlhag, E. , Kokosar, M. , Sazonova, A. , Behre, C. J. , Lind, M. , Ohlsson, C. , Højlund, K. , and Benrick, A. (2016) Changes in HbA1c and circulating and adipose tissue androgen levels in overweight‐obese women with polycystic ovary syndrome in response to electroacupuncture. Obesity Science & Practice, 2: 426–435. doi: 10.1002/osp4.78.
References
- 1. Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91: 48–53. [DOI] [PubMed] [Google Scholar]
- 2. Diamanti‐Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33: 981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev 2008; 24: 520–532. [DOI] [PubMed] [Google Scholar]
- 4. Manneras L, Cajander S, Holmang A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007; 148: 3781–3791. [DOI] [PubMed] [Google Scholar]
- 5. Sverrisdottir YB, Mogren T, Kataoka J, Janson PO, Stener‐Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 2008; 294: E576–E581. [DOI] [PubMed] [Google Scholar]
- 6. Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity‐related hypertension. Hypertension 2006; 48: 787–796. [DOI] [PubMed] [Google Scholar]
- 7. Manneras‐Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 2011; 96: E304–E311. [DOI] [PubMed] [Google Scholar]
- 8. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM‐Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012; 97: 28–38.e25. [DOI] [PubMed] [Google Scholar]
- 9. Legro RS, Dodson WC, Kris‐Etherton PM, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015; 100: 4048–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kagitani F, Uchida S, Hotta H. Afferent nerve fibers and acupuncture. Auton Neurosci 2010; 157: 2–8. [DOI] [PubMed] [Google Scholar]
- 11. Stener‐Victorin E. Hypothetical physiological and molecular basis for the effect of acupuncture in the treatment of polycystic ovary syndrome. Mol Cell Endocrinol 2013; 373: 83–90. [DOI] [PubMed] [Google Scholar]
- 12. Jedel E, Labrie F, Oden A, et al. Impact of electro‐acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2011; 300: E37–E45. [DOI] [PubMed] [Google Scholar]
- 13. Johansson J, Redman L, Veldhuis PP, et al. Acupuncture for ovulation induction in polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2013; 304: E934–E943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stener‐Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low‐frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 2009; 297: R387–R395. [DOI] [PubMed] [Google Scholar]
- 15. Johansson J, Yi F, Shao R, Lonn M, Billig H, Stener‐Victorin E. Intense acupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of polycystic ovary syndrome. Am J Physiol Endocrinol Metab 2010; 299: E551–E559. [DOI] [PubMed] [Google Scholar]
- 16. Stener‐Victorin E, Fujisawa S, Kurosawa M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J Appl Physiol 2006; 101: 84–91. [DOI] [PubMed] [Google Scholar]
- 17. Stener‐Victorin E, Baghaei F, Holm G, et al. Effects of acupuncture and exercise on insulin sensitivity, adipose tissue characteristics, and markers of coagulation and fibrinolysis in women with polycystic ovary syndrome: secondary analyses of a randomized controlled trial. Fertil Steril 2012; 97: 501–508. [DOI] [PubMed] [Google Scholar]
- 18. Benrick A, Maliqueo M, Johansson J, et al. Enhanced insulin sensitivity and acute regulation of metabolic genes and signaling pathways after a single electrical or manual acupuncture session in female insulin‐resistant rats. Acta Diabetol 2014; 51: 963–972. [DOI] [PubMed] [Google Scholar]
- 19. Sato M, Dehvari N, Oberg AI, et al. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes 2014; 63: 4115–4129. [DOI] [PubMed] [Google Scholar]
- 20. Torres‐Rosas R, Yehia G, Pena G, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014; 20: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 2010; 7: e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rotterdam ESHRE/ASRM‐Sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41–47. [DOI] [PubMed] [Google Scholar]
- 23. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223. [DOI] [PubMed] [Google Scholar]
