Summary
Objective
The aim of this study was to examine differences in rates of non‐caloric beverage adoption by participants classified as sweet likers (SLs) or sweet dislikers (measured using a behavioural tasting task).
Methods
Data are a sub‐study from a 6‐month, three‐group, randomized weight loss trial (CHOICE) (body mass index 36.3 ± 5.8 kg m−2, 84% female, aged 42.2 ± 10.9 years, 53% African‐American) comparing the replacement of caloric beverages with either non‐caloric sweetened beverages (diet) or water (water) compared with a control group. This sub‐study, which included participants within the water (n = 106) and diet (n = 103) groups only, examined whether SLs (n = 33 water; n = 37 diet) varied in their adherence to caloric beverage recommendations compared with sweet dislikers (n = 73 water; n = 76 diet) over the 6‐month study.
Results
Diet intake and sweet‐liking data collected on 190 (3 months) and 169 participants (6 months) were used for analysis. The interaction between SL status and beverage group (diet vs. water) approached significance (P = 0.06) at 3 months but not 6 months. Caloric beverage intake (% energy) at 3 months was significantly higher in SLs within the water group (9.7 ± 1.4%) compared with SLs in the diet group (5.4 ± 1.0%, P = 0.03).
Conclusions
Results suggest that SL status may affect the rate in reduction of caloric beverages when water is the recommended substitution. Future studies should explore tailoring beverage recommendations to tasting profile.
Keywords: Beverages, dietary adherence, tasting preference, water
Preference for sweet foods may be partly determined by genetic influences 1 but may also be affected by altered sensitivity to reward 2 or a heightened sensitivity to the mood‐altering impacts of consuming sweet foods and beverages 3. There have been varying results regarding the role of these tasting profiles in affecting health outcomes, body weight and dietary patterns 4, 5, 6. There are several different methods that can be used to identify these sweet‐tasting patterns and sweet preferences, such as a behavioural tasting task of different sweetened solutions 7, sweet foods preference checklists 5 or possibly genotyping for chromosome 16 p11.2 8. Preference for or dislike of sweet foods and beverages may be related to dietary intake and body weight. Those individuals who are classified as sweet likers (SLs) – having a high preference for sweet solutions or foods 9 – are more likely to be dependent on alcohol 10, 11, 12 and also show greater preference for sweet foods and beverages 9, 13. Results linking sweet taste preference and body weight, however, have not shown a strong relationship 4, 12.
Although some studies have shown potential predictors of weight loss success, such as limited prior weight loss attempts 14, high quality of life 14 and reported readiness to attempt weight loss 15, few studies have examined how baseline tasting preferences may affect an individual's ability to adopt new dietary recommendations as part of a weight loss intervention. Adherence to dietary recommendations is a strong predictor of weight loss 16, and it is possible that different tasting profiles may moderate this effect 17. The role of sweet preference on the ability to improve dietary outcomes, achieve weight loss and adhere to a behavioural weight loss intervention has not been previously explored. It is important to determine ways to tailor dietary recommendations for weight loss that will lead to increased adherence and better weight loss outcomes. Therefore, the purpose of the present study is to examine whether SLs and sweet dislikers (SDLs) differ in their ability to adopt a beverage reduction and substitution recommendation as a way to achieve weight loss.
The present investigation is a sub‐study of an overall weight loss intervention examining changes in caloric beverage intake as a weight control strategy compared with an attention control group. Results of the main study, which have been described elsewhere 18, found that those participants assigned to replace their caloric beverage intake with a non‐caloric beverage (water and diet beverage groups combined) were twice as likely to have achieved a 5% weight loss at 6 months as those in the control group (OR: 2.07, χ2 4.2, P < 0.05). This paper includes those participants randomly assigned to one of the two beverage replacement interventions and examines the relationship of SL status and beverage intervention group with change in caloric beverage intake.
