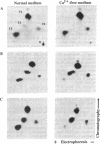
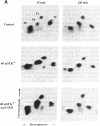
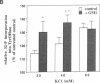
Abstract
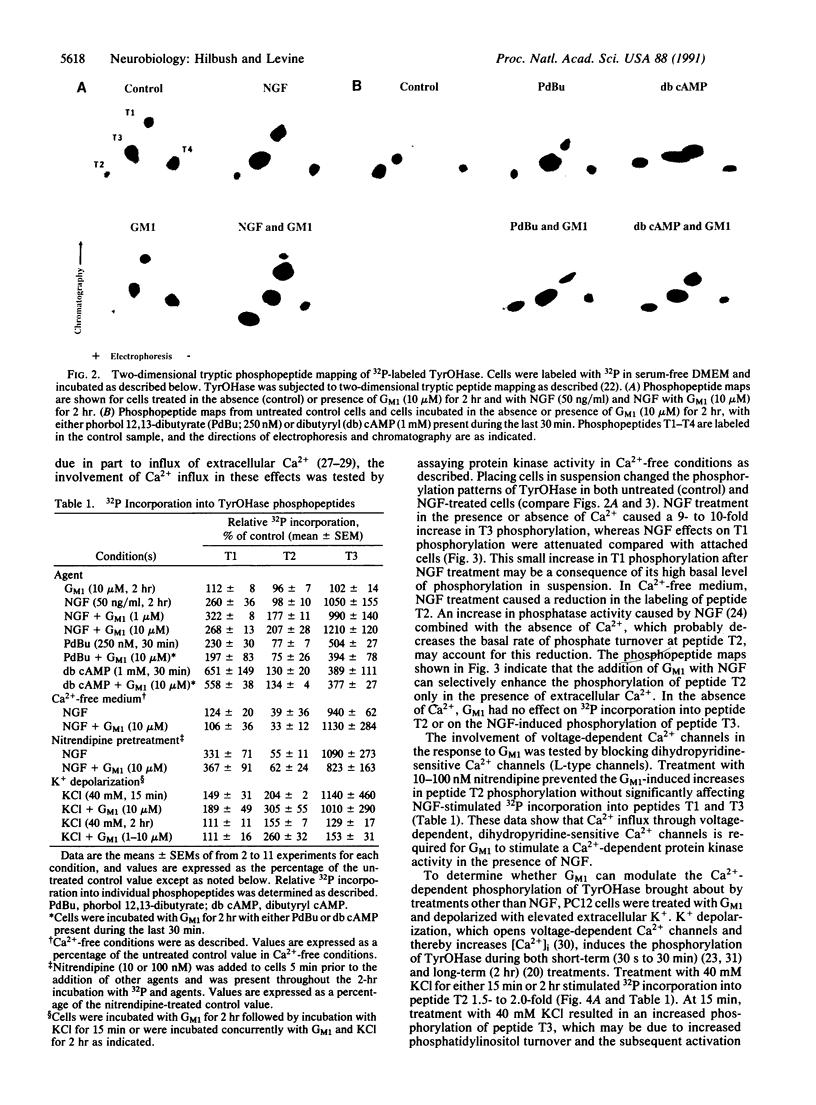
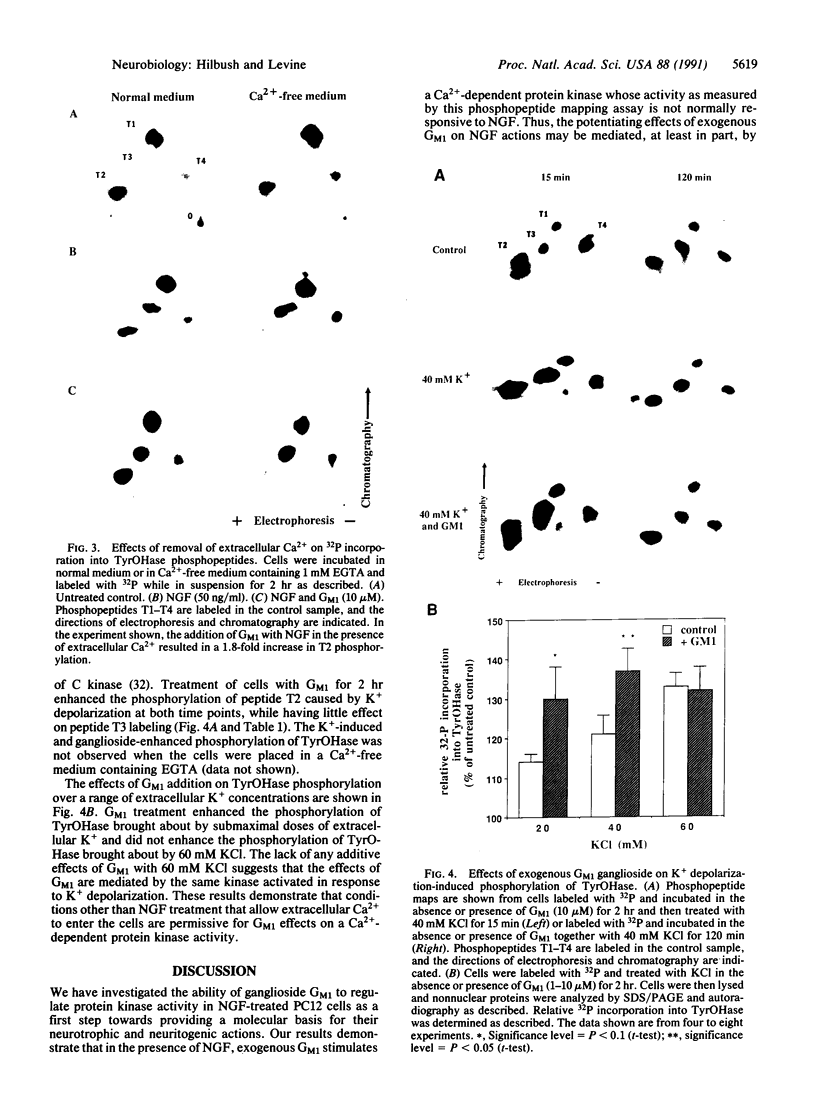
We have investigated the ability of exogenous gangliosides to modulate nerve growth factor (NGF) signal transduction in PC12 cells. The effects of exogenous ganglioside GM1 on multiple protein kinase activities were assayed by analyzing site-specific serine phosphorylation of tyrosine hydroxylase (TyrOHase) by two-dimensional phosphopeptide mapping. In the presence of NGF, exogenous GM1 (1-10 microM) increased 32P incorporation into TyrOHase phosphopeptide T2, a Ca2+/calmodulin-dependent protein kinase substrate whose phosphorylation is not normally affected by NGF treatment. In the absence of NGF, GM1 treatment had no significant effects on TyrOHase phosphorylation. The removal of extracellular Ca2+ or blockade of dihydropyridine-sensitive Ca2+ channels prevented the GM1-induced increases in 32P incorporation into phosphopeptide T2. Exogenous GM1 also potentiated K+ depolarization-induced increases in the phosphorylation of TryOHase. These results suggest that the stimulatory effects of exogenous GM1 ganglioside on NGF actions may be due to its ability to potentiate a Ca(2+)-dependent signaling pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Farber I. C., Shahar A., Grinvald A. Localization of voltage-sensitive calcium channels along developing neurites: their possible role in regulating neurite elongation. Dev Biol. 1982 Dec;94(2):351–365. doi: 10.1016/0012-1606(82)90353-0. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986 Feb 15;261(5):2434–2440. [PubMed] [Google Scholar]
- Cahill A. L., Horwitz J., Perlman R. L. Phosphorylation of tyrosine hydroxylase in protein kinase C-deficient PC12 cells. Neuroscience. 1989;30(3):811–818. doi: 10.1016/0306-4522(89)90172-3. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Hardie D. G., Vulliet P. R. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986 Aug 15;261(23):10489–10492. [PubMed] [Google Scholar]
