Abstract
Co-existing disordered and ordered (raft) membrane domains exist in Borrelia burgdorferi, the causative agent of Lyme disease. However, although B. burgdorferi contains cholesterol lipids, it lacks sphingolipids—a crucial component of rafts in eukaryotes. To define the principles of ordered lipid domain formation in Borrelia, the domain forming properties of vesicles composed of its three major lipids, acylated cholesteryl galactoside (ACGal), monogalactosyl diacyglycerol (MGalD), and phosphatidylcholine (PC) and/or their mixtures were studied. Anisotropy and fluorescence resonance energy transfer measurements were used to assay membrane order and ordered-domain formation. ACGal had the highest potential to form ordered domains. Interestingly, mixtures of ACGal with B. burgdorferi PC formed ordered domains more readily than mixtures of ACGal with MGalD. This appears to reflect the relatively high level of saturation observed for B. burgdorferi PC, as vesicles containing ACGal and PC, but in which the unsaturated lipid dioleoyl PC was substituted for Borrelia PC, failed to form ordered domains. In addition, the properties of ACGal were compared to those of cholesterol. Depending on what other lipids were present, ordered-domain formation in the presence of ACGal was greater than or equal to that in the presence of cholesterol. Giant unilamellar vesicles formed from ACGal-containing mixtures showed rounded domain shapes similar to those in analogous vesicles containing cholesterol, indicative of liquid-ordered state rather than solid-like gel-state domain formation. Over all, principles of ordered-domain formation in B. burgdorferi appear to be very similar to those in eukaryotes, with saturated PC taking the place of sphingolipids, but with ACGal being the main lipid component inducing ordered-domain formation.
Introduction
Lipid rafts in eukaryotic cells are tightly packed, liquid-ordered (Lo)-state microdomains that are rich in sterol and sphingolipids. They are believed to co-exist with loosely packed liquid-disordered (Ld) domains rich in unsaturated lipids (1, 2). Lipid rafts have been very well studied in model membranes and eukaryotic membranes (1, 2, 3, 4, 5, 6, 7, 8, 9, 10). Sorting membrane proteins into domains can either cluster proteins in like domains or segregate proteins from one another into different domains. Thus, lipid rafts can control protein-protein interactions, and are believed to play a very important role in a variety of biological functions, contributing to membrane trafficking, infection, and platforms for cell signaling, especially in immune cells (8, 9, 11, 12, 13).
Bacteria cannot synthesize cholesterol, and so had not been thought to contain lipid rafts. However, cholesterol, presumably acquired from the host or environment, is present in bacteria such as Mycoplasma, Ehrlichia, Anaplasma, Brachyspira, Helicobacter, and Borrelia (14, 15, 16, 17, 18). In addition, other bacteria have molecules with cholesterol-like properties (e.g., hopanoids), and may use them to form membrane microdomains (19, 20, 21, 22).
The presence of cholesterol in Borrelia burgdorferi raised the possibility that it might have lipid rafts. B. burgdorferi is a Gram-negative-like, pathogenic spirochete that causes Lyme disease. It is transmitted to mammals through the bite of infected Ixodes ticks (23, 24). It has a relatively high amount of lipids, ∼20% of the cell dry weight (25). Eleven lipids have been identified in total lipid extracts from B. burgdorferi (26, 27, 28, 29). Instead of having lipopolysaccharide in its outer membrane, B. burgdorferi has three lower-molecular-weight glycolipids (28). These glycolipids constitute ∼55–60% of the total lipids. Two contain cholesterol: cholesterol-β-D-galactopyranoside (CGal), which has cholesterol attached to galactose through a glycosidic bond, and cholesteryl 6-O-acyl-β-D-galactopyranoside (ACGal/BbGL-I), which is CGal with one acyl chain attached to the 6 carbon of the galactose. The third glycolipid is mono-α-galactosyl-diacylglycerol (MGalD/BbGL-II). It has two acyl chains and a galactose attached to the glycerol (26, 27, 28, 29) (Fig. S1 in the Supporting Material). The most abundant glycolipids are ACGal and MGalD, with ACGal comprising ∼25% of the total lipid (27, 29). Two major phospholipids, phosphatidylcholine (PC) and phosphatidylglycerol (PG), comprise >20% of total B. burgdorferi lipids. Other lipids are more minor species, including free cholesterol and cholesterol ester. The fatty acid composition for the major lipids PC, PG, ACGal, and MGalD shows that palmitic, stearic, oleic, and to a lesser extent linoleic acyl chains are present, with the exact acyl composition dependent upon the lipid species (26, 27). The saturated acyl chain level is >50% for PC and PG and as high as ∼70% for ACGal (27), suggesting that these lipids can participate in ordered-domain formation (see below). MGalD has been reported to have ∼50% saturated acyl chains (27), and might have one saturated and one unsaturated acyl chain per molecule, similar to mammalian phospholipids. It should be noted that Borrelia does not synthesize cholesterol or long-chain-saturated fatty acids, so the culture medium or host cells must provide them (25). Borrelia does contain proteins that synthesize ACGal, MGalD, PC, and PG (30, 31, 32). Other Borrelia species have a lipid profile similar to that of B. burgdorferi. B. garinii and B. afzelii have ACGal (29), and B. hermsii contains 6-O-acyl-β-glucopyranoside (ACGlu), in which a glucose replaces galactose (29).
The high amount of cholesterol lipids in Borrelia makes it a good model to study the formation of co-existing ordered and disordered domains in bacteria. Recent studies show that B. burgdorferi has lipid rafts. Lipid microdomains were directly observed by transmission electron microscope (TEM) studies both in lab-cultured B. burgdorferi and B. burgdorferi from infected mice (33), and their proteome was characterized (34). In model membrane vesicles composed of B. burgdorferi whole lipid extracts, co-existing ordered and disordered domains were detected by fluorescence resonance energy transfer (FRET) and a high level of membrane order by 1,6-diphenyl-1,3,5-hexatriene (DPH) fluorescence anisotropy (33). Ordered domains are often detergent resistant (7), so isolation of detergent-resistant membrane (DRM) from B. burgdorferi also supported the hypothesis that they form lipid rafts (33).
Additional studies were undertaken to obtain definitive evidence of raft formation in Borrelia (35). TEM, FRET, and detergent resistance were used to study sterol dependence on raft formation in sterol-substituted B. burgdorferi cells (35). Co-existing ordered and disordered domains were detected by FRET in living Borrelia. In agreement with sterol effects on lipid rafts composed of mammalian lipids (36, 37), strongly raft-supporting sterols maintained domain formation ability in Borrelia, whereas raft-inhibiting sterols disrupted raft formation (35). Another property of Borrelia domains that was found to be shared with ordered lipid rafts formed by eukaryotic lipids was selective accumulation of lipids with saturated acyl chains in ordered domains (35).