Methods
The methods for this study have been described elsewhere 18, 19. Briefly, overweight men and women (aged 18–65 years; body mass index [BMI] 25.0–49.9 kg m−2) who reported consuming at least 285 kcals per day of caloric beverages were randomized to one of the three conditions: (i) water provision, (ii) non‐caloric sweetened beverage provision and (iii) attention control as part of the Choose Healthy Options Consciously Everyday (CHOICE) study, a 6‐month, three‐group, randomized clinical weight loss trial. The present study examines the two beverage replacement groups (water vs. diet) only. Two days of unannounced, 24‐h food recalls were collected at baseline and included one weekday and one weekend day. Interviews were conducted by trained interviewers at the Nutrition Epidemiology Core of the UNC Clinical Nutrition Research Center (Grant Number: DK56350). Dietary intake data were collected and analysed using Nutrition Data System for Research software versions 2007 and 2008 developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Final calculations were completed using Nutrition Data System for Research version 2008. The 2 d of dietary intake were averaged, and energy from caloric beverages was calculated by examining the total kcals from all caloric beverages consumed (averaged for the 2 d). Percent energy from caloric beverages was used for analyses as a way to standardize beverage intake among the four beverage and SL/SDL groups, taking into account total energy intake. In addition, percent energy from caloric beverages was also used because energy intake decreased over the course of the 6‐month study among participants, and the objective of this sub‐study was to examine the percentage of calories coming from caloric beverages while taking into account total energy. The University of North Carolina at Chapel Hill Institutional Review Board approved the study, and all participants gave written informed consent.
Body weight was obtained using a calibrated digital scale (Tanita BWB 800) with participants in hospital gowns, without shoes, and height was measured using a wall‐mounted stadiometer (Perspective Enterprises, Inc., Kalamazoo, MI). Methods of assessing sweet preferences are described in detail elsewhere 19. In summary, SL status was assessed using a behavioural preference task with varying levels of sucrose solutions 3. To estimate each subject's sensitivity and hedonic response to sweet taste, five concentrations of sucrose solution (0.05, 0.10, 0.21, 0.42 and 0.83 molarity [m]) were presented five times in a predetermined random order (25 total tastings) used for all participants. These solution concentrations are similar in taste to a 12‐oz coffee with two packets of sugar (0.05 m), a mixture of half sweet and half unsweet tea (0.10 m), sweet tea (0.21 m), lemonade or orange soda (0.42 m) and a glucose tolerance test beverage (0.83 m). Participants were asked to rate each solution's pleasurableness, answering the question, ‘How much do you like the taste?’ and marking the answer on a 200‐mm analogue scale with the poles labelled ‘Disliked very much’ and ‘Liked very much’, and the midpoint labelled ‘Neither liked nor disliked’. Participants who gave the highest score for the 0.83 m sucrose solution were classified as SLs based on Kampov‐Polevoy's protocol 3.
Main outcomes of the CHOICE trial, which have been reported elsewhere, found that participants in the two beverage substitution groups (water and diet groups combined) were twice as likely to have achieved a 5% weight loss at 6 months as those in the control group (OR: 2.07, χ2 4.2, P < 0.05) 18. Participants in both the water and diet groups reduced their energy intake from caloric beverages to a greater degree than the control group. Reduction of caloric beverage intake was significantly correlated with weight loss at both three (r = 0.17, P < 0.05) and six (r = 0.19, P < 0.01) months.
Statistical methods
The overall CHOICE study was powered to compare the attention control with each of the beverage groups separately. The present paper represents a sub‐study of that project, in which only participants in the diet and beverage groups with complete information on both SL status and caloric beverage consumption were included. Analysis of variance and chi‐squared test of independence were used to assess differences among groups at baseline. Logistic regression was used to examine differential drop‐out rates among SL status within each beverage group, adjusting for age and race. Pearson's correlations examined the relationship between the continuous variables of changes in weight and caloric beverage intake. Analysis of changes in percent energy from beverage intake was performed using sas version 9.2 (SAS institute Inc., Cary, NC). For the model, along with the main effects of treatment group by time (as a categorical variable with the three assessment time points), race, baseline age and SL status were included in the mixed‐effect model. Least‐square means were obtained from the mixed‐effect models. For better efficiency, water and diet beverage consumption were jointly modelled 20, with an indicator variable taking a value of 1 for water consumption and 0 for diet beverage. Square root transformation was applied to the beverage consumption variable to avoid the violation of normality assumption of residuals.