- 24. Manneras L, Cajander S, Lonn M, Stener‐Victorin E. Acupuncture and exercise restore adipose tissue expression of sympathetic markers and improve ovarian morphology in rats with dihydrotestosterone‐induced PCOS. Am J Physiol Regul Integr Comp Physiol 2009; 296: R1124–R1131. [DOI] [PubMed] [Google Scholar]
- 25. Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener‐Victorin E. Low‐frequency electro‐acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone‐induced polycystic ovary syndrome. Endocrinology 2008; 149: 3559–3568. [DOI] [PubMed] [Google Scholar]
- 26. Nilsson ME, Vandenput L, Tivesten A, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high‐sensitive gas chromatography‐tandem mass spectrometry. Endocrinology 2015: en20141890. [DOI] [PubMed] [Google Scholar]
- 27. Prieto‐Garcia L, Egecioglu E, Studer E, Westberg L, Jerlhag E. Ghrelin and GHS‐R1A signaling within the ventral and laterodorsal tegmental area regulate sexual behavior in sexually naive male mice. Psychoneuroendocrinology 2015; 62: 392–402. [DOI] [PubMed] [Google Scholar]
- 28. Moro C, Pasarica M, Elkind‐Hirsch K, Redman LM. Aerobic exercise training improves atrial natriuretic peptide and catecholamine‐mediated lipolysis in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009; 94: 2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendes R, Sousa N, Almeida A, et al. Exercise prescription for patients with type 2 diabetes—a synthesis of international recommendations: narrative review. Br J Sports Med 2015. [DOI] [PubMed] [Google Scholar]
- 30. Powers MA, Bardsley J, Cypress M, et al. Diabetes self‐management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care 2015; 38: 1372–1382. [DOI] [PubMed] [Google Scholar]
- 31. Nahin RL, Dahlhamer JM, Taylor BL, et al. Health behaviors and risk factors in those who use complementary and alternative medicine. BMC Public Health 2007; 7: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008; 294: E15–E26. [DOI] [PubMed] [Google Scholar]
- 33. Osler ME, Fritz T, Caidahl K, Krook A, Zierath JR, Wallberg‐Henriksson H. Changes in gene expression in responders and nonresponders to a low‐intensity walking intervention. Diabetes Care 2015; 38: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 34. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA 2001; 286: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 35. Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab 2011; 96: E48–E56. [DOI] [PubMed] [Google Scholar]
- 36. Hutchison SK, Teede HJ, Rachon D, Harrison CL, Strauss BJ, Stepto NK. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia 2012; 55: 1424–1434. [DOI] [PubMed] [Google Scholar]
- 37. Gay JL, Buchner DM, Schmidt MD. Dose–response association of physical activity with HbA1c: Intensity and bout length. Prev Med 2016; 86: 58–63. [DOI] [PubMed] [Google Scholar]
- 38. Committee PaGA. Physical activity Guidelines Advisory Committee Report. Washington, DC, US 2008.
- 39. Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 2007; 192: 585–594. [DOI] [PubMed] [Google Scholar]
- 40. Ng TB, Wong CM. Epidermal and nerve growth factors manifest antilipolytic and lipogenic activities in isolated rat adipocytes. Comp Biochem Physiol B 1985; 81: 687–689. [DOI] [PubMed] [Google Scholar]
- 41. Masoudi R, Ioannou MS, Coughlin MD, et al. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J Biol Chem 2009; 284: 18424–18433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hasegawa Y, Suehiro A, Higasa S, Namba M, Kakishita E. Enhancing effect of advanced glycation end products on serotonin‐induced platelet aggregation in patients with diabetes mellitus. Thromb Res 2002; 107: 319–323. [DOI] [PubMed] [Google Scholar]
- 43. Bello NT, Hajnal A. Alterations in blood glucose levels under hyperinsulinemia affect accumbens dopamine. Physiol Behav 2006; 88: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Study flow chart.
Supplemental Table 1: Acupuncture points, stimulation, localization, tissue in which needles were inserted and innervation areas. The two sets were alternated every other treatment
Supporting info item
Supporting info item