These studies show that ordered domains exist in Borrelia, and they share characteristics similar to those expected for lipid rafts in eukaryotic cells. What is less clear is which Borrelia lipids participate in raft formation, and whether the principles of lipid participation in raft formation are similar in bacteria and eukaryotes. Triton X-100 solubilized PC, PG, and MGalD, whereas ACGal, CGal, and free cholesterol were found predominantly in the DRM fraction (33). This suggests that the latter lipids participate in raft formation. Since Borrelia does not have sphingolipids and ACGal has both an acyl chain and cholesterol in its structure, one possibility is that ACGal plays the roles of both cholesterol and sphingolipids (35). However, because detergents can perturb membrane structure, detergent insolubility can only provide a rough picture of Borrelia raft composition (6, 7).
Therefore, we undertook studies to investigate how different B. burgdorferi lipids participate in domain formation. To do this, we prepared model membrane vesicles composed of various combinations of B. burgdorferi lipids. Model membrane vesicles have proven to be very useful systems for defining the abilities of lipids to form ordered domains in membranes and identifying conditions in which such domains exist as nanoscale-size domains (38, 39, 40, 41, 42, 43, 44, 45). Using model membrane vesicles, we found that the major Borrelia lipids differ greatly in their ability to support ordered-domain/raft formation. As in eukaryotes, ordered-domain/raft formation was found to be dependent upon the presence of cholesterol-containing lipids and lipids with saturated acyl chains. This confirmed that the general principles of raft formation are the same in Borrelia and eukaryotes. However, some interesting contrasts between the structures and roles of individual lipids participating in ordered-domain formation were also identified.
Materials and Methods
Materials
Porcine brain sphingomyelin (bSM), chicken egg sphingomyelin (eSM), 1,2-dimyristoleoylphosphatidylcholine (DMoPC), 1,2-dioleoyl-phosphatidylcholine (DOPC), 1,2-dipalmitoyl-phosphatidylcholine (DPPC), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), cholesterol, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DPPE), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (rhod-DOPE) were purchased from Avanti Polar Lipids (Alabaster, AL). 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl) (pyrene-DPPE) was purchased from Molecular Probes (Eugene, OR). Anthracene and DPH were purchased from Sigma-Aldrich (St. Louis, MO). ACGal, MGalD, and B. burgdorferi PC (Bb.PC) were extracted and isolated from B31 as described below. Lipids and probes were dissolved in chloroform and stored at −20°C. The concentrations of commercial unlabeled lipids were quantified by dry weight. Concentrations of fluorescent lipids were determined by absorbance in methanol using εNBD-DPPE = 21,000 M−1 cm−1 at 460 nm, εpyrene-DPPE = 35,000 M−1 cm−1 at 350 nm, εrhod-DOPE = 95,000 M−1 cm−1 at 560 nm, and εDPH = 84,800 M−1 cm−1 at 352 nm.
Methods
Borrelia cultures and lipid extraction, purification, and quantification
B. burgdorferi wild-type strain B31, mutant B313, and B. hermsii were grown in homemade BSK-II medium supplemented with 6% (v/v) rabbit serum (Sigma-Aldrich) at 33°C. B. burgdorferi were washed three times after resuspending in 1× phosphate-buffered saline (10 mM sodium phosphate and 150 mM sodium chloride (pH 7.8 ± 0.2)). Then, lipids were extracted with chloroform/methanol using the Bligh and Dyer method (46). The lipid extracts were dried under a nitrogen gas stream and then dissolved in chloroform. Lipids in whole-lipid extracts were separated by chromatography on high-performance thin-layer chromatography plates in CHCl3/methanol (85:15 v/v). Multiple lanes with lipid were loaded onto each plate. One lane was used to localize the migration of individual lipid bands by transferring a small amount of the lipid-bearing silica gel to a transparent tape and then staining the tape with iodine vapor. The three major lipids, Bb.PC, ACGal, and MGalD, were well separated, and the silica gel containing them was scraped off the high-performance thin-layer chromatography plate. Lipid was eluted from the Bb.PC-containing silica gel three times using (at 2× silica gel volume) CHCl3/methanol/H2O (54:37:4 v/v); ACGal and MGalD were eluted from silica gel similarly, but using CHCl3/methanol (85:15 v/v). Each extract was placed in glass Corex centrifuge tubes and then centrifuged at 5000 rpm for 10 min in a Sorvall RC-5 centrifuge. Supernatants were collected after each centrifugation and pooled. To remove residual silica gel, the pooled extracts for each lipid were filtered twice, each time over fresh VWR qualitative filter paper. The filtrates were then dried and dissolved in chloroform. Lipid concentration was measured by dry weight and then, more accurately, by NMR using anthracene as a reference standard. To do this, a known concentration of anthracene, 648 μM, was freshly prepared (anthracene can be volatile) in deuterated chloroform. This was added to a small aliquot of lipid that had been dried under nitrogen to give an estimated ∼800 μM lipid.
The 1H-NMR spectra of lipids were acquired at 850 MHz with a 30° pulse, a delay time between acquisitions of 10 s to avoid saturation, and 32 acquisitions. The areas of lipid peaks corresponding to known numbers of protons per molecule were then compared to the area of anthracene proton peaks to calculate lipid concentration.
Model membrane vesicle preparation
Lipid vesicles were prepared by sonication. Borrelia lipids and fluorescent probes were mixed in glass tubes and then dried under nitrogen for 5–10 min followed by high vacuum for 1 h. Phosphate-buffered saline heated to 70°C was added to dried lipids that had been preheated to 70°C for 5 min. The samples were then sonicated in a bath sonicator (Laboratory Supplies, Hicksville, NY), typically for 5–10 min, but in all cases until dried lipids were no longer stuck to the bottom of the glass tube and the sample appeared to be homogeneously dispersed. Samples were cooled down to room temperature before the measurements were taken.
Fluorescence measurements
Fluorescence was measured in a SPEX FluoroLog 3 spectrofluorometer (Jobin-Yvon, Edison, NJ), equipped with an automated polarization apparatus, using quartz semi-microcuvettes (excitation path length, 10 mm; emission path length, 4 mm), as previously (47).
Temperature dependence of fluorescence anisotropy. Samples containing ∼50 μM Borrelia lipids with 0.1 mol % DPH were prepared as described above. Fluorescence anisotropy was measured from 16°C to 60°C at 4°C intervals. Due to the high level of DPH fluorescence, background values were negligible and were not subtracted. Between intervals, samples were heated and fluorescence anisotropy was measured once the temperature stabilized, as monitored by a probe thermometer (probe diameter ∼1/16 inch; YSI Series 400, YSI Instruments, Yellow Springs, OH) placed in a cuvette. Average values and standard deviations calculated from three different experiments are reported.
