SUMMARY
Background
Published data regarding temporal trends in vancomycin-resistant enterococci (VRE) prevalence within specific regions or healthcare systems are scarce.
Aim
To characterize temporal trends and risk factors for healthcare-associated infections caused by VRE.
Methods
The study included all adult discharges occurring from 2006 to 2014 with an enterococcal infection from three hospitals in a large academic healthcare system. Bivariate analyses were used to identify statistically significant factors associated with vancomycin-susceptible or -resistant infection. Statistically significant variables were included in a final logistic regression model. Trends assessed whether the proportion of enterococcal infections resistant to vancomycin changed over time.
Findings
The sample included 10,186 adults with first-time healthcare-associated enterococcal infection. Significant risk factors (P ≤ 0.05) for VRE in the final logistic regression model included: tertiary 1 hospital, intensive care unit length of stay, higher Charlson Comorbidity Index, previous immunosuppressive or chemotherapeutic medications, previous hospitalization, renal failure, malignancy, longer length of stay prior to infection, taking an antibiotic prior to infection, being female, and having an infection in winter or spring. Between 2006 and 2014, the rate of resistance varied from 37.1 to 42.9% but there were no significant differences in the proportion resistant to vancomycin over time (P = 0.36).
Conclusion
Research targeted at risk factors is important to decrease the amount of VRE infections.
Keywords: Vancomycin-resistant, enterococci, Risk factors, Temporal trends
Introduction
Healthcare-associated infections (HCAIs) are frequent (4% of hospitalized patients), often preventable, and associated with medical devices: central line-associated bloodstream infection (BSI), surgical site infection (SSI), catheter-associated urinary tract infection (UTI), and ventilator-associated pneumonia.1,2 Enterococci are one causative agent of HCAIs; these bacteria are a component of the faecal flora and may be contracted by direct or indirect by faecal–oral transmission; invasive devices are another important portal of entry.3 Enterococcus faecium accounts for the majority of vancomycin-resistant infections and Enterococcus faecalis constitutes 2–20% of vancomycin-resistant enterococci (VRE) isolates.3,4
Similar to other infectious agents, populations largely affected by VRE include the elderly, those with compromised immune systems, and critically ill patients in the intensive care unit (ICU).5 Other risk factors include prolonged length of hospital stay, previous exposure to vancomycin, anti-cancer chemotherapeutic agents, immunosuppressants and anti-inflammatory drugs, renal insufficiency, malignancies, comorbidities, and surgical procedures.4–6
According to the Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance (NNIS) system, the proportion of HCAIs caused by VRE rose from 0.3% to 7.9% between 1992 and 2004.7 In addition, infections caused by VRE have been associated with higher treatment costs, prolonged morbidity, and greater mortality rates.8 However, information regarding temporal trends in VRE prevalence within specific regions or healthcare systems is scarce. Therefore, the aim of this study was to characterize temporal trends and risk factors for HCAIs caused by VRE during a nine-year period (2006–2014) in a large academic healthcare system in New York City.
Methods
Study population
The study was conducted as part of a federally funded project, ‘Health Information Technology to Reduce Healthcare-Associated Infection’ (National Institute of Nursing Research, National Institutes of Health; R01NR010822). In this project a clinical research database was created of patients hospitalized within three adult acute tertiary care hospitals of the largest healthcare system in New York City. This analysis included all adult (≥18 years) patient discharges from the three hospitals between 2006 and 2014. The hospitals comprised two-tertiary/quaternary care hospitals, designated as hospital 1 and hospital 2 (about 650 and 910 beds), and an approximately 220-bed community hospital designated as hospital 3.
