Abstract
Background
Preoperative/neoadjuvant therapy (NT) is increasingly utilized for the treatment of pancreas cancer (PDAC). However, little data exist regarding to inform the use of additional postoperative therapy following NT. The lymph node ratio (LNR) is a prognostic marker of oncologic outcomes after NT and resection. In this study, we evaluated the effectiveness of postoperative therapy following NT, stratified by LNR.
Methods
A prospective tumor registry database was queried to identify patients with PDAC who underwent resection following NT from 1990–2008. Clinicopathologic factors were compared to identify associations with overall survival (OS) and time to recurrence (TTR) based on postoperative chemotherapy status.
Results
Thirty-six (14%) of the 263 patients received additional postoperative therapy. There were no differences in the pathologic characteristics between patients that received postoperative chemotherapy and those who did not. The median LNR was 0.12 for patients with N+ disease. Following NT, the administration of postoperative therapy was associated with improved median OS (72 vs. 33 months; p=0.008) for patients with a LNR <0.15. There was no association between postoperative chemotherapy and OS for patients with LNR ≥ 0.15. Multivariate analysis demonstrated that the administration of postoperative systemic therapy in patients with a low LNR was associated with a reduced risk of death (HR 0.49; p=0.02).
Conclusion
Postoperative chemotherapy after NT in patients with low LNR is associated with improved oncologic outcomes.
INTRODUCTION
It is well recognized that completion of multimodality therapy is associated with improved oncologic outcomes compared to surgery alone in the treatment of pancreatic ductal adenocarcinoma (PDAC)1–4. However, the timing of chemotherapy and radiation in relationship to surgical resection is still widely debated. Historically, the treatment of PDAC has included a surgery-first approach followed by post-operative adjuvant therapy. Barriers to the completion of multimodality therapy completion include early cancer progression and postoperative major complications1. Administration of chemotherapy and/or radiation therapy prior to surgical resection (neoadjuvant therapy, NT) has been suggested as an alternative to overcome some of these issues5,6. Theoretical benefits of NT include also early treatment of micrometastatic disease, selection of patients likely to benefit from surgical resection, and a potential for tumor downstaging.
Following upfront surgical resection, the administration of adjuvant therapy (chemotherapy and/or radiation) for PDAC is associated with improved oncologic outcomes2,3,7. Current National Comprehensive Cancer Network (NCCN) guidelines recommend NT for patients with borderline-resectable tumors8 with consideration of additional systemic chemotherapy at the discretion of the practitioner. For patients who received NT, data and objective criteria supporting additional therapy after surgery are lacking.
Metastatic disease in peri-pancreatic lymph nodes remains one of the strongest prognostic factors for survival following surgical resection8. The lymph node ratio (LNR), defined as the number of lymph nodes with metastatic disease among the total number of lymph nodes retrieved, has been validated in patients with PDAC9–14. Recently, we showed that the LNR is a useful prognostic indicator in patients with potentially resectable PDAC treated with NT13. Interestingly, following NT, patients with a LNR=0 and LNR 0.01–0.14 were found to have equivalent overall survival (OS) and time to recurrence (TTR), whereas patients with a persistently elevated LNR following NT had significantly reduced OS and shorter time to recurrence13.
In this study, we sought to determine the impact of postoperative therapy in patients with PDAC following the administration of NT and surgical resection. To test this hypothesis, we critically examined the associations between LNR, postoperative chemotherapy use, recurrence and overall survival (OS) in patients with PDAC who underwent potentially curative resection following NT.
METHODS
This retrospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. We retrieved clinical data on all patients who underwent preoperative therapy followed by surgical resection for PDAC between 1990–2008 from our prospectively maintained institutional pancreatic tumor database15. We excluded from analysis patients with a final diagnosis of invasive adenocarcinoma arising in an intraductal papillary mucinous neoplasm or mucinous cystadenocarcinoma, patients with other non-pancreatic periampullary adenocarcinoma (n=12), patients who had chemotherapy or radiation prior to presentation at our institution (n=35), and patients who received intra-operative radiation (IORT, n=109).