Temperature dependence of FRET. F samples had vesicles composed of a mixture of Borrelia lipids and/or other unlabeled lipids, FRET donor, and FRET acceptor. Fo samples were prepared with the same lipids, but lacking the FRET acceptor. Background samples for F samples lacked the donor, whereas background samples for Fo samples lacked donor and acceptor. One of either of two donor-acceptor pairs was used: NBD-DPPE/rhod-DOPE or pyrene-DPPE/rhod-DPPE. The mol % of labeled lipids in the “F sample”-labeled vesicles was either 0.1 mol % NBD-DOPE/2 mol % rhod-DOPE or 0.1 mol % pyrene-DPPE/5 mol % rhod-DPPE. The fluorescence of one each of the F, Fo, and background samples as a function of temperature were measured in a four-sample cuvette holder every 4°C from 16°C to 60°C, as described above, with an excitation λ of 460 nm and emission λ of 534 nm for NBD-DOPE, or an excitation of λ = 350 nm and emission of λ = 379 nm for pyrene-DPPE. F/Fo values were calculated after subtraction of backgrounds. The ordered-domain melting temperature was defined by the midpoint of a sigmoid fit of F/Fo versus temperature curve using SlideWrite Plus software (Advanced Graphics Software, Encinitas, CA), of the type F/Fo = a + b/[1 + exp(−(x − c)/d)], where a equals the limiting value of F/Fo at low temperature, a + b equals the limiting value of F/Fo at high temperature, c equals the midpoint temperature (Tm), and d is a parameter describing the sharpness of the sigmoidal curve as a function of temperature. Average values and standard deviations calculated from three different experiments are reported.
Changes in F/Fo when temperature was increased to 60°C were found to be reversible when heated samples were cooled to 16°C in representative cases (whole B. burgdorferi strain B31 lipids, 1:1:1 (mol/mol) ACGal/MGalD/Bb.PC, and pure ACGal).
It should be noted that the Tm values could be influenced by the inclusion of a few mol % acceptor. However, previous studies show that a few mol % rhod-DOPE does not affect Tm significantly (47). In addition, we found little difference between melting behavior using pyrene-DPPE in 1:1:1 mol/mol SM/POPC/chol with 5 mol % rhod-DOPE acceptor, studied here, and that seen for the same lipid mixture with 2 mol % rhod-DOPE in a previous study (45). Furthermore, the studies here are concerned more with comparing different lipids than with the exact absolute values of Tm.
Laser-scanning microscopy
Giant unilamellar vesicles (GUVs) composed of 1:1 (mol/mol) eSM/DMoPC with different amounts of cholesterol or ACGal were prepared using the electroformation method (48). The fluorescently labeled lipid rhod-DOPE (0.02 mol %) was used as a marker. It preferentially partitions into disordered domains, allowing the visualization of domains (49). To prepare GUVs, the desired lipid combinations were dissolved in chloroform at a total lipid concentration of 5 mM, and a small volume (2 μL) was spread on indium-tin-oxide-coated coverslips. The solvent was then completely evaporated under high vacuum for 1 h and placed in a home-built flow chamber. Then a 2 mm spacer and second coverslip were placed on top of the first coverslip, with contact sites completely sealed by grease. After 200–300 μL distilled water was injected into the chamber, a voltage of 1.2 V at 10 Hz was applied for 2 h at 60°C. Samples were observed under a ConfoCor 2 confocal microscope (Carl Zeiss, Oberkochen, Germany) immediately after they were cooled down to room temperature, and rhod-DOPE fluorescence was detected using a 543 nm laser (red channel).
Measurement of vesicle size
The measurement of vesicle size after the fluorescence experiments was carried out at 23°C using a Protein Solutions DynaPro 99 dynamic light-scattering instrument (Wyatt Technology, Santa Barbara, CA). Vesicles contained Borrelia lipids used for the FRET or anisotropy experiments diluted to 5–10 μM. Size and FRET measurements were carried out the same day. Data were analyzed with the software Dynamics version 5.25.44 (Protein Solutions, Charlottesville, VA).
Results
The whole Borrelia lipid extracts and individual Borrelia lipids have various abilities to exist in an ordered state, as assayed by fluorescence anisotropy
Previous studies have shown that raft-like ordered lipid microdomains exist in B. burgdorferi membranes in vitro and in vivo (33). To understand the molecular origin of lipid raft formation, the properties of model membrane vesicles composed of various mixtures of B. burgdorferi lipids, as well as those of isolated lipids, were studied. Mildly sonicated vesicles were prepared to give vesicles with diameters in the range 100–200 nm for almost all the lipid mixtures studied (Table S1 in the Supporting Material). Larger and more heterogeneous vesicles were formed by pure ACGal and 1:1 ACGal/MGalD. That ACGal formed intact vesicles was confirmed by the fact that when a fluorescently labeled NBD lipid was incorporated into them, half of the NBD groups were inaccessible to an external reducing agent (Fig. S2). Vesicles with only MGalD were so heterogeneous that no size estimate was possible. This may reflect their greater ability, relative to that of most other lipids, to form structures in a non-bilayer state when pure (50, 51).
To define the ability to form an ordered-state membrane, DPH fluorescence anisotropy was measured. In the solid-like gel and liquid-ordered states, anisotropy is high (∼0.3), whereas in the liquid-disordered state, DPH anisotropy is much lower (∼0.05–0.15), with the exact value being somewhat temperature dependent, because membrane disorder increases as temperature increases.
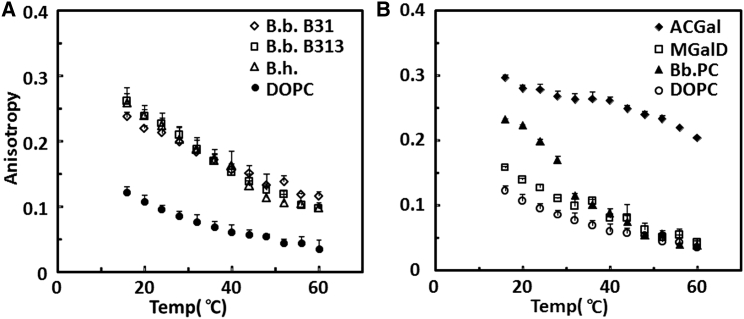
Fig. 1 shows the temperature dependence of anisotropy for vesicles composed of whole lipid extracts from different Borrelia and for individual B. burgdorferi lipids as temperature was increased from 16°C to 60°C. Fig. 1 A shows the behavior of whole lipid extracts from B. burgdorferi strains B31 and B313 and from B. hermsii. B31 is the wild-type organism, used here to extract and isolate individual lipids for later studies. B313 is the mutant that lacks two raft-associated proteins, OspA and OspB, which contribute to the thermal stability of raft domains in vivo (52). B. hermsii causes tick-borne relapsing fever. It has a lipid composition similar to that of B. burgdorferi, except with ACGlu in place of ACGal, as noted above. All of the whole lipid extracts show a high level of anisotropy, especially at lower temperatures, indicative of a high degree of membrane order, in contrast to DOPC vesicles, which are shown as a standard for lipid bilayers in the disordered, Ld, state. The observation that model membranes from the lipids of wild-type and B313 strains have similar levels of anisotropy suggests that the outer surface proteins themselves modulate membrane order in vivo, rather than outer-surface-protein deletions influencing lipid properties by altering lipid composition.
Figure 1.
Ability of whole Borrelia lipid extracts and individual Borrelia lipids to form vesicles with ordered-state bilayers as assayed by fluorescence anisotropy. Samples contained 0.1 mol % DPH in vesicles containing 50 μM lipid. (A) Whole lipid extracts from B. burgdorferi strain B31 (diamonds), B. burgdorferi strain B313 (squares), and B. hermsii (triangles). DOPC (circles) is shown for comparison. (B) Individual lipids ACGal (diamonds), MGalD (squares), B. burgdorferi PC (triangles), and DOPC (circles). The average and standard deviation from three samples are shown.