Data collection
The study database was constructed using electronic data from clinical and administrative systems shared between the study institutions.9 The database drew information from numerous sources, including patients’ electronic health record, laboratory and medication administration records, and included admission and discharge data, International Classification of Diseases Ninth Revision Clinical Modification (ICD-9-CM) codes, age, sex, comorbidities, and surgical procedures. A subset of this larger dataset was used, with the primary outcome of interest being the first healthcare-associated (i.e. occurring >2 days after hospital admission) BSI, UTI, or SSI caused by E. faecalis or E. faecium. Infections were identified using a combination of time-stamped microbiology laboratory records and ICD-9-CM procedure and diagnosis codes, based on modified criteria from the CDC National Healthcare Safety Network (NHSN).10
Bloodstream infection was defined as positive E. faecalis or E. faecium blood culture with no positive culture with the same organism at another body site within the previous 14 days. In a previous study we verified that the definition of BSI was 100% congruent with a review of 122 medical records.11 UTI was defined as a positive E. faecalis or E. faecium urine culture with ≥105 colony-forming units (cfu) per mL and no more than one other species of micro-organism, or 103–105 cfu/mL plus pyuria. SSI was defined as positive E. faecalis or E. faecium wound culture within 30 days of an ICD-9-CM-documented National Healthcare Safety Network (NHSN) operative procedure.12 Additionally, individual patient records were linked using their medical record number, and each patient’s first positive VRE infection was used to remove patients with multiple cases of VRE infections. The year and season of each infection (winter, December–February; spring, March–May; summer, June–August; autumn, September–November) were also recorded. Vancomycin susceptibility patterns were obtained from the clinical microbiology reports.
Patients’ demographic characteristics and medical conditions were collected from electronic sources. The institution’s time-stamped electronic medication administration record was used to determine whether patients received antibiotics or other medications that might increase patient risk of infection (chemotherapeutic agents, immunosuppressants, and anti-inflammatory drugs), which we term ‘high-risk medications’ at least 24 h before infection. Comorbidities (diabetes, renal failure, and malignancies) and the Charlson Comorbidity Index were collected.13 Other data collected included patients’ age, sex, length of hospitalization, hospital location, length of stay prior to infection (calculated as the difference between first date of infection and date of admission), ICU stay prior to admission, prior within-network hospitalization, and admission from a nursing facility.
Identification of organisms was done in the study institution’s clinical microbiology laboratory using Clinical and Laboratory Standards Institute standards; additionally, Vitek2 (bioMérieux, Inc., Durham, NC, USA) and/or E-test were used for antibiotic susceptibility testing.14,15
Statistical analysis
The frequency of BSI, UTI, and SSI caused by vancomycin-susceptible and -resistant E. faecalis and E. faecium were recorded by year. Initially, bivariate analyses using χ2-tests for categorical variables or simple logistic regression for continuous variables were used to identify statistically significant (P ≤ 0.05) factors associated with a susceptible or resistant enterococcal infection, including age, sex, hospital, ICU stay prior to infection, Charlson Comorbidity Index, diabetes, renal failure, malignancy, length of hospital stay prior to infection, antibiotic and high-risk medication use prior to infection, season and year (2006–2014), prior stay in a nursing facility, prior in-network hospitalization, infection site (BSI, UTI, or SSI), and medical invasive device. Those variables that were statistically significant in the bivariate analyses were included in a multivariate logistic regression model in a stepwise forward fashion. All analyses were performed using SAS version 9.4. The Cochran–Armitage test for trends was used to assess whether the proportion of E. faecalis and E. faecium infections resistant to vancomycin changed over time.
Results
A total of 10,186 adults with first-time healthcare-associated enterococcal infections were identified from 2006 through 2014: 4094 patients (40.2%) with VRE and 6092 (59.8%) with susceptible strains. Differences between patients with antimicrobial-resistant versus -susceptible enterococcus infections are summarized in Table I. In the final multivariable model (Table II), the significant risk factors of resistance were tertiary 1 hospital vs tertiary 2 (59.4% and 34.1%, respectively; odds ratio: 2.57; 95% confidence interval: 2.30–2.88), having stayed in the ICU (52.1%; 1.54; 1.38–1.71), higher Charlson Comorbidity Index score (1.04; 1.02–1.06), prior high-risk medications (43.5%; 8.19; 5.6–11.26), prior hospitalization (41.9%; 1.52; 1.37–1.68), renal failure (48.9%; 1.75; 1.58–1.94), having a malignancy (52.6%; 1.46; 1.29–1.66), longer length of stay prior to infection (1.03; 1.02–1.03), taking an antibiotic prior to infection (48.3%; 23.72; 17.55–32.07), being female (42.3%; 1.29; 1.17–1.43), and having an infection in winter or spring versus summer or autumn (41.7% and 38.4%, respectively; P = 0.009).