Treatment Sequencing and Therapy
Prior to the initiation of NT, patients underwent a comprehensive staging evaluation including physical exam, cross-sectional imaging and measurement of CA 19–9. Patients received external-beam radiation (typically 30 or 50.4 Gy) with the chemoradiation regimens shown in Table 1. Systemic chemotherapy was delivered prior to chemoradiation in selected cases or as part of a clinical trial 5 (Table 1). All patients underwent restaging evaluation after completion of preoperative therapy. Criteria for surgical resection following completion of NT included performance status sufficient for major abdominal surgery, optimized comorbidities, and no radiographic or intraoperative detection of tumor progression 16. Pancreaticoduodenectomy was performed using a standardized technique 17,18. The decision to administer adjuvant therapy was influenced by the presence of high-risk factors, enrollment in a clinical trial, or perceived benefit of adjuvant therapy. Patients were staged according to the MD Anderson definitions of resectability 19.
Table 1.
Clinicopathologic characteristics of patients with pancreatic adenocarcinoma treated with preoperative therapy by post-operative chemotherapy status#.
| Clinical factor | Entire Cohort | + post-operative therapy | − post-operative therapy | p* |
|---|---|---|---|---|
| N | 263 | 36 | 227 | |
| Gender, n (%) | 0.11 | |||
| Male | 142 (54) | 15 (42) | 127 (56) | |
| Female | 121 (46) | 21 (58) | 100 (44) | |
| Age, median (range), y | 64 (35–85) | 67 (35–85) | 64 (39–85) | 0.59 |
| Anatomic Classification, n (%) | 0.001 | |||
| Potentially Resectable | 218 (82) | 22 (62) | 196 (87) | |
| Borderline | 41 (16) | 13 (35) | 28 (12) | |
| Locally Advanced | 4 (2) | 1 (3) | 3 (1) | |
| Re-operative, n (%) | ||||
| Pre-referral exploration | 13 (5) | 1 (3) | 12 (5) | 1 |
| Pre-referral bypass | 25 (9) | 3 (8) | 22 (10) | 1 |
| Preoperative Therapy, n (% positive) | ||||
| Chemoradiation, n (% positive) | 263 (100) | 36 (100) | 227 (100) | 0.004 |
| Gemcitabine, n (%) | 160 (60) | 22 (60) | 138 (61) | |
| 5-FU | 48 (18) | 0 | 48 (21) | |
| Capecitabine, n (%) | 41 (16) | 13 (37) | 28 (12) | |
| Other | 14 (6) | 1(3) | 13 (6) | |
| Chemotherapy, n (% positive) | 128 (49) | 22 (59) | 106 (48) | 0.11 |
| Gemcitabine/Cisplatin, n (%) | 93 (72) | 16 (70) | 77 (73) | |
| Gemcitabine, n (%) | 10 (8) | 2 (10) | 8 (7) | |
| Capecitabine or Gemcitabine + bevacizumab, n (%) | 10 (8) | 2 (10) | 8 (7) | |
| Gem/Cis/erlotinib | 5 (4) | 0 | 5 (5) | |
| Other | 10 (8) | 2 (10) | 8 (8) | |
| Postoperative Therapy, n (% positive) | N/A | |||
| Chemotherapy, n (% positive) | 36 (14) | 36 (14) | N/A | |
| Gemcitabine, n (%) | 16 (44) | 16 (44) | N/A | |
| Gemcitabine/Cisplatin, n (%) | 6 (16) | 6 (16) | N/A | |
| Gem/erlotinib | 6 (16) | 6 (16) | N/A | |
| Gem/bevacizumab, n (%) | 3 (8) | 3 (8) | N/A | |
| Other | 6 (16) | 6 (16) | N/A | |
| Lymph node status, n (%) | 0.82 | |||
| N0 | 134 (51) | 19 (53) | 115 (51) | |
| N1 | 129 (49) | 17 (47) | 112 (49) | |
| Perineural invasion, n (%) | 0.31 | |||
| Negative | 50 (22) | 10 (27) | 40 (33) | |
| Positive | 177 (78) | 25 (73) | 152 (67) | |
| Unavailable | 36 (--) | 0 | 36 (--) | |
| Median number of lymph nodes examined, n (range) | 19 (2–50) | 20 (3–41) | 18 (2–50) | 0.16 |
| Number of Positive Lymph Nodes, median (range) | 0 (1–20) | 0 (0–2) | 0 (0–20) | 0.79 |
| Lymph Node Ratio, All; median (range) | 0 (0–0.54) | 0 (0–0.54) | 0.03 (0–0.53) | 0.36 |
| Lymph Node Ratio, N1; median (range) | 0.12 (0.02–0.54) | 0.13 (0.03–0.54) | 0.13 (0.02–0.53) | 0.89 |
CA19-9, lymphovascular invasion, margin status, grade, resection type, blood loss and vascular resection evaluated and not significantly different between the groups.
