Abstract
Current models of lipid rafts propose that lipid domains exist as nanoscale compositional fluctuations and these fluctuations can potentially be stabilized into larger domains, consequently better compartmentalizing cellular functions. However, the mechanisms governing stabilized raft assembly and function remain unclear. Here, we test the role of glycolipid crosslinking as a raft targeting and ordering mechanism using the well-studied raft marker cholera toxin B pentamer (CTxB) that binds up to five GM1 glycosphingolipids to enter host cells. We show that when applied to cell-derived giant plasma membrane vesicles, a variant of CTxB containing only a single functional GM1 binding site exhibits significantly reduced partitioning to the ordered phase compared to wild-type CTxB with five binding sites. Moreover, monovalent CTxB does not stabilize membrane domains, unlike wild-type CTxB. These results support the long-held hypothesis that CTxB stabilizes raft domains via a lipid crosslinking mechanism and establish a role for crosslinking in the partitioning of CTxB to ordered domains.
Main Text
The plasma membrane is hypothesized to contain dynamic subdiffraction-sized coexisting liquid-ordered (lo, raft) and liquid-disordered (ld) phases (1). Although not readily apparent in intact cells, micron-sized lo and ld phases have been observed in isolated giant plasma membrane vesicles (GPMVs). It has been proposed that these micron-sized phase separations are related to the nanoscale organization of membranes (2). Thus, GPMVs can be used as a model system for understanding the origins of lipid-mediated membrane organization (2, 3). Several proteins are known to associate with the lo phase in GPMVs, suggesting they are selectively targeted to raftlike domains (4). However, mechanisms that control the partitioning of proteins to rafts remain incompletely understood.
Cholera toxin (CTx), an AB5 toxin, is a well-known lipid raft marker that has been used extensively to study the origins and functionality of lipid rafts (5). Its homopentameric B subunit (CTxB) recognizes and binds up to five molecules of its glycolipid receptor ganglioside GM1 (5). Previous studies have established that CTxB partitions into the lo phase, defining it as a raft marker (6, 7, 8). (Working definitions of terminologies used are in the Supporting Material.) This preference of CTxB for the lo phase is thought to arise in part from the intrinsic phase preference of GM1 itself (9). Moreover, binding of CTxB can drive the formation of both nanoscale and large-scale domains in cell-derived vesicles and model membranes that are close to a demixing point (6, 7, 8, 10). Thus, CTxB not only functions as a raft marker but also as a domain inducer and raft stabilizer. It is widely assumed that these properties of CTxB depend on its ability to bind and cluster multiple GM1 molecules (6). However, this model remains to be formally tested. In this Letter, we test this hypothesis by comparing the behavior of wild-type forms of CTx and CTxB that can bind up to five GM1 molecules with corresponding monovalent variants (mCTx and mCTxB) that can bind only a single GM1 (11).
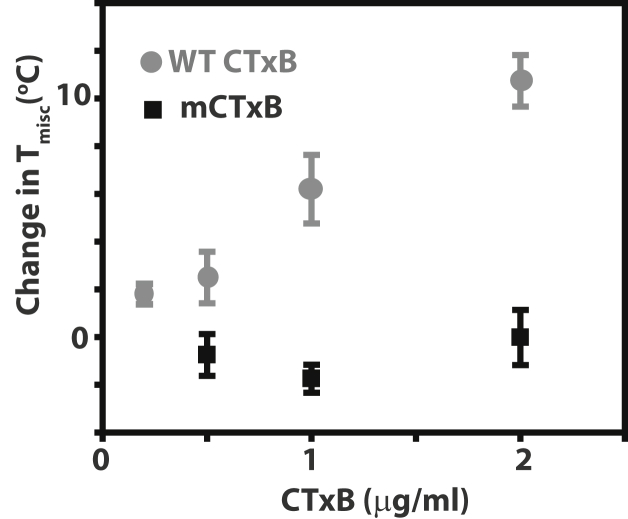
We first examined whether the ability of CTxB to bind multiple copies of its glycolipid receptor stabilizes micron-sized domains in GPMVs. To test this, we compared the effect of wild-type (WT) versus mCTxB binding on the miscibility transition temperature (Tmisc) of GPMVs. Tmisc is defined as the temperature at which 50% of the GPMVs contain coexisting lo and ld phases and provides a measure of the energetics of mixing behavior of the two phases (12, 13). Treatment of GPMVs with WT CTxB has been previously reported to increase Tmisc, indicating that rafts are stabilized in the presence of CTxB (7). Consistent with this, we found that GPMVs isolated from COS-7 cells and subsequently treated with WT CTxB exhibited an increased Tmisc. This change in Tmisc was dependent on the concentration of CTxB (Fig. 1). Similar results were obtained in a second cell line and when COS-7 cells were labeled with CTxB before GPMV isolation (Fig. S3 in the Supporting Material). In contrast, binding of mCTxB to the GPMVs did not change Tmisc over the range of concentrations studied (Fig. 1). Thus, while WT CTxB binding stabilizes microscopic domains in the membrane, mCTxB is incapable of doing so.