Fig. 1 B shows the results of DPH anisotropy measurements in vesicles composed of individual B. burgdorferi lipids. We concentrated on ACGal, MGalD, and Bb.PC because CGal and B. burgdorferi PG were present in only small amounts (Fig. S3). ACGal forms vesicles with a high degree of order. In contrast, MGalD formed model membranes with a low degree of order, similar to that of DOPC. Bb.PC vesicles had an intermediate degree of order at lower temperature, which gradually decreased to a low degree of order similar to that of DOPC as temperature was increased. This suggests that ACGal and Bb.PC are the lipids most likely to form ordered domains.
The whole Borrelia lipid extracts and individual Borrelia lipids have various abilities to form co-existing ordered- and disordered-domain states, as detected by FRET
Although anisotropy can reveal the degree of membrane order, it cannot distinguish directly between a homogeneous membrane with high or intermediate levels of order and one that has co-existing ordered and disordered domains. To directly detect the segregation into ordered and disordered domains, FRET was used. FRET can detect domains by utilizing donor and acceptor pairs that partition differently into ordered and disordered domains. For FRET experiments, pyrene-DPPE was used as a probe that can partition to a significant degree into ordered domains, and rhod-DOPE was used as a probe that partitions strongly into disordered domains. (It should be noted that the donor does not have to preferentially partition into the ordered domains to detect domains when the acceptor strongly partitions into the disordered domains. Under conditions in which the extent of FRET is high, the donor need only partition into the ordered domains to a significant degree (47).) In membranes segregated into domains, donor fluorescence (F/Fo) is higher than in homogeneous membranes that lack domains. Typically, because ordered domains “melt” at higher temperatures, and the lipids become homogeneous, strong donor fluorescence disappears as temperature is increased. The “melting” temperature (Tm) can be defined as the mid-point (point of maximum slope) of the F/Fo-versus-temperature curve. This generally corresponds to the temperature above which the ordered domains have melted. Below this temperature, there is usually a mixture of ordered and disordered domains. A state in which the bilayer is fully ordered is generally formed only at or below 0°C when a lipid having acyl chains with a cis unsaturated double bond is present in large amounts and so is below the experimental temperature range.
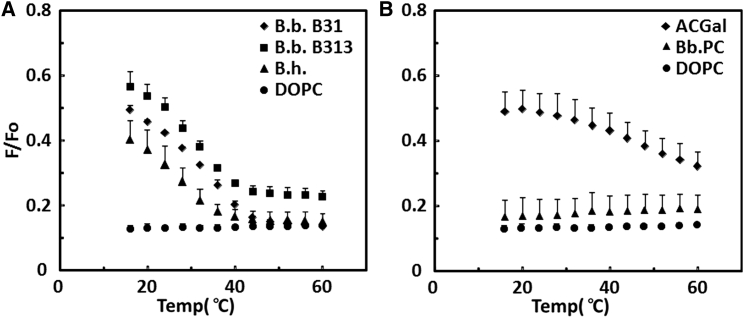
Fig. 2 A shows the ability of the Borrelia whole lipid extracts to form domains using the FRET assay. The whole lipid extracts showed weak FRET at low temperature, indicative of co-existing ordered and disordered domains. The dependence of FRET upon temperature showed that the B31 strain and B313 strain of B. burgdorferi form ordered domains with a similar thermal stability (Tm ∼30–32°C). B. hermsii lipids formed ordered domains with slightly less thermal stability (Tm ∼27°C). The control all-Ld sample composed of DOPC showed strong FRET (low F/Fo) that was largely temperature independent, as expected. Notice that at high temperature, F/Fo values for all samples were similar, indicative of the presence of homogeneous Ld-state lipids.
Figure 2.
Ability of whole Borrelia lipid extracts and individual Borrelia lipids to form vesicles with ordered-state bilayers as assayed by FRET. (A) Whole lipid extracts from B. burgdorferi strain B31 (diamonds), B. burgdorferi strain B313 (squares), and B. hermsii (triangles). DOPC (circles) is shown for comparison. (B) Individual lipids ACGal (diamonds), B. burgdorferi PC (triangles), and DOPC (circles). F/Fo is the ratio of fluorescence in vesicles with donor (0.1 mol % pyrene-DPPE) and acceptor (5 mol % rhod-DOPE) to that in vesicles containing only acceptor, after background values were subtracted. The average and standard deviation from three samples are shown.
The ability of the individual Borrelia lipids to form ordered domains was examined by FRET, as shown in Fig. 2 B. FRET curves for ACGal vesicles showed weak FRET at low temperature, again indicative of co-existing ordered and disordered domains. F/Fo decreased as temperature increased, but remained somewhat elevated even at 60°C, indicating that the ordered domains present had not completely melted, consistent with the anisotropy results. Surprisingly, given the somewhat elevated anisotropy seen at lower temperatures in Bb.PC vesicles, vesicles containing only Bb.PC gave curves very similar to those with DOPC alone, consistent with a lack of ordered-domain formation. However, this seems to be an artifact of the particular donor used. When FRET was repeated using DPH as a donor (Fig. S4), FRET curves showed a transition at a temperature similar to that detected by anisotropy. Thus, the Bb.PC forms co-existing ordered and disordered domains at low temperature.
Reproducible FRET measurements could not be made in vesicles composed of MGalD. This may be a problem with inhomogeneous mixing of the FRET probe lipids with MGalD. Combined, the DPH anisotropy and FRET results indicate that the ability to form ordered-state bilayers decreases in the order ACGal > Bb.PC > MGalD ∼ DOPC. It should be noted that FRET and anisotropy studies do not distinguish between Lo domains and solid-like gel domains, both of which are ordered states, an issue we examined by microscopy (see below).
Effect of lipid composition in mixtures of Borrelia lipids on the ability to form ordered domains, as assayed by FRET
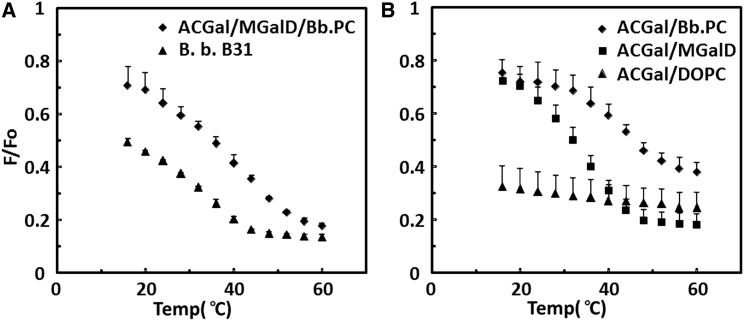
To define more precisely which lipids contribute to ordered-domain formation, we prepared lipid vesicles with different combinations of B. burgdorferi lipids and again assayed domain formation using FRET. Fig. 3 A shows that a 1:1:1 mixture of ACGal, MGalD, and Bb.PC, the three most abundant lipids of B. burgdorferi (27), forms ordered domains. As in the vesicles composed of B. burgdorferi whole lipid extracts, there is a large, sigmoidal decrease in F/Fo as temperature increases. The domain melting temperature derived from these curves shows that the ordered domains in the vesicles composed of the 1:1:1 mixture are even more thermally stable (Tm 39°C) than domains in vesicles prepared from B. burgdorferi whole lipid extracts (Tm 32°C). This strongly suggests that these three lipids have the most important roles in ordered-domain formation. The difference in thermal stability between the 1:1:1 mixture and whole lipid extracts presumably reflects the difference in lipid proportions and composition relative to the whole lipid mixture.