Table I.
Factors associated with HCAI caused by vancomycin-susceptible and -resistant Enterococcus faecalis and Enterococcus faecium in three New York City hospitals, 2006–2014 (univariate analysis)
| Factor | Resistant to vancomycin | P-valuea | |
|---|---|---|---|
|
|
|||
| Yes | No | ||
| Total | 4094 (40.2%) | 6092 (59.8%) | |
| Mean (range) age (years) | 64.7 (0–107) | 65.7 (0–104) | 0.003a |
| Sex | <0.0001a | ||
| Male | 1714 (41.9%) | 2845 (46.7%) | |
| Female | 2380 (58.1%) | 3247 (53.3%) | |
| Hospital | <0.001a | ||
| Community | 286 (7%) | 996 (16.4%) | |
| Tertiary 1 | 1814 (44.3%) | 1238 (20.3%) | |
| Tertiary 2 | 1994 (48.7%) | 3858 (63.3%) | |
| ICU prior to infection | <0.0001a | ||
| Yes | 2029 (49.6%) | 1868 (30.7%) | |
| No | 2065 (50.4%) | 4224 (69.3%) | |
| Mean (range) CCI | 3.37 (0–19) | 2.75 (0–16) | <0.0001a |
| Mean (range) days of stay prior to infection | 15.83 (0–889) | 5.46 (0–348) | <0.0001a |
| High risk medicationb prior to infection | <0.0001a | ||
| Yes | 4043 (98.8%) | 5420 (89%) | |
| No | 51 (1.2%) | 672 (11%) | |
| Prior stay in nursing facility | 0.93 | ||
| Yes | 262 (6.4%) | 387 (6.4%) | |
| No | 3832 (93.6%) | 5705 (96.6%) | |
| Prior in-network hospitalization | <0.0001a | ||
| Yes | 2697 (65.9%) | 3735 (61.3%) | |
| No | 1397 (34.1%) | 2357 (38.7%) | |
| Antibiotic use prior to infection | <0.0001a | ||
| Yes | 4048 (98.9%) | 4328 (71%) | |
| No | 46 (1.1%) | 1794 (29%) | |
| Site of infection | <0.0001a | ||
| Bloodstream | 928 (22.7%) | 1078 (17.7%) | |
| Urinary tract | 2926 (71.5%) | 4356 (71.5%) | |
| SSI | 240 (5.9%) | 658 (10.8%) | |
| Season of infection onset | 0.009a | ||
| Winter | 1084 (26.5%) | 1523 (25%) | |
| Spring | 1186 (29%) | 1647 (27%) | |
| Summer | 944 (23%) | 1497 (24.6%) | |
| Autumn | 880 (21.5%) | 1425 (23.4%) | |
| Diabetes | 0.30 | ||
| Yes | 1220 (29.8%) | 1874 (30.8%) | |
| No | 2868 (70.2%) | 4208 (69.2%) | |
| Renal failure | <0.0001 | ||
| Yes | 2185 (53.5%) | 2286 (37.6%) | |
| No | 1903 (46.5%) | 3796 (62.4%) | |
| Malignancy | <0.0001 | ||
| Yes | 1302 (31.9%) | 1171 (19.3%) | |
| No | 2786 (68.2%) | 4911 (80.8%) | |
| Year of infection onset | 0.009 | ||
| 2006 | 545 (13.3%) | 848 (13.9%) | |
| 2007 | 615 (15%) | 817 (13.4%) | |
| 2008 | 448 (10.9%) | 761 (12.5%) | |
| 2009 | 485 (11.9%) | 823 (13.5%) | |
| 2010 | 500 (12.2%) | 666 (10.9%) | |
| 2011 | 423 (10.3%) | 618 (10.1%) | |
| 2012 | 362 (8.8%) | 537 (8.8%) | |
| 2013 | 383 (9.4%) | 525 (8.6%) | |
| 2014 | 333 (8.1%) | 497 (8.2%) | |
HCAI, healthcare-associated infection; CCI, Charlson Comorbidity Index; SSI, surgical site infection.