+ post-operative chemotherapy vs. -post-operative chemotherapy
Follow-up
Following resection, patients were evaluated every 3–4 months using a standard surveillance protocol, including physical examination; chest radiography and abdominal CT. Intervals were extended to 6 months after 2 years. The development of a new low-density mass or abdominal lymphadenopathy in the region of the resected pancreas or mesenteric root was considered locoregional recurrence in this area. Radiographic evidence of a low-density mass in the liver or lungs or new-onset ascites was considered evidence for distant recurrence. Biopsy of recurrence was rarely performed. Only the first site(s) of recurrent disease was documented for this study.
Statistical Analysis
Categorical variables were compared using χ2 or Fisher’s exact test. Wilcoxon’s rank sum test (t-test) and the Kruskal-Wallis test (ANOVA) were used to compare the distributions of continuous variable among different groups. Time to recurrence (TTR) is defined as from the time of surgery to the time of first locoregional or distant recurrence or to the time of last contact. Overall survival (OS) was defined as from the time of diagnosis to the time of death or to the time of last contact. The distributions of TTR and OS were estimated by the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazards regression model, in which a backward elimination process was used for variable selection with an entry and removal limit of P<0.1 and P<0.05, respectively, was applied to identify independent factors associated with OS and TTR. All tests are two sided. P-values less than 0.05 were considered statistically significant. All analyses are conducted using SAS (version 9.1, Cary, NC) software and S-Plus (Insightful Corp., Seattle, WA).
RESULTS
Demographic and Pathologic Characteristics
A total of 263 patients with PDAC who underwent preoperative chemoradiation met inclusion criteria and were included in the study. Demographic and treatment characteristics are shown in Table 1. Based on MDACC definition of resectability19, 218 patients (82%) had potentially resectable disease and 16% had anatomically borderline resectable disease at presentation. A median of 19 lymph nodes were examined per patient with 134 patients (51%) having ypN0 disease after NT. Of the patients that had N1 disease, the median LNR was 0.12 and was similar between patients that did and did not receive postoperative therapy.
Systemic Chemotherapy and Cancer-Related Outcomes
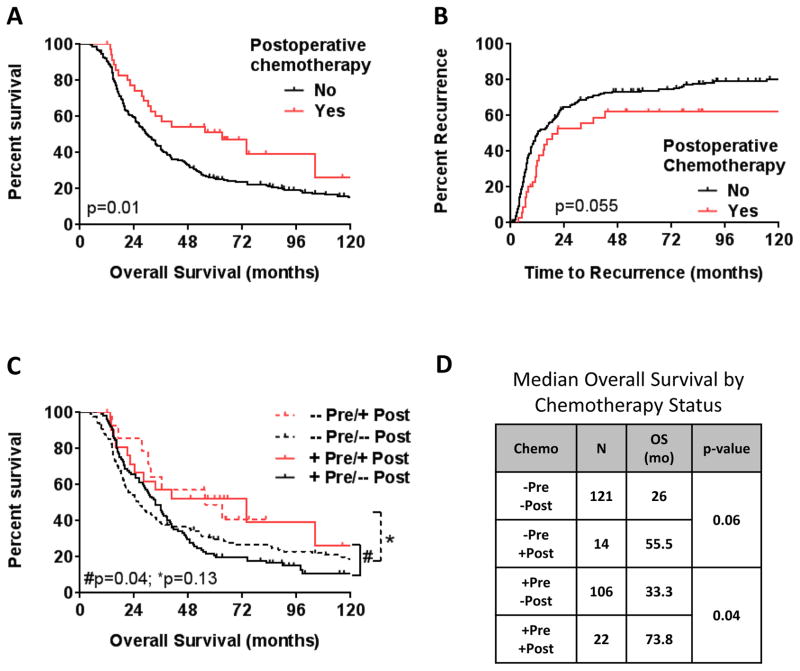
At a median follow-up of 31 months (115 months for survivors), median overall survival (OS) was 31 months and median time to recurrence (TTR) was 13 months (data not shown). Of the 263 patients in the study cohort, 36 (14%) underwent additional postoperative systemic therapy, with the majority receiving gemcitabine-based therapy (Table 1). For the entire cohort, administration of postoperative chemotherapy was associated with improved median OS compared to no postoperative therapy (63 vs. 30 months, respectively; p=0.01; Fig. 1A). In addition, by univariate analysis, postoperative chemotherapy was marginally significantly associated with TTR compared to no postoperative therapy (21 vs. 12 months, respectively; p=0.055; Fig. 1B).