Figure 3.
Comparison of ordered domain formation as a function of lipid composition in vesicles containing binary and ternary Borrelia lipid combinations detected by FRET. (A) Ordered domain formation in vesicles in a ternary lipid mixture versus whole lipid extract. Samples contained 1:1:1 mol/mol ACGal/MGalD/Bb.PC (diamonds) or B. burgdorferi B31 lipids (triangles). (B) Ordered-domain formation in vesicles with 1:1 mol/mol binary lipid combinations. Samples contained: ACGal/Bb.PC (diamonds), ACGal/MGalD (squares), or ACGal/DOPC (triangles). FRET was performed as in Fig. 2. The average and standard deviation from three samples are shown.
Fig. 3 B illustrates the domain-forming properties of vesicles composed of various 1:1 mol/mol binary mixtures containing B. burgdorferi lipids. Sigmoidal F/Fo curves were observed for both 1:1 ACGal/Bb.PC and 1:1 ACGal/MGalD. The vesicles formed from 1:1 ACGal/Bb.PC exhibited a higher Tm (43°C) than those formed from 1:1 ACGal/MGalD (Tm 34°C). (The relatively high F/Fo value at high temperature in the 1:1 ACGal/Bb.PC vesicles could reflect residual ordered domains, perhaps formed by ACGal, or an artifact of a slightly inhomogeneous distribution of lipids in different vesicles.) Ordered domains were not detected in vesicles composed of 1:1 ACGal/DOPC. This was confirmed by anisotropy, which showed low values over the entire temperature range studied. These results indicate that Bb.PC contributes to ordered-domain formation to a greater degree than MGalD does, and that MGalD supports ordered-domain formation more than DOPC does. It also confirms that the type of PC has an important effect on domain formation. A similar dependence on PC composition was observed in vesicles composed of ternary lipid mixtures containing 1:1:1 mol/mol ACGal/MGalD/PC. The Tm values for the 1:1:1 ACGal/MGalD/Bb.PC vesicles (Tm 39°C) were much higher than that for 1:1:1 ACGal/MGalD/DOPC (Tm <10°C) (Fig. S5).
Double-bond content of lipids
The difference between Bb.PC and DOPC involves a difference in acyl chain composition. DOPC has two monounsaturated acyl chains, i.e., two double bonds per molecule. The number of double bonds in Bb.PC was assayed using 1H NMR (Fig. S6). The double bond/acyl chain ratio in Bb.PC was ∼0.25. This indicates that 75% or more of the Bb.PC acyl chains were saturated, slightly higher than the number reported in a previous study (27). This ratio means that the minimum fraction of Bb.PC molecules with two saturated acyl chains is 50%. This is a lower limit, because the fraction of PC with two saturated acyl chains would be higher if some of the remaining Bb.PC molecules have two unsaturated or one polyunsaturated acyl chain (27). Consistent with the level of saturation in Bb.PC, vesicles composed of 1:1:1 (mol/mol) ACGal/MGalD/PC in which PC was a 1:2 (mol/mol) mixture of DPPC (which has no double bonds) and POPC formed ordered domains with thermal stability similar to that of ternary mixtures using Bb.PC (data not shown).
1H NMR analysis of double-bond content indicated∼1.5 double bonds per MGalD molecule (Fig. S6), slightly more than the number measured in previous studies (27) (Fig. S3). This is consistent with each MGalD molecule having one saturated and one unsaturated acyl chain. ACGal had ∼0.3 acyl chain double bonds per molecule, in agreement with previous studies (27) (Fig. S6).
Comparison of ACGal and cholesterol ability to form ordered domains
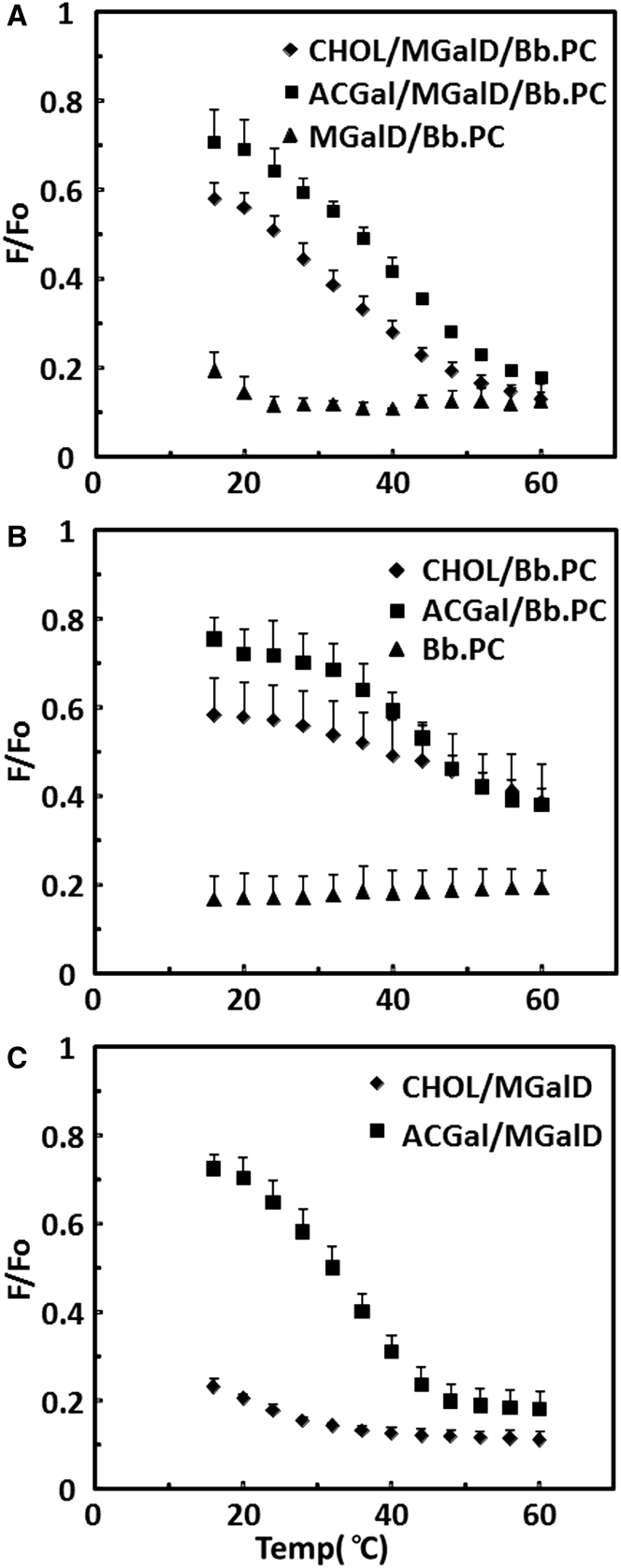
Since cholesterol and ACGal differ significantly in their chemical structure, their role in supporting membrane domain formation could be different. To investigate this, we used FRET to compare their influence on domain formation in vesicles in which they were mixed with Borrelia lipids. Fig. 4 A compares domain formation in vesicles composed of 1:1:1 mol/mol ACGal/MGalD/Bb.PC to that in vesicles composed of 1:1:1 cholesterol/MGalD/Bb.PC and to that in vesicles lacking cholesterol lipids. Domain formation and thermal stability in the presence of ACGal (Tm ∼39°C) and cholesterol (Tm ∼32°C) were similar. This shows that the acyl chain and galactose of ACGal are not absolutely required for domain formation in these mixtures. However, domains did not form, at least above 20°C, in 1:1 MGalD/Bb.PC vesicles lacking cholesterol lipids. The difference in F/Fo values at low temperature in mixtures containing cholesterol and ACGal could reflect a greater degree of donor and acceptor segregation due to a higher degree of ordered-domain formation in the vesicles with ACGal compared to those with cholesterol or a greater difference in the partitioning of donor and acceptor into ordered domains.