Figure 1.
WT CTxB but not mCTxB increases Tmisc of GPMVs isolated from COS-7 cells. See text for details. Data represent mean ± SE (N ≥ 3).
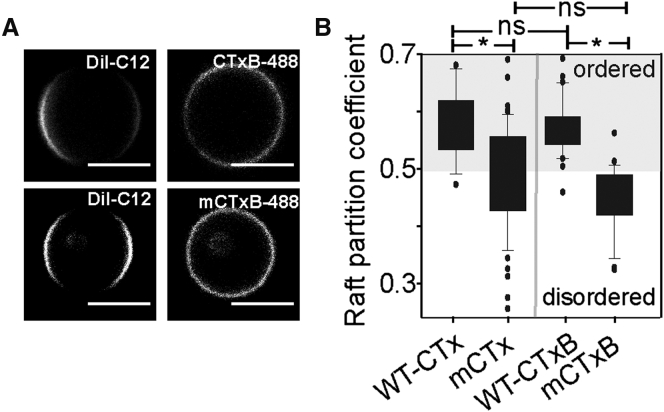
We next compared the phase preference of WT and monovalent CTx and CTxB (refer to Supporting Materials and Methods and Fig. S4 for details). As expected (2), WT CTxB and CTx exhibited lo phase preference (Fig. 2). In contrast, mCTx and mCTxB, which cannot cluster GM1, had reduced raft preference (Fig. 2). This difference in partitioning was not a consequence of the decreased avidity of binding of monovalent compared to the WT toxins (Fig. S4). Thus, preferential partitioning of CTx and CTxB into the lo phase appears to be an emergent behavior dependent on its binding to multiple molecules of GM1.
Figure 2.
Monovalent and WT variants of CTx and CTxB exhibit different phase partitioning preferences in GPMVs derived from COS-7 cells. (A) Representative images of GPMVs labeled with WT or monovalent CTxB and the ld phase marker DiI-C12. Scale bars, 5 μm. (B) Quantification of raft partition coefficients for WT and monovalent CTx and CTxB.
To confirm if clustering is sufficient to drive raft partitioning and raft stabilization, we examined the effects of antibody crosslinking. Crosslinking of mCTxB increased the Tmisc of the GPMVs (Fig. S5) and induced mCTxB to strongly partition into the lo phase (Figs. S6 and S7), consistent with the role of clustering in enabling raft partitioning.
Our results have two major implications for our understanding of raft stabilization and partitioning mechanisms. First, they strongly suggest that the previously observed stabilization of microscopic phases by CTxB (6, 7, 8) indeed has its origins in the ability of CTxB to bind multiple molecules of GM1. While the impact of toxin binding is observed at the micron scale in our experiments, this likely translates into differences in plasma membrane organization at the nanoscale under physiological conditions (refer to the Supporting Material for further discussion).
Second, we find that the mere binding of CTxB to GM1 is not sufficient to ensure its preference for partitioning into the lo phase. One potential explanation for these findings is that clustering of GM1 induced by CTxB binding increases its lo phase preference. This model stems from earlier experimental observations that CTxB binds initially to the disordered phase in GUVs and with increased time partitions into the lo phase (14). Such a rationale is further supported by our current findings that an increase in clustering induced through antibody crosslinking of the toxin increases raft partitioning. However, additional factors could also potentially contribute to differences in the phase preference of WT and monovalent CTxB. For example, binding of CTxB to GM1 is cooperative (15, 16), depends on the orientation of the GM1 headgroup (17, 18, 19), and is sensitive to the local density and clustering of GM1 (17, 20). Another major factor that can impact the partitioning of CTxB is the structure of GM1’s ceramide moiety (21). Thus, even though clustering of GM1 by CTxB stabilizes large-scale domains and is linked to CTxB’s phase preference, properties of GM1 itself could also influence these behaviors.