Numbers in strata may not equal total due to missing values.
Continuous variables assessed using simple logistic regression (Wald χ2). Categorical variables assessed using Pearson χ2.
High-risk medications include chemotherapeutic agents, immunosuppressants, and anti-inflammatory drugs.
Table II.
Significant risk factors for vancomycin-resistant enterococcus infection (logistic regression analysis)
| Factor | OR | 95% CI |
|---|---|---|
| Hospital | ||
| Tertiary 2 | Reference | Reference |
| Community | 0.63 | 0.54–0.74 |
| Tertiary 1 | 2.58 | 2.30–2.88 |
| ICU stay | 1.54 | 1.38–1.71 |
| Charlson Comorbidity Index | 1.04 | 1.02–1.06 |
| Prior high-risk medication | 8.19 | 5.96–11.26 |
| Prior hospitalization | 1.52 | 1.37–1.68 |
| Renal failure | 1.75 | 1.58–1.94 |
| Maglignancy | 1.46 | 1.29–1.66 |
| Antibiotic use prior to infection | 23.72 | 17.55–32.07 |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.29 | 1.17–1.43 |
| Season | ||
| Summer | Reference | Reference |
| Spring | 1.16 | 1.02–1.33 |
| Autumn | 1.04 | 0.90–1.19 |
| Winter | 1.17 | 1.02–1.34 |
| Length of stay prior to infection | 1.03 | 1.02–1.03 |
OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
Of the total number of first-time enterococcus infections over the study period from 2006 to 2014, the rate of resistance varied from 37.1% to 42.9% (Figure 1). However, there were no significant differences in the proportion resistant to vancomycin over time (two-sided Cochran–Armitage trend: P = 0.36).
Figure 1.
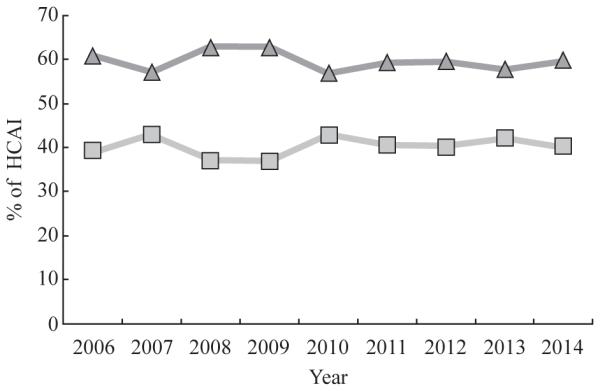
Percentages of Enterococcus faecalis and Enterococcus faecium healthcare-associated infection (HCAI) that were susceptible (triangles) and resistant (squares) to vancomycin per year in three New York City Hospitals, 2006–2014. Pearson χ2-test for independence across years: P = 0.009. Two-sided Cochran–Armitage test for trend: P = 0.36.
Discussion
The overall proportion of VRE HCAI in this study was slightly higher than that reported by CDC for US hospitals during the same time-period (40.2% versus 30%).16 Many of the risk factors identified in this study were consistent with those previously reported, including previous exposure to antibiotics, greater acuity, more comorbidities, prior hospitalizations and longer length of hospital stay.17–24 One study found that prior exposure to vancomycin increased the odds of VRE infection between 2.3- and 11.0-fold.20 Other studies have also reported that antibiotic exposure prior to infection, renal failure, and presence of malignancy were significant risk factors for VRE infection.18–21 Furthermore, prior ICU stay, longer length of stay prior to infection, and prior hospitalization have all been found to be significant risk factors for a VRE infection.23,24 The current literature also supports a previous stay in a nursing facility as a risk factor for VRE infection, but our study found no such association (P = 0.93).25–27 One possible reason for this is that patients acquired the infection at the nursing home, and thus it would have been identified on admission and not considered a hospital-associated infection.28,29
In addition to risk factors previously identified, we also found that rates of resistance were significantly higher in one tertiary care study hospital, even when confounding by other known risks factors was controlled. This was unexpected since both hospitals are part of the same network and share the same infection prevention staff, procedures, and policies. This association might be due to environmental or host factors unaccounted for in these analyses; or, despite similar policies, implementation of infection control practices such as antimicrobial prescribing or barrier precautions may have varied between the two hospitals.30–32 This finding highlights the importance of local surveillance to identify institution- or unit-specific problems and priorities for infection prevention.