Figure 4.
Comparison of the effects of ACGal and cholesterol on ordered-domain formation when mixed with other Borrelia lipids assayed by FRET. (A) Effect of sterol lipids on ordered-domain formation in vesicles with a 1:1 mol/mol MGalD/Bb.PC mixture with 33 mol % cholesterol (diamonds) or 33 mol % ACGal (squares), and without sterol (triangles). (B) Effect of sterol lipids on FRET in vesicles containing Bb.PC with 50 mol % cholesterol (diamonds) or 50 mol % ACGal (squares), or Bb.PC vesicles without sterol (triangles). (C) Effect of sterol lipids on ordered-domain formation in vesicles containing MGalD with 50 mol % cholesterol (diamonds) or 50 mol % ACGal (squares). FRET was performed as in Fig. 2. The average and standard deviation from three samples are shown.
The ability of ACGal and cholesterol to support ordered-domain formation was also studied in vesicles containing only Bb.PC or MGalD. As shown in Fig. 4 B, vesicles containing 1:1 ACGal/Bb.PC and cholesterol/Bb.PC showed ordered-domain formation, with similar thermal stability (Tm ∼43°C and ∼45°C, respectively). In contrast, Fig. 4 C shows that in vesicles containing 1:1 cholesterol/MGalD, there is very little domain formation over the entire experimental range, whereas in 1:1 ACGal/MGalD vesicles, ordered-domain formation is obvious, with Tm ∼34°C. Thus, when mixed with a lipid with a very low ability to form ordered domains, ACGal can support ordered-domain formation much better than cholesterol.
ACGal forms slightly larger domains than cholesterol, as assayed using FRET donor and acceptor pairs with different Ro values
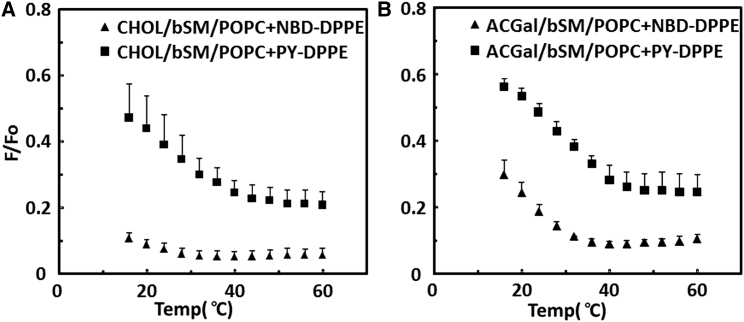
Some lipids form vesicles with only tiny nanodomains (47). To distinguish these from larger domains, FRET using different donor and acceptor pairs with different critical interaction distances (Ro values) can be used. This is true because FRET pairs cannot readily detect domains when the domain radius is smaller than Ro (47). In contrast, when domains are large, they can be detected by FRET pairs with both large and small Ro values. For these experiments, we used as FRET pairs NBD-DPPE/rhod-DOPE, with an effective Ro of 4.9 nm, and pyrene-DPPE/rhod-DOPE, with an effective Ro of 2.6 nm (47). Only the latter can detect domains in the 2.5–5 nm range.
Fig. 5 A compares domain formation using these two FRET pairs. The mixture 1:1:1 cholesterol/bSM/POPC was previously shown to form 2.5- to 5-nm domains over a wide temperature range (47). In agreement with this, domain formation is clearly detected using the pyrene-DPPE/rhod-DOPE FRET pair, but not as clearly with the NBD-DPPE/rhod-DOPE FRET pair. In contrast, Fig. 5 B shows that domain formation is readily detected with both FRET pairs for vesicles composed of 1:1:1 ACGal/bSM/POPC. This indicates that ACGal/bSM/POPC forms larger domains than cholesterol/bSM/POPC.
Figure 5.
Comparison of FRET using a small Ro donor (pyrene-DPPE) and a large Ro donor (NBD-DPPE) in vesicles composed of 1:1:1 mol/mol cholesterol lipids/bSM/POPC. (A) FRET in vesicles containing cholesterol. (B) FRET in vesicles containing ACGal. Shown are 1:1:1 (CHOL or ACGal)/bSM/POPC with NBD-DPPE donor and rhod-DOPE acceptor (triangles) and 1:1:1 (CHOL or ACGal)/bSM/POPC with pyrene-DPPE donor and rhod-DOPE acceptor (squares). F samples contained 0.1 mol % pyrene-DPPE and 5 mol % rhod-DOPE, or 0.1 mol % NBD-DPPE and 2 mol % rhod-DOPE. Fo samples contained 0.1 mol % pyrene-DPPE or NBD-DPPE. FRET was performed as in Fig. 2. The average and standard deviation from three samples are shown. F/Fo is the ratio of fluorescence in vesicles with donor and acceptor to that in vesicles containing only acceptor, after background values subtracted. The average from three samples and standard deviations are shown.
Both ACGal and cholesterol form Lo ordered domains
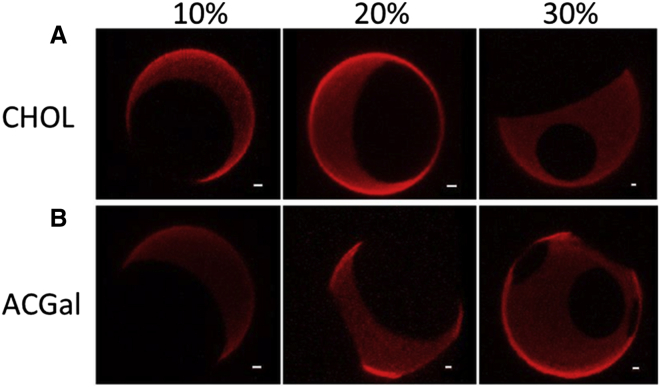
FRET studies do not distinguish between Lo domains, which have rounded shapes, and solid-like gel domains, which have highly irregular shapes. These ordered states can be distinguished by microscopy under conditions in which domains are very large. To visualize domain shape, microscopy studies were carried out on GUVs containing cholesterol or ACGal mixed with 1:1 (mol/mol) eSM/DMoPC. The latter lipids were chosen because they form very large domains (53). (Borrelia lipid domains in GUVs were not large enough to see.) Fig. 6 shows the formation of rounded ordered domains (unstained due to the strong partition of the fluorescent probe into the disordered domains) with both ACGal and cholesterol over the entire range of sterol concentrations studied (10–30 mol %). Similar large domains were also seen at 40 mol % ACGal (data not shown). This shows that the ordered domains are Lo domains. Although behavior in Borrelia lipid mixtures could be different, based on these observations, it is likely that ACGal forms Lo domains.