Figure 1.
Kaplan-Meier estimate of (A) overall survival and (B) time to recurrence for patients who underwent preoperative therapy stratified by post-operative chemotherapy status. (C) Stratification by preoperative (pre) and postoperative (post) systemic chemotherapy status. (D) Median overall survival estimates (months) by systemic chemotherapy status.
49% (n=128) of patients received preoperative systemic chemotherapy prior to the initiation of preoperative chemoradiation. To further evaluate the association of postoperative chemotherapy with survival, patients were further stratified based on preoperative and postoperative chemotherapy administration (Fig. 1C & D). For patients who received postoperative therapy (Fig. 1C; red lines), there was no significant difference in survival with the addition of preoperative therapy (+Pre/+Post) compared to postoperative therapy alone (−Pre/+Post). However, for patients who received preoperative chemotherapy (Fig. 1C; solid lines), the administration of additional of postoperative therapy (+Pre/+Post) was associated with improved OS compared to preoperative chemotherapy alone (+Pre/−Post; p=0.04).
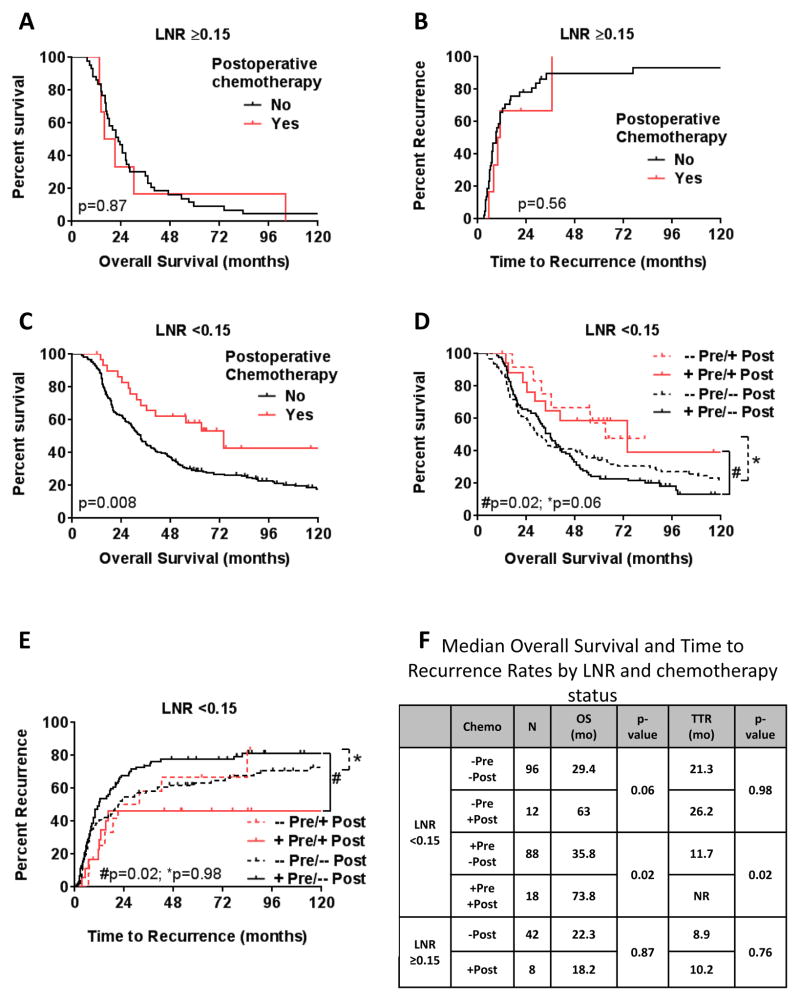
To further evaluate the benefit of postoperative systemic therapy following NT, survival outcomes were evaluated based on lymph node status. Previously, our group has demonstrated differences in oncologic outcomes following NT based on post-therapy LNR13,20. Therefore, we divided patients into 2 groups using our the previously-established LNR cutoff of 0.1513. Patients with a high LN burden following preoperative therapy (LNR ≥0.15) had poor OS and TTR, regardless of the administration of additional postoperative systemic therapy (Fig. 2A, B & F). In patients with a low-LNR (0–0.14), administration of postoperative systemic therapy was associated with improved OS compared to patients who did not receive postoperative systemic therapy (72 months vs. 33 months, respectively, p=0.008; Fig. 2C). Further, patients with N0 disease that received additional postoperative chemotherapy following NT had median OS of 73 months compared to 34 months for those that did not (p=0.09; data not shown).
Figure 2.
Kaplan-Meier estimate of (A, C) overall survival and (B, D) time to recurrence for patients who underwent preoperative therapy with a LNR ≥ 0.15 (A & B) and a LNR <0.15 (C & D) by postoperative chemotherapy status. (E) Stratification of patients with LNR <0.15 by preoperative (pre) and postoperative (post) systemic chemotherapy status. (F) Median overall survival estimates (months) by systemic chemotherapy status and LNR.