Finally, patients hospitalized during winter and spring seasons were significantly more likely to have a resistant infection when compared to those admitted during summer and autumn. This could be the result of the increase in patient volume in the colder months or, as noted by Nelson, there may be changes in the immune system associated with seasonality.33
The results highlight that there was no significant reduction in the proportion of enterococcal infections that were vancomycin resistant. One contributing factor could be the ability of VRE to persist on surfaces for long periods of time.34–36 For example, Huang and colleagues found the odds of acquiring VRE when staying in a room where a previous VRE-positive patient stayed were 1.4-fold greater than the odds of acquiring VRE when a VRE-negative patient had stayed in that room.37 A second contributing factor may be related to antibiotic prescribing patterns of clinicians and the excessive or unnecessary use of broad-spectrum agents such as vancomycin, emphasizing the importance of antimicrobial stewardship programmes. Results of such programmes would not be expected to be immediate, and would likely take some years to have an impact.
In addition to confirming previously identified risk factors for VRE, the results of this study highlight the importance of identifying local infection prevention practices and patterns of resistance, since, even within a single healthcare system, our findings varied significantly across hospitals. Furthermore, this study emphasizes the need for high-level surveillance in which microbiology results, clinical information, and admission details are gathered into a single patient record across hospitals for ongoing accurate surveillance of risk factors for resistance. This is supported by the problem of emerging antibiotic resistance that has been difficult to combat, which supports the importance of moving into patient information systems that collect these data items in an integrated manner. Finally, it is clear that the proportion of infections resistant to vancomycin is not significantly different, emphasizing the importance of antimicrobial stewardship programmes.
Acknowledgments
Funding sources
None.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. New Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Type of Healthcare-Associated Infections j HAI j CDC. 2015 Available at: http://www.cdc.gov/HAI/infectionTypes.html [last accessed November 2015]
- 3.Handwerger S, Raucher B, Altarac D, et al. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 4.Prasad K, Tripathi A, Shukla S, Singh A. Prevalence, outcome and risk factor associated with vancomycin-resistant Enterococcus faecalis and Enterococcus faecium at a tertiary care hospital in northern India. Indian J Med Microbiol. 2016;34:38–45. doi: 10.4103/0255-0857.174099. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention CDC – VRE in Healthcare Settings – HAI. 2015 Available at: http://www.cdc.gov/HAI/organisms/vre/vre.html [last accessed November 2015]
- 6.Boyce JM. Vancomycin-resistant enterococcus: detection, epidemiology, and control measures. Infect Dis Clin North Am. 1997;11:367–384. doi: 10.1016/s0891-5520(05)70361-5. [DOI] [PubMed] [Google Scholar]
- 7.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 8.Calfee D, Giannetta E, Durbin L, Germanson T, Farr B. Control of endemic vancomycin-resistant enterococcus among inpatients at a university hospital. Clin Infect Dis. 2003;37:326–332. doi: 10.1086/376624. [DOI] [PubMed] [Google Scholar]
- 9.Apte M, Neidell M, Furuya EY, Caplan D, Larson E. Using electronically available inpatient hospital data for research. Clin Transl Sci. 2011;4:338–345. doi: 10.1111/j.1752-8062.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Ippolito P, Larson EL, Furuya EY, Liu J, Seres DS. Utility of electronic medical records to assess the relationship between parenteral nutrition and central line-associated bloodstream infections in adult hospitalized patients. J Parenter Enteral Nutr. 2014;39:929–934. doi: 10.1177/0148607114536580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Healthcare Safety Network . Surgical Site Infection Surveillance (SSI) Center for Disease Control and Prevention; 2013. Available at: http://apic.org/Resource_/TinyMceFileManager/Academy/ASC_101_resources/Surveillance_NHSN/ASCA_NHSN_SSI_Surveillance_2013.pdf [last accessed April 2016] [Google Scholar]