Figure 6.
Fluorescence micrographs of GUVs show formation of coexisting Lo and Ld domains in the presence of cholesterol or ACGal. (A) GUVs composed of 1:1 eSM/DMoPC with 10–30 mol % of cholesterol. (B) GUVs composed of 1:1 eSM/DMoPC with 10–30 mol % of ACGal. GUVs contain 0.02 mol % rhod-DOPE as a marker of Ld domains. White lines indicate a scale bar representing 1 μm. To see this figure in color, go online.
Discussion
Effect of chemical structure upon the ability of Borrelia lipids to form lipid rafts
Cholesterol lipids
Previous DRM studies and TEM data identified ACGal, CGal, and free cholesterol as major lipids of ordered domains in Borrelia (33). The importance of ACGal, the most abundant cholesterol lipid in Borrelia, is confirmed in this study by the observation from anisotropy that it has the strongest ability to form vesicles in an ordered state, and by FRET measurements showing that it spontaneously forms ordered (and co-existing disordered) membrane domains with a very high thermal stability. The high (∼70%) level of ACGal acyl saturation (27), which we confirmed, is one factor contributing to its strong ability to form ordered domains. Presumably, the disordered domains present in ACGal membranes are formed by the fraction of ACGal molecules with unsaturated acyl chains. Although it contains ACGlu in place of ACGal, B. hermsii lipids formed lipid rafts with stability similar to those formed from B. burgdorferi. This is not a surprise, as acyl steryl glycosides, ACGlu-like molecules found in plants, also have the ability to stabilize the ordered domains in model membrane vesicles (54).
Bb.PC and MGalD
Since we know that detergent resistance is a very rough method of determining lipid raft composition (6, 7), low inclusion in DRM does not rule out a role for other Borrelia lipids in ordered-domain formation. In fact, despite its low level in DRM, Bb.PC, which has a high level of acyl chain saturation, was able to support ordered-domain formation when mixed with ACGal. This was not true with DOPC, which has fully unsaturated acyl chains. It is likely that the fraction of Bb.PC molecules that have fully saturated acyl chains, which comprise at least 50% of the Bb.PC molecules, interact with the saturated subpopulation of ACGal molecules to form ordered domains. MGalD, which has a lower level of saturated fatty acids than ACGal and Bb.PC, had a much lower ability to form ordered domains than ACGal and Bb.PC, although it was able to participate in ordered-domain formation to a greater extent than DOPC.
Overall, ordered-domain formation in Borrelia appears to arise from the combined properties of ACGal and, presumably, the sub-population of Bb.PC with only saturated acyl chains. This is analogous to the situation in eukaryotes, in which the combination is sphingolipid (which has a saturated acyl chain) and cholesterol. However, the role Bb.PC may be less important than that of sphingolipids, whereas the role of ACGal is more important than of cholesterol.
Comparison of the properties of ACGal and cholesterol
The similarities and differences between ACGal and cholesterol were interesting. We found that both form Lo-type ordered domains. In the presence of other lipids that can participate in ordered-domain formation, their behavior seemed similar. They formed ordered domains with similar thermal stability. However, there were also some differences. ACGal could form membranes with co-existing ordered and disordered domains by itself. This presumably reflects the presence of the acyl chain in ACGal. Cholesterol can only participate in ordered-domain formation if another molecule with raft-forming abilities is present.
Generalizing raft-formation principles from Borrelia to other bacteria and eukaryotes
Studies of the raft-forming principles in Borrelia help illustrate similarities and differences between membrane domains in prokaryotes and eukaryotes. The general principle governing the formation of tightly packed ordered domains by interaction between lipids with saturated acyl chains and sterol (1) appears to be similar in Borrelia and eukaryotes. These principles may apply to other bacteria. Even though cholesterol is not necessary for its growth, the Gram-negative spirochete Helicobacter pylori also has three kinds of cholesterol glycolipids (15). In addition, lipid domains have been reported in the Gram-positive bacterium Staphyloccus aureus. Interestingly, the S. aureus lipid staphyloxanthin is structurally analogous to ACGal, but with a rigid polyisoprene lacking rings in place of cholesterol (55). It should be interesting to study the physical and chemical basis of domain formation in these bacteria. Understanding how lipids and proteins function in raft formation could aid studies of the biological function of lipid-raft infection and pathogenesis. Experiments in which the synthesis of raft-forming lipids is blocked, or in which the acyl chain composition of the raft-forming lipids is altered, may be of particular value in determining how altering rafts alters biological function and protein-lipid interactions.
Author Contributions
E.L. conceptualized the general project, designed research, analyzed data, and wrote the manuscript; Z.H. designed research, performed research, analyzed data, and wrote the manuscript; A.M.T. designed and performed research; and J.L.B. conceptualized the general project.
Acknowledgments
This work was supported by National Institutes of Health grants GM 099892 (to E.L.) and AI 027044 (to J.L.B.).
Editor: Klaus Gawrisch.
Footnotes
Six figures and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)31040-2.