Results
Descriptions of the entire study sample have been described elsewhere 18. There were no differences in any of the baseline demographic characteristics among the three intervention groups in the main trial analyses 18. For the present analysis, only participants belonging to the two beverage groups that had complete diet data at each time point and baseline sweet‐liking data were included (209 participants at baseline, 190 participants at 3 months and 169 participants at 6 months). Within the two beverage groups combined, 33% (n = 70) were classified as SLs. Controlling for age, race and gender, there were no differences in SL/SDL status in those who discontinued the study at either three (P = 0.20) or six (P = 0.39) months, and there was no significant interaction between SL status and beverage group in predicting study drop‐out at either time point (P = 0.61 at 3 months; P = 0.78 at 6 months). Baseline demographic characteristics, BMI and energy intake by SL status and beverage group are presented in Table 1. There was no difference in baseline SL status between the two beverage groups. There were no differences in baseline characteristics among the four possible beverage groups and SL tasting patterns, with the exception of age. SLs in the diet beverage group were significantly younger than SDLs in the water group. There were also no differences in total energy intake between SLs and SDLs in either the water or diet group at baseline (P = 0.84), 3 months (P = 0.97), or 6 months (P = 0.74).
Table 1.
Baseline characteristics, body mass index and energy intake of sweet likers and sweet dislikers within each arm
| Water group SLs | Water group SDLs | Diet beverage group SLs | Diet beverage SDLs | Both beverage groups combined | |
|---|---|---|---|---|---|
| n | 33 | 73 | 37 | 66 | 209 |
| Age (mean years ± SD) | 39.7 ± 10.9 | 45.0 ± 10.1 | 39.1 ± 10.1* | 42.4 ± 11.8 | 42.3 ± 11.0 |
| Sex | |||||
| Female | 28 (85%) | 66 (90%) | 31 (84%) | 51 (77%) | 176 (84%) |
| Male | 5 | 7 | 6 | 15 | 33 |
| Race, ethnicity | |||||
| African‐American | 25 (76%) | 40 (55%) | 24 (65%) | 27 (41%) | 116 (56%) |
| Caucasians | 7 | 29 | 12 | 35 | 83 |
| Other | 1 | 4 | 1 | 4 | 10 |
| Education | |||||
| Less than a college degree | 21 (64%) | 32 (44%) | 17 (46%) | 26 (39%) | 96 (46%) |
| College degree or greater | 12 | 41 | 20 | 40 | 113 |
| Mean body mass index (mean kg m−2 ± SD) | 36.5 ± 4.9 | 35.5 ± 5.4 | 36.3 ± 5.8 | 36.1 ± 6.4 | 36.0 ± 5.7 |
| Energy intake from caloric beverages (%kcal) (SD) | 15.4 ± 7.3 | 16.4 ± 8.5 | 17.5 ± 8.4 | 15.9 ± 9.3 | 16.3 ± 8.6 |
Significantly different from Water group non‐Sweet Likers.
SD, standard deviation; SLs, sweet likers; SDLs, sweet dislikers.
The interaction between SL status and beverage intervention group (diet vs. water) demonstrated a trend at 3 months (P = 0.059) but not at 6 months (P = 0.65). At 3 months, adjusted mean intakes of caloric beverages (% energy) were significantly higher in SLs within the water group (9.7 ± 1.4%) compared with SLs in the diet group (5.4 ± 1.0%, P = 0.03) (Figure 1). There were no differences in the adjusted mean intake of caloric beverages among SDLs in the diet and water groups at 3 months.
Figure 1.
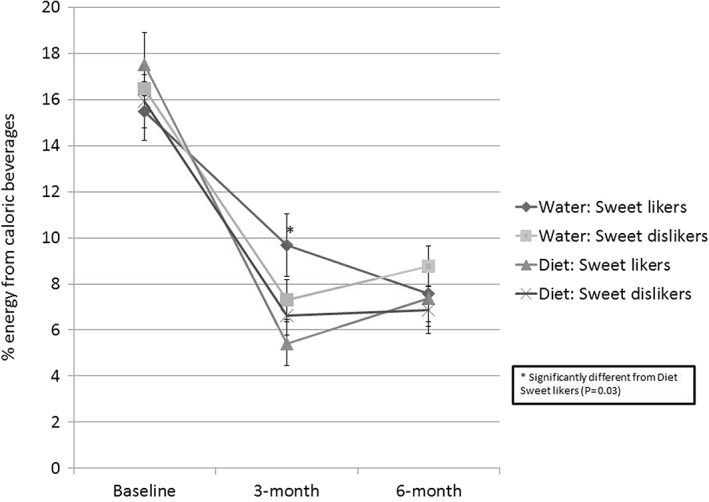
Percent energy from caloric beverages at three‐time points among sweet likers and beverage groups.