Patients with a LNR <0.15 were then further stratified based on preoperative chemotherapy status. For patients that received preoperative chemotherapy (Fig. 2D, E & F; solid lines), the administration of additional of postoperative therapy (+Pre/+Post) was associated with improved OS and TTR compared to preoperative chemotherapy alone (+Pre/−Post; p=0.02).
Univariate and multivariate analyses were performed to identify factors independently associated with OS. On multivariate analysis, pre-referral laparotomy and a high LNR were associated with reduced OS (Table 2). The addition of postoperative chemotherapy following NT was associated with reduced risk of death (HR 0.61, CI 0.37–1; p=0.017) (Table 2). Further stratification of patients by LNR (Table 3) demonstrated that administration of postoperative chemotherapy was associated with a reduced risk of death (HR 0.48, 95% CI 0.28–0.82; p=0.007) in patients with a reduced LNR, compared to patients who did not receive additional postoperative systemic chemotherapy. There was no observed benefit of additional postoperative chemotherapy following NT in patients with a high LNR.
Table 2.
Multivariate Analysis of Overall Survival after Preoperative Chemoradiation*
| Parameter | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|
| Pre-referral laparotomy | Negative | Ref. | 0.019 |
| Positive | 2.04 (1.13–3.69) | ||
| Postop chemotherapy | Negative | Ref. | 0.017 |
| Positive | 0.61 (0.37–1) | ||
| LNR | 0 | Ref. | - |
| 0.01–0.14 | 0.97 (0.69–1.33) | 0.45 | |
| ≥ 0.15 | 1.87 (1.32–2.67) | 0.002 |
Included in multivariate model but not significant: anatomic classification, CA19-9, perineural invasion
Table 3.
Multivariate Analysis of Overall Survival after Preoperative Chemoradiation by LNR.
| Parameter | Median (months) | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| <0.15 | −Postop | 32.8 | Ref. | 0.007 |
| +Postop | 72.1 | 0.48 (0.28–0.82) | ||
| ≥ 0.15 | −Postop | 22.3 | Ref. | 0.79 |
| +Postop | 18.2 | 1.15 (0.47–2.64) |
DISCUSSION
There is increased use of preoperative therapy for the treatment of PDAC. However, data supporting additional postoperative systemic therapy following surgical resection are lacking. In the current study, we found that the administration of additional postoperative systemic chemotherapy following NT was associated with improved overall survival and time to recurrence in patients with limited LN involvement (LNR <0.15). Interestingly, patients with significant LN burden following NT did not appear to benefit from the administration of additional systemic therapy following preoperative chemoradiation.