For CTx, it is known that loss of even a single GM1 binding site results in significant loss of toxicity (11, 22). This loss of toxicity has been hypothesized to reflect a requirement for multivalent binding to scaffold GM1 into nanodomains to support subsequent membrane trafficking steps (11). Our results demonstrating a role of multivalent binding in CTxB’s raft partitioning and domain stabilization strongly support this hypothesis. Finally, we note that many toxins and viruses utilize a multivalent glycolipid binding strategy to gain access to host cells (23, 24). Further, clustering of glycolipids has been hypothesized to play a role in many cellular functions (25, 26, 27, 28). Our results validate the underlying assumption in these biological scenarios that clustering of glycolipids enables raft partitioning and stabilization.
Author Contributions
A.K.K., K.R., W.I.L., and M.G.J. designed research; K.R., T.H.W., D.J.C., and M.G.J. performed research; K.R. contributed analytic tools; K.R., T.H.W., and M.G.J. analyzed data; K.R., A.K.K., M.G.J., W.I.L, and D.J.C. wrote the article; and all authors reviewed the final manuscript.
Acknowledgments
Supported by NIH grant No. R01 GM106720 (to A.K.K.), the Stanley Cohen Innovation Fund (to A.K.K.), and grant Nos. DK048106, DK084424, and DK034854 (to W.I.L.) from the Harvard Digestive Disease Center.
Editor: Claudia Steinem.
Footnotes
Supporting Materials and Methods, seven figures, and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)31035-9.
Supporting Citations
References (29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47) appear in the Supporting Material.
Supporting Material
References
- 1.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart T., Hammond A.T., Webb W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veatch S.L., Cicuta P., Baird B. Critical fluctuations in plasma membrane vesicles. ACS Chem. Biol. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta P., Hammond A., Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim. Biophys. Acta. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day C.A., Kenworthy A.K. Functions of cholera toxin B-subunit as a raft cross-linker. Essays Biochem. 2015;57:135–145. doi: 10.1042/bse0570135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingwood D., Ries J., Simons K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc. Natl. Acad. Sci. USA. 2008;105:10005–10010. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson S.A., Stinson B.M., Baumgart T. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim. Biophys. Acta. 2010;1798:1427–1435. doi: 10.1016/j.bbamem.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond A.T., Heberle F.A., Feigenson G.W. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sezgin E., Levental I., Schwille P. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim. Biophys. Acta. 2012;1818:1777–1784. doi: 10.1016/j.bbamem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Štefl M., Šachl R., Hof M. Dynamics and size of cross-linking-induced lipid nanodomains in model membranes. Biophys. J. 2012;102:2104–2113. doi: 10.1016/j.bpj.2012.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jobling M.G., Yang Z., Holmes R.K. A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway. MBio. 2012;3:1–9. doi: 10.1128/mBio.00401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray E., Karslake J., Veatch S.L. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys. J. 2013;105:2751–2759. doi: 10.1016/j.bpj.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghunathan K., Ahsan A., Veatch S.L. Membrane transition temperature determines cisplatin response. PLoS One. 2015;10:e0140925. doi: 10.1371/journal.pone.0140925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacia K., Schwille P., Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin H., Kitova E.N., Klassen J.S. Measuring positive cooperativity using the direct ESI-MS assay. Cholera toxin B subunit homopentamer binding to GM1 pentasaccharide. J. Am. Soc. Mass Spectrom. 2014;25:104–110. doi: 10.1007/s13361-013-0751-5. [DOI] [PubMed] [Google Scholar]
- 16.Lencer W.I., Chu S.H., Walker W.A. Differential binding kinetics of cholera toxin to intestinal microvillus membrane during development. Infect. Immun. 1987;55:3126–3130. doi: 10.1128/iai.55.12.3126-3130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J., Yang T., Cremer P.S. GM1 clustering inhibits cholera toxin binding in supported phospholipid membranes. J. Am. Chem. Soc. 2007;129:5954–5961. doi: 10.1021/ja069375w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahfoud R., Manis A., Lingwood C.A. A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins. J. Biol. Chem. 2010;285:36049–36059. doi: 10.1074/jbc.M110.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingwood D., Binnington B., Simons K. Cholesterol modulates glycolipid conformation and receptor activity. Nat. Chem. Biol. 2011;7:260–262. doi: 10.1038/nchembio.551. [DOI] [PubMed] [Google Scholar]