Overall, baseline consumption of various beverages (grams of water, unsweetened coffee or tea, diet sodas and energy from fruit drinks and soda) did not differ between SLs and SDLs. Controlling for age, race and gender, the models were not significant for water intake (P = 0.08) or diet soda (P = 0.10), and there were no significant differences in baseline consumption of unsweetened coffee (P = 0.85), unsweetened tea (P = 0.36) or fruit drinks (P = 0.35) between SLs and SDLs.
Because the recommended beverage substitution strategy used in the CHOICE trial involved both reduction of caloric beverages and replacement with non‐caloric beverages, adoption of specific beverage replacement recommendations (replacing with water or diet beverages) was also examined in models controlling for age, race and baseline beverage consumption of the examined beverage. In both the water and diet groups, there were no differences in consumption of the prescribed beverage (e.g. water consumption in the water group) or the non‐prescribed beverage (e.g. water consumption in the diet group) among SLs or SDLs (all P > 0.05).
Discussion
Behavioural weight loss interventions are a successful way to help people achieve clinically meaningful weight loss (i.e. 5% weight loss by 1 year) 21, but large variations in weight loss among participants usually remain unexplained. The present study sought to explore potential reasons for these variations in rates of adoption. In the 6‐month randomized CHOICE beverage reduction trial, participants assigned to beverage replacement groups (water and diet combined) had better weight loss success than the control group 18. The findings of the present study lend support to the hypothesis that being an SL may result in slower adoption of caloric beverage reduction recommendations when water is recommended instead of non‐caloric sweet beverages (diet beverages). These findings were observed at 3 months but not at 6 months, suggesting that SLs in the water group took more time to adjust to caloric beverage reduction recommendations. Furthermore, with the exception of SLs in the water group, all groups and tasting profiles had a reduction in intervention adherence from 3 to 6 months.
Both beverage arms of the intervention recommended the reduction of caloric beverage consumption. This study found that within the two beverage replacements arms, a reduction of caloric beverages was associated with weight loss 18. Other studies have found associations between sugar‐sweetened beverage consumption and weight gain 22, 23 and reduced caloric beverage consumption and weight loss maintenance 24. Our study suggests that reduction of caloric beverages is a potential simple strategy people can use to achieve clinically meaningful weight loss.
Studies have found that SLs are more likely to be consumers of sweet desserts 9, 13 and alcohol 10 than SDLs and feel less control over eating when consuming sweet foods 3. This is one of the first studies to examine caloric beverage intake by SL status. SLs in the water group reduced their caloric beverages less than SDLs in the diet group, suggesting a greater difficulty with discontinuing caloric beverages among participants who had a high sweet preference. This translated, on average, to a difference of 63 more kcal per day from beverages among SLs in the water group compared with SLs in the diet group at 3 months. By 6 months, this interaction was no longer apparent, namely because SLs in the water group eventually reduced their beverage intake to the same levels as the other groups.
Interestingly, although the SLs in the water group had difficulty adopting the caloric beverage reduction recommendation, they were the only group to continue to reduce their beverage intake between 3 and 6 months. SLs in the diet group and SDLs in both the diet and water groups had reduced adherence to the caloric beverage reduction recommendations from months 3 to 6. Reduced adherence to study‐related dietary recommendations – and subsequent weight regain – is common during behavioural weight loss interventions 25, 26. Adding more intensive patient–counsellor contact 25 and increasing the number of group sessions 21 have both been shown to help with added weight loss and maintenance and may be useful strategies for facilitating continued adherence to beverage reduction recommendations. This finding also points to the need for further research examining whether tailoring beverage substitution recommendations or other dietary change recommendations should be based on SL status. For example, SLs might be encouraged to initially adopt non‐caloric sweet beverages or non‐caloric, flavoured waters and SDLs might succeed with initial substitution with water. Overall, the increases in energy from caloric beverages from months 3 to 6 were not large, but the decreases seen in SLs in the diet group and SDLs in the diet and water groups did not continue, demonstrating that longer‐term adherence to recommendations can be difficult regardless of tasting profile.