The use of preoperative treatment sequencing strategies is associated with several potential advantages. Patients treated with NT have reduced non-therapeutic laparotomy rates, increased margin-negative resection rates, improved multimodality completion rates, and sterilization of peri-pancreatic lymph nodes1,2,13,21,22. Current NCCN guidelines support the use of NT for patients with borderline resectable PDAC and recognize it as an alternative for patients with potentially resectable disease, especially for cases in which there is a high likelihood of a margin-positive resection 8. Our preferred treatment approach for patients with PDAC has been a NT approach, when feasible. Forty-nine percent of patients received preoperative chemotherapy prior to chemoradiation. Interestingly, in the current study, the addition of preoperative chemotherapy to preoperative chemoradiation was not associated with changes in survival or recurrence, which is consistent with previous studies5.
The use of postoperative systemic chemotherapy following surgical resection of PDAC is associated with improved survival and has been demonstrated in several randomized trials7,23,24. However, the use of postoperative chemotherapy following NT has not been well described. In this study, the addition of postoperative systemic therapy following NT was associated with significantly improved OS (72 months) compared to patients that did not receive postoperative systemic therapy (OS: 33 months). Although there was significant variability in the type of chemotherapy administered, the majority of patients received a gemcitabine-based regimen. However, when patients were further stratified based on amount of LN involvement, the addition of postoperative chemotherapy was associated with improved outcomes only in patients who had a low LNR (<0.15). Patients with a persistently high LNR (≥0.15) following NT had poor survival, despite additional systemic therapy. Although this may appear counter-intuitive, it is likely that patients who had a persistently elevated LNR after NT represented a cohort of patients with unfavorable tumor biology, for whom standard cytotoxic systemic therapy of the study period was ineffective. This is not to suggest that these patients would not benefit from further treatment. Rather, we would suggest that this group of patients should be considered for clinical trials, as they have demonstrated that their tumors are insensitive to the conventional chemotherapy of the study period.
These data should be evaluated in the context of their limitations. First, this was a non-randomized, retrospective cohort study of selected patients treated at a single institution and the number of patients that received postoperative chemotherapy was small. However, to our knowledge, this is the first report on patients treated with postoperative systemic chemotherapy following NT. Although retrospective and single-institution, we believe the data presented in this manuscript provides insight into an, as yet unanswered, clinical decision-making dilemma. Guidelines are present as evidence to the lack of level 1 data. Current NCCN guidelines recommend preoperative therapy for patients with borderline resectable tumors, but specifically comment that data are lacking regarding the use of additional therapy postoperatively. The data presented in the current manuscript explores this question and provides background for a more formal evaluation of this clinically important question. An alternative explanation for our results is that patients who were able to receive postoperative therapy had more favorable tumor biology and less surgical complications, which is why they were able to receive additional therapy. Additionally, it is possible that there was a clustering of postoperative therapy based on the treating medical oncologist. However, there were 9 medical oncologists involved in the administration of additional postoperative therapy in the 36 patients that received therapy, with a range of 1 to 12 patients per physician. Finally, although the results in this study are encouraging, these patients represent a heterogeneous patient population, with variability in the chemoradiation and chemotherapy regimens administered, highlighting the importance of clinical trials to answer these important questions.
In conclusion, our data demonstrate that the administration of additional postoperative systemic chemotherapy following NT is associated with improved oncologic outcomes. This benefit is greatest in patients with low-volume LN disease following preoperative therapy, likely indicating favorable tumor biology. LNR should be used as stratification variable or inclusion criteria in clinical trials evaluating novel adjuvant systemic therapies.
Synopsis.
Little data exist to inform the use of postoperative chemotherapy following preoperative therapy for pancreas cancer. In this study, the administration of postoperative therapy was associated with improved survival for patients with a lymph node ratio < 0.15.