Supporting Material
References
- 1.London E. Insights into lipid raft structure and formation from experiments in model membranes. Curr. Opin. Struct. Biol. 2002;12:480–486. doi: 10.1016/s0959-440x(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 2.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Fiedler K., Parton R.G., Simons K. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J. 1994;13:1729–1740. doi: 10.1002/j.1460-2075.1994.tb06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown D. Structure and function of membrane rafts. Int. J. Med. Microbiol. 2002;291:433–437. doi: 10.1078/1438-4221-00150. [DOI] [PubMed] [Google Scholar]
- 5.Brown D.A., London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 6.London E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim. Biophys. Acta. 2005;1746:203–220. doi: 10.1016/j.bbamcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.London E., Brown D.A. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim. Biophys. Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 8.Luo C., Wang K., Zhao Q.S. The functional roles of lipid rafts in T cell activation, immune diseases and HIV infection and prevention. Cell. Mol. Immunol. 2008;5:1–7. doi: 10.1038/cmi.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S.N., Brown D.A., London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 11.Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 12.He H.T., Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:525–530. doi: 10.1038/embor.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratajczak M.Z., Adamiak M. Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking. Leukemia. 2015;29:1452–1457. doi: 10.1038/leu.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haque M., Hirai Y., Oguma K. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J. Bacteriol. 1995;177:5334–5337. doi: 10.1128/jb.177.18.5334-5337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirai Y., Haque M., Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J. Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin M., Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith P.F. Biosynthesis of cholesteryl glucoside by Mycoplasma gallinarum. J. Bacteriol. 1971;108:986–991. doi: 10.1128/jb.108.3.986-991.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trott D.J., Alt D.P., Stanton T.B. The search for Brachyspira outer membrane proteins that interact with the host. Anim. Health Res. Rev. 2001;2:19–30. [PubMed] [Google Scholar]
- 19.López D., Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–1902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sáenz J.P., Grosser D., Simons K. Hopanoids as functional analogues of cholesterol in bacterial membranes. Proc. Natl. Acad. Sci. USA. 2015;112:11971–11976. doi: 10.1073/pnas.1515607112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sáenz J.P., Sezgin E., Simons K. Functional convergence of hopanoids and sterols in membrane ordering. Proc. Natl. Acad. Sci. USA. 2012;109:14236–14240. doi: 10.1073/pnas.1212141109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez D. Molecular composition of functional microdomains in bacterial membranes. Chem. Phys. Lipids. 2015;192:3–11. doi: 10.1016/j.chemphyslip.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Benach J.L., Bosler E.M., Kaslow R.A. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 24.Burgdorfer W., Barbour A.G., Davis J.P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 25.Johnson R.C. The spirochetes. Annu. Rev. Microbiol. 1977;31:89–106. doi: 10.1146/annurev.mi.31.100177.000513. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Menachem G., Kubler-Kielb J., Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossain H., Wellensiek H.J., Lochnit G. Structural analysis of glycolipids from Borrelia burgdorferi. Biochimie. 2001;83:683–692. doi: 10.1016/s0300-9084(01)01296-2. [DOI] [PubMed] [Google Scholar]
- 28.Schröder N.W., Schombel U., Schumann R.R. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J. Biol. Chem. 2003;278:33645–33653. doi: 10.1074/jbc.M305799200. [DOI] [PubMed] [Google Scholar]
- 29.Stübs G., Fingerle V., Schröder N.W. Acylated cholesteryl galactosides are specific antigens of borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J. Biol. Chem. 2009;284:13326–13334. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostberg Y., Berg S., Bergström S. Functional analysis of a lipid galactosyltransferase synthesizing the major envelope lipid in the Lyme disease spirochete Borrelia burgdorferi. FEMS Microbiol. Lett. 2007;272:22–29. doi: 10.1111/j.1574-6968.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Morales F., Schobert M., Geiger O. Pathways for phosphatidylcholine biosynthesis in bacteria. Microbiology. 2003;149:3461–3471. doi: 10.1099/mic.0.26522-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang X.G., Scagliotti J.P., Hu L.T. Phospholipid synthesis in Borrelia burgdorferi: BB0249 and BB0721 encode functional phosphatidylcholine synthase and phosphatidylglycerolphosphate synthase proteins. Microbiology. 2004;150:391–397. doi: 10.1099/mic.0.26752-0. [DOI] [PubMed] [Google Scholar]
- 33.LaRocca T.J., Crowley J.T., Benach J.L. Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement-independent antibody. Cell Host Microbe. 2010;8:331–342. doi: 10.1016/j.chom.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo A., Pérez A., Benach J.L. The lipid raft proteome of Borrelia burgdorferi. Proteomics. 2015;15:3662–3675. doi: 10.1002/pmic.201500093. [DOI] [PubMed] [Google Scholar]
- 35.LaRocca T.J., Pathak P., London E. Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts. PLoS Pathog. 2013;9:e1003353. doi: 10.1371/journal.ppat.1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Megha, London E. Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): implications for lipid raft structure and function. Biochemistry. 2004;43:1010–1018. doi: 10.1021/bi035696y. [DOI] [PubMed] [Google Scholar]
- 37.Xu X., London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 38.Konyakhina T.M., Feigenson G.W. Phase diagram of a polyunsaturated lipid mixture: brain sphingomyelin/1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine/cholesterol. Biochim. Biophys. Acta. 2016;1858:153–161. doi: 10.1016/j.bbamem.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konyakhina T.M., Wu J., Feigenson G.W. Phase diagram of a 4-component lipid mixture: DSPC/DOPC/POPC/chol. Biochim. Biophys. Acta. 2013;1828:2204–2214. doi: 10.1016/j.bbamem.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J., Wu J., Feigenson G.W. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol. Biochim. Biophys. Acta. 2007;1768:2764–2776. doi: 10.1016/j.bbamem.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feigenson G.W., Buboltz J.T. Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys. J. 2001;80:2775–2788. doi: 10.1016/S0006-3495(01)76245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleecker J.V., Cox P.A., Keller S.L. Thickness mismatch of coexisting liquid phases in noncanonical lipid bilayers. J. Phys. Chem. B. 2016;120:2761–2770. doi: 10.1021/acs.jpcb.5b10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beattie M.E., Veatch S.L., Keller S.L. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 2005;89:1760–1768. doi: 10.1529/biophysj.104.049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veatch S.L., Keller S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak P., London E. The effect of membrane lipid composition on the formation of lipid ultrananodomains. Biophys. J. 2015;109:1630–1638. doi: 10.1016/j.bpj.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 47.Pathak P., London E. Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation. Biophys. J. 2011;101:2417–2425. doi: 10.1016/j.bpj.2011.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Q., London E. Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O. J. Biol. Chem. 2013;288:1340–1352. doi: 10.1074/jbc.M112.415596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiantia S., Schwille P., London E. Asymmetric GUVs prepared by MβCD-mediated lipid exchange: an FCS study. Biophys. J. 2011;100:L1–L3. doi: 10.1016/j.bpj.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koynova R., Caffrey M. Phases and phase transitions of the glycoglycerolipids. Chem. Phys. Lipids. 1994;69:181–207. doi: 10.1016/0009-3084(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 51.Mannock D.A., Lewis R.N., Gruner S.M. An analysis of the relationship between fatty acid composition and the lamellar gel to liquid-crystalline and the lamellar to inverted nonlamellar phase transition temperatures of phosphatidylethanolamines and diacyl-α-D-glucosyl glycerols. Eur. Biophys. J. 2001;30:537–554. doi: 10.1007/s00249-001-0185-z. [DOI] [PubMed] [Google Scholar]
- 52.Toledo A., Crowley J.T., Benach J.L. Selective association of outer surface lipoproteins with the lipid rafts of Borrelia burgdorferi. MBio. 2014;5 doi: 10.1128/mBio.00899-14. e00899-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Q., London E. Transmembrane protein (perfringolysin o) association with ordered membrane domains (rafts) depends upon the raft-associating properties of protein-bound sterol. Biophys. J. 2013;105:2733–2742. doi: 10.1016/j.bpj.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grosjean K., Mongrand S., Gerbeau-Pissot P. Differential effect of plant lipids on membrane organization: specificities of phytosphingolipids and phytosterols. J. Biol. Chem. 2015;290:5810–5825. doi: 10.1074/jbc.M114.598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White D.C., Frerman F.E. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J. Bacteriol. 1968;95:2198–2209. doi: 10.1128/jb.95.6.2198-2209.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.