This study has several strengths. It is among the first to use a sweet preference tasting task and resulting phenotype (SL status) to examine adoption of a specific dietary recommendation for weight loss. The study used a randomized design (by beverage group) and contained a diverse sample. SL status was assessed by a behavioural taste task versus relying on a checklist of foods, which often assess attitudes towards the concept of foods and not the food itself 5. Dietary data were collected using two unannounced 24‐h recalls, which is a reliable method of measuring dietary intake 27. The study also provided beverages to ensure availability and promote consumption.
The current research also has some limitations. Participants were all overweight or obese and were mostly female, so the results may not be applicable to persons with a lower BMI or to males. Participants were also all caloric beverage consumers at baseline, which – although allowing us to intervene on this dietary behaviour – may have resulted in a population different from people who do not consume caloric beverages. Although the overall study recruited a large population, the groups were smaller in this sub‐study, as participants were subdivided into SL status within each beverage arm. This may have limited the power to detect differences. Although several smaller studies have examined differences in SL status by race, to date, there has been no large, nationally representative study conducted to examine prevalence of SL status by race. Lastly, only one behavioural task was used to assess SL status versus adding more objective measures, such as genetic tests.
Adherence to recommendations that are key components of most behavioural weight loss programmes (e.g. attendance at meetings, increased exercise and self‐monitoring of dietary intake) has been found to be a strong predictor of long‐term weight loss success 28. Predicting why some people within the context of a behavioural obesity treatment programme adopt new dietary recommendations while others struggle is an important area of research for continued exploration. Interpersonal differences in tasting preferences may assist with more tailored recommendations that could improve adherence within behavioural weight loss treatment programmes. The present study provides evidence on how tasting preferences at baseline are related to adoption of new dietary recommendations. These findings may help guide how dietary recommendations in behavioural weight loss trials can be customized based on sweet preference.
In conclusion, within the context of a minimal weight loss intervention that solely emphasized the substitution of water or non‐caloric sweetened beverages for caloric beverages, SL status emerged as a potential predictor of rate of intervention adoption when participants were assigned to replace caloric beverages with water. Reduction of caloric beverages was related to weight loss in both beverage groups. A pattern emerged that suggests more research is needed to determine whether SLs would have more success with adherence to a beverage reduction intervention if they replaced caloric beverages with diet (sweet) beverages. There are health benefits to drinking water 29, 30, so SLs may use diet beverages as a small part of their overall beverage intake while still regularly drinking water or using diet beverages as a way to transition to water. Future studies may wish to examine whether tailoring beverage recommendations based on SL status assists with more rapid and lasting adoption. In addition, because reducing dietary simple sugar intake may have an impact on perceived sweet intensity 31, future studies should also examine whether interventions that reduce sweetened beverages can also lead to changes in perceptions of sweet taste intensity.
Disclosure statement
All authors read and approved the final manuscript. The authors have no conflicts of interests to declare with regard to this manuscript. None of the authors has or had any financial or personal relationships with the company or organization sponsoring the research at the time the research was conducted.
Acknowledgements
This study was supported by an investigator‐initiated research grant to the University of North Carolina from Nestlé Waters USA and also by the UNC Interdisciplinary Obesity Center (NIH T32 MH075854). Water was also provided by Nestlé.
Turner‐McGrievy, G. , Wang, X. , Popkin, B. , and Tate, D. F. (2016) Tasting profile affects adoption of caloric beverage reduction in a randomized weight loss intervention. Obesity Science & Practice, 2: 392–398. doi: 10.1002/osp4.64.