- 13.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute CLSI Publishes New Antimicrobial Susceptibility Testing Standards. Available at: http://clsi.org/blog/2015/01/08/clsi-publishes-new-antimicrobial-susceptibilitytesting-standards/ [last accessed August 2016]
- 15.bioMérieux Vitek 2 Healthcare. http://www.biomerieux-usa.com/clinical/vitek-2-healthcare [last accessed August 2016]
- 16.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2013. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 17.Byers KE, Anglim AM, Anneski CJ, et al. A hospital epidemic of vancomycin-resistant enterococcus: risk factors and control. Infect Control Hosp Epidemiol. 2001;22:140–147. doi: 10.1086/501880. [DOI] [PubMed] [Google Scholar]
- 18.Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, caseecontrol study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis. 2000;36:145–158. doi: 10.1016/s0732-8893(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 19.Lautenbach E, Bilker WB, Brennan PJ. Enterococcal bacteremia: risk factors for vancomycin resistance and predictors of mortality. Infect Control Hosp Epidemiol. 1999;20:318–323. doi: 10.1086/501624. [DOI] [PubMed] [Google Scholar]
- 20.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, Gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136:834–844. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- 21.Shay DK, Maloney SA, Montecalvo M, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 22.McGregor JC, Kim PW, Perencevich EN, et al. Utility of the chronic disease score and Charlson comorbidity index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol. 2005;161:483–493. doi: 10.1093/aje/kwi068. [DOI] [PubMed] [Google Scholar]
- 23.Tornieporth NG, Roberts RB, John J, Hafner A, Riley LW. Risk factors associated with vancomycin-resistant Enterococcus faecium infection or colonization in 145 matched case patients and control patients. Clin Infect Dis. 1996;23:767–772. doi: 10.1093/clinids/23.4.767. [DOI] [PubMed] [Google Scholar]
- 24.Carmeli Y, Eliopoulos G, Samore M. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant enterococcus. Emerg Infect Dis. 2002;8:802–807. doi: 10.3201/eid0808.010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren DK, Nitin A, Hill C, Fraser VJ, Kollef MH. Occurrence of co-colonization or co-infection with vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol. 2004;25:99–104. doi: 10.1086/502357. [DOI] [PubMed] [Google Scholar]
- 26.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 27.Tacconelli E, Cataldo MA. Vancomycin-resistant enterococci (VRE): transmission and control. Int J Antimicrob Agents. 2008;31:99–106. doi: 10.1016/j.ijantimicag.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Fisch J, Lansing B, Wang L, et al. New acquisition of antibiotic-resistant organisms in skilled nursing facilities. J Clin Microbiol. 2012;50:1698–1703. doi: 10.1128/JCM.06469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strausbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. Antimicrobial resistance in long-term-care facilities. Infect Control Hosp Epidemiol. 1996;17:129–140. doi: 10.1086/647257. [DOI] [PubMed] [Google Scholar]
- 30.Siegel JD, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. 2007 doi: 10.1016/j.ajic.2007.10.007. Available at: http://www.cdc.gov/ hicpac/pdf/isolation/Isolation2007.pdf [last accessed September 2016] [DOI] [PMC free article] [PubMed]
- 31.Weinstein RA, Hayden MK. Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin Infect Dis. 2000;31:1058–1065. doi: 10.1086/318126. [DOI] [PubMed] [Google Scholar]
- 32.Goodman ER, Piatt R, Bass R, Onderdonk AB, Yokoe DS, Huang SS. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol. 2008;29:593–599. doi: 10.1086/588566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Bonilla HF, Zervos MJ, Kauffman CA. Long-term survival of vancomycin-resistant Enterococcus faecium on a contaminated surface. Infect Control Hosp Epidemiol. 1996;17:770–771. doi: 10.1086/647230. [DOI] [PubMed] [Google Scholar]
- 35.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:1–8. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martone WJ. Spread of vancomycin-resistant enterococci: why did it happen in the United States? Infect Control Hosp Epidemiol. 1998;19:539–545. doi: 10.1086/647870. [DOI] [PubMed] [Google Scholar]
- 37.Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Archs Intern Med. 2006;166:1945–1951. doi: 10.1001/archinte.166.18.1945. [DOI] [PubMed] [Google Scholar]