Acknowledgments
Financial Support: This research has been supported by the Khalifa Bin Zayed Al Nahyan Foundation and the Various Donor Pancreatic Cancer Research Fund at the University of Texas MD Anderson Cancer Center, the Ruth L. Kirschstein National Research Service Award NIH T32 CA009599 and the NIH/NCI Cancer Center Support Grant P30CA016672. We thank Mano Sundar for her management of our departmental pancreatic cancer database.
Abbreviations
- LNR
lymph node ratio
- NT
preoperative/neoadjuvant therapy
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
This work was presented as a poster presentation at the American College of Surgeons Clinical Congress in Chicago, IL, October 2012.
References
- 1.Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2014 Jan;18(1):16–24. doi: 10.1007/s11605-013-2412-1. discussion 24–15. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Annals of surgical oncology. 2009 Apr;16(4):836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S. : utilization, outcomes, and the effect of hospital volume. Cancer. 2007 Sep 15;110(6):1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 4.Colbert LE, Hall WA, Nickleach D, et al. Chemoradiation therapy sequencing for resected pancreatic adenocarcinoma in the National Cancer Data Base. Cancer. 2014 Feb 15;120(4):499–506. doi: 10.1002/cncr.28530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jul 20;26(21):3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 6.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jul 20;26(21):3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. Jama. 2013 Oct 9;310(14):1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines) 2014. Pancreatic adenocarcinoma Version 2.2104. [Google Scholar]
- 9.Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, Hall RI. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J Surg. 2010 Apr;34(4):768–775. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]
- 10.La Torre M, Cavallini M, Ramacciato G, et al. Role of the lymph node ratio in pancreatic ductal adenocarcinoma. Impact on patient stratification and prognosis. J Surg Oncol. 2011 Nov 1;104(6):629–633. doi: 10.1002/jso.22013. [DOI] [PubMed] [Google Scholar]
- 11.Murakami Y, Uemura K, Sudo T, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. Journal of the American College of Surgeons. 2010 Aug;211(2):196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007 May;141(5):610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Roland CL, Yang AD, Katz MHG, et al. Neoadjuvant Therapy is Associated with a Reduced Lymph Node Ratio in Patients with Potentially Resectable Pancreatic Cancer. Annals of Surgical Oncology. 2014 Oct 29; doi: 10.1245/s10434-014-4192-6. Epub. [DOI] [PMC free article] [PubMed]
- 14.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Annals of surgical oncology. 2008 Jan;15(1):165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 15.Hwang RF, Wang H, Lara A, et al. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Annals of surgical oncology. 2008 May;15(5):1356–1366. doi: 10.1245/s10434-008-9833-1. [DOI] [PubMed] [Google Scholar]
- 16.Tzeng CW, Fleming JB, Lee JE, et al. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol. 2012 Jun;19(6):2045–2053. doi: 10.1245/s10434-011-2211-4. [DOI] [PubMed] [Google Scholar]
- 17.Yen TW, Abdalla EK, Pisters PW, Evans DB. Pancreaticoduodenectomy. Sandury: Jones and Bartlett; 2005. [Google Scholar]
- 18.Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012 Jan;16(1):68–78. doi: 10.1007/s11605-011-1748-7. discussion 78–69. [DOI] [PubMed] [Google Scholar]
- 19.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. Journal of the American College of Surgeons. 2008 May;206(5):833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012 Jan 1;118(1):268–277. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 21.Pingpank JF, Hoffman JP, Ross EA, et al. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2001 Mar-Apr;5(2):121–130. doi: 10.1016/s1091-255x(01)80023-8. [DOI] [PubMed] [Google Scholar]
- 22.White RR, Hurwitz HI, Morse MA, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol. 2001 Dec;8(10):758–765. doi: 10.1007/s10434-001-0758-1. [DOI] [PubMed] [Google Scholar]
- 23.Ghaneh P, Neoptolemos JP. Conclusions from the European Study Group for Pancreatic Cancer adjuvant trial of chemoradiotherapy and chemotherapy for pancreatic cancer. Surgical oncology clinics of North America. 2004 Oct;13(4):567–587. vii–viii. doi: 10.1016/j.soc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. The New England journal of medicine. 2004 Mar 18;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]