- 20.Šachl R., Amaro M., Hof M. On multivalent receptor activity of GM1 in cholesterol containing membranes. Biochim. Biophys. Acta. 2014;1853:850–857. doi: 10.1016/j.bbamcr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Chinnapen D.J.F., Hsieh W.T., Lencer W.I. Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev. Cell. 2012;23:573–586. doi: 10.1016/j.devcel.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf A.A., Jobling M.G., Lencer W.I. Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster ganglioside GM1 molecules. Infect. Immun. 2008;76:1476–1484. doi: 10.1128/IAI.01286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewers H., Smith R.W., Johannes L. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010;12:11–18. doi: 10.1038/ncb1999. [DOI] [PubMed] [Google Scholar]
- 24.Römer W., Berland L., Johannes L. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 25.Ledeen R.W., Wu G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 2015;40:407–418. doi: 10.1016/j.tibs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Ekyalongo R.C., Nakayama H., Iwabuchi K. Organization and functions of glycolipid-enriched microdomains in phagocytes. Biochim. Biophys Acta. 2015;1851:90–97. doi: 10.1016/j.bbalip.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K., Yamaguchi T., Matsuzaki K. Mechanism of amyloid β-protein aggregation mediated by GM1 ganglioside clusters. Biochemistry. 2011;50:6433–6440. doi: 10.1021/bi200771m. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa N., Iwabuchi K., Arikawa-Hirasawa E. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 2009;122:289–299. doi: 10.1242/jcs.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.London E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim. Biophys. Acta. 2005;1746:203–220. doi: 10.1016/j.bbamcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Wenz J.J., Barrantes F.J. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 2003;42:14267–14276. doi: 10.1021/bi035759c. [DOI] [PubMed] [Google Scholar]
- 31.Levental K.R., Lorent J.H., Levental I. Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys. J. 2016;110:1800–1810. doi: 10.1016/j.bpj.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putzel G.G., Schick M. Theory of raft formation by the cross-linking of saturated or unsaturated lipids in model lipid bilayers. Biophys. J. 2009;96:4935–4940. doi: 10.1016/j.bpj.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machta B.B., Veatch S.L., Sethna J.P. Critical Casimir forces in cellular membranes. Phys. Rev. Lett. 2012;109:138101. doi: 10.1103/PhysRevLett.109.138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honigmann A., Sadeghi S., Vink R. A lipid bound actin meshwork organizes liquid phase separation in model membranes. eLife. 2014;3:e01671. doi: 10.7554/eLife.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edidin M. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 36.Simons K., Vaz W.L.C. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 37.de Almeida R.F.M., Loura L.M., Prieto M. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J. Mol. Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Sun H., Chen L., Fang W. Nanodomain formation of ganglioside GM1 in lipid membrane: effects of Cholera toxin mediated crosslinking. Langmuir. 2015;31:9105–9114. doi: 10.1021/acs.langmuir.5b01866. [DOI] [PubMed] [Google Scholar]
- 39.Tian A., Baumgart T. Sorting of lipids and proteins in membrane curvature gradients. Biophys. J. 2009;96:2676–2688. doi: 10.1016/j.bpj.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safouane M., Berland L., Bassereau P. Lipid cosorting mediated by Shiga toxin induced tubulation. Traffic. 2010;11:1519–1529. doi: 10.1111/j.1600-0854.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- 41.Day C.A., Baetz N.W., Kenworthy A.K. Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis. Traffic. 2015;16:572–590. doi: 10.1111/tra.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jobling M.G., Holmes R.K. Identification of motifs in cholera toxin A1 polypeptide that are required for its interaction with human ADP-ribosylation factor 6 in a bacterial two-hybrid system. Proc. Natl. Acad. Sci. USA. 2000;97:14662–14667. doi: 10.1073/pnas.011442598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jobling M.G., Holmes R.K. Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Mol. Microbiol. 1991;5:1755–1767. doi: 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 44.Merritt E.A., Sarfaty S., Hol W.G. Structural studies of receptor binding by cholera toxin mutants. Protein Sci. 1997;6:1516–1528. doi: 10.1002/pro.5560060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujinaga Y., Wolf A.A., Lencer W.I. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulum. Mol. Biol. Cell. 2003;14:4783–4793. doi: 10.1091/mbc.E03-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liljeqvist S., Ståhl S., Murby M. Fusions to the cholera toxin B subunit: influence on pentamerization and GM1 binding. J. Immunol. Methods. 1997;210:125–135. doi: 10.1016/s0022-1759(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 47.Kang D., Gho Y.S., Kang C. Highly sensitive and fast protein detection with Coomassie brilliant blue in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Bull. Korean Chem. Soc. 2002;23:1511–1512. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.