References
- 1. Hayes JE, Feeney EL, Allen AL. Do polymorphisms in chemosensory genes matter for human ingestive behavior? Food Quality and Preference 2013; 30: 202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite 2007; 48: 12–9. [DOI] [PubMed] [Google Scholar]
- 3. Kampov‐Polevoy AB, Alterman A, Khalitov E, Garbutt JC. Sweet preference predicts mood altering effect of and impaired control over eating sweet foods. Eat Behav 2006; 7: 181–7. [DOI] [PubMed] [Google Scholar]
- 4. Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 2009; 90: 800S–3S. [DOI] [PubMed] [Google Scholar]
- 5. Drewnowski A. Taste preferences and food intake. Annu Rev Nutr 1997; 17: 237–53. [DOI] [PubMed] [Google Scholar]
- 6. Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr 2008; 28: 367–88. [DOI] [PubMed] [Google Scholar]
- 7. Kampov‐Polevoy AB, Garbutt JC, Davis CE, Janowsky DS. Preference for higher sugar concentrations and tridimensional personality questionnaire scores in alcoholic and nonalcoholic men. Alcohol Clin Exp Res 1998; 22: 610–4. [DOI] [PubMed] [Google Scholar]
- 8. Keskitalo K, Knaapila A, Kallela M, et al. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr 2007; 86: 55–63. [DOI] [PubMed] [Google Scholar]
- 9. Methven L, Xiao C, Cai M, Prescott J. Rejection thresholds (RjT) of sweet likers and dislikers. Food Quality and Preference 2016; 52: 74–80. [Google Scholar]
- 10. Kampov‐Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high‐concentration sucrose solution in alcoholic men. A J Psychiatry 1997; 154: 269–70. [DOI] [PubMed] [Google Scholar]
- 11. Garbutt JC, Osborne M, Gallop R, et al. Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol Alcohol 2009; 44: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krahn D, Grossman J, Henk H, Mussey M, Crosby R, Gosnell B. Sweet intake, sweet‐liking, urges to eat, and weight change: relationship to alcohol dependence and abstinence. Addict Behav 2006; 31: 622–31. [DOI] [PubMed] [Google Scholar]
- 13. Drewnowski A, Henderson SA, Levine A, Hann C. Taste and food preferences as predictors of dietary practices in young women. Public Health Nutr 1999; 2: 513–9. [DOI] [PubMed] [Google Scholar]
- 14. Teixeira PJ, Going SB, Houtkooper LB, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord 2004; 28: 1124–33. [DOI] [PubMed] [Google Scholar]
- 15. Kong W, Langlois M‐F, Kamga‐Ngandé C, Gagnon C, Brown C, Baillargeon J‐P. Predictors of success to weight‐loss intervention program in individuals at high risk for type 2 diabetes. Diabetes Res Clin Pract 2010; 90: 147–53. [DOI] [PubMed] [Google Scholar]
- 16. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005; 293: 43–53. [DOI] [PubMed] [Google Scholar]
- 17. Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav 2008; 95: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tate DF, Turner‐McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012; 95: 555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner‐McGrievy G, Tate D, Moore D, Popkin B. Taking the bitter with the sweet: relationship of supertasting and sweet preference with metabolic syndrome and dietary intake. J Food Sci 2013; 78: S336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diggle PJ, Heagerty P, Liang K‐Y, Zeger SL. Analysis of longitudinal data 2 Ed. Oxford University Press: Oxford: 2002. [Google Scholar]
- 21. Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011; 11: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malik VS, Schulze MB, Hu FB. Intake of sugar‐sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006; 84: 274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta‐analysis. Am J Public Health 2007; 97: 667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phelan S, Wing RR, Loria CM, Kim Y, Lewis CE. Prevalence and predictors of weight‐loss maintenance in a biracial cohort: results from the coronary artery risk development in young adults study. Am J Prev Med 2010; 39: 546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am 2011; 34: 841–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garner DM, Wooley SC. Confronting the failure of behavioral and dietary treatments for obesity. Clin Psychol Rev 1991; 11: 729–80. [Google Scholar]
- 27. Lagerros YT, Mucci LA, Bellocco R, Nyren O, Balter O, Balter KA. Validity and reliability of self‐reported total energy expenditure using a novel instrument. Eur J Epidemiol 2006; 21: 227–36. [DOI] [PubMed] [Google Scholar]
- 28. Wadden TA, Neiberg RH, Wing RR, et al. Four‐year weight losses in the look AHEAD study: factors associated with long‐term success. Obesity 2011; 19: 1987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev 2010; 68: 505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev 2010; 68: 439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wise PM, Nattress L, Flammer LJ, Beauchamp GK. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am J Clin Nutr 2016; 103: 50–60. [DOI] [PubMed] [Google Scholar]