- Chan K. F. Ganglioside-modulated protein phosphorylation. Partial purification and characterization of a ganglioside-stimulated protein kinase in brain. J Biol Chem. 1987 Apr 15;262(11):5248–5255. [PubMed] [Google Scholar]
- Cimino M., Benfenati F., Farabegoli C., Cattabeni F., Fuxe K., Agnati L. F., Toffano G. Differential effect of ganglioside GM1 on rat brain phosphoproteins: potentiation and inhibition of protein phosphorylation regulated by calcium/calmodulin and calcium/phospholipid-dependent protein kinases. Acta Physiol Scand. 1987 Jun;130(2):317–325. doi: 10.1111/j.1748-1716.1987.tb08143.x. [DOI] [PubMed] [Google Scholar]
- Cremins J., Wagner J. A., Halegoua S. Nerve growth factor action is mediated by cyclic AMP- and Ca+2/phospholipid-dependent protein kinases. J Cell Biol. 1986 Sep;103(3):887–893. doi: 10.1083/jcb.103.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G., Fabris M., Gorio A. Gangliosides enhance neurite outgrowth in PC12 cells. Brain Res. 1983 Jun;284(2-3):215–221. doi: 10.1016/0165-3806(83)90006-8. [DOI] [PubMed] [Google Scholar]
- Fukunaga K., Miyamoto E., Soderling T. R. Regulation of Ca2+/calmodulin-dependent protein kinase II by brain gangliosides. J Neurochem. 1990 Jan;54(1):103–109. doi: 10.1111/j.1471-4159.1990.tb13288.x. [DOI] [PubMed] [Google Scholar]
- Gazzotti G., Sonnino S., Ghidoni R. Normal-phase high-performance liquid chromatographic separation of non-derivatized ganglioside mixtures. J Chromatogr. 1985 Dec 4;348(2):371–378. doi: 10.1016/s0021-9673(01)92475-6. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Otis L. C., Yu R. K., DeLorenzo R. J. Calcium/ganglioside-dependent protein kinase activity in rat brain membrane. J Neurochem. 1985 Apr;44(4):1229–1234. doi: 10.1111/j.1471-4159.1985.tb08748.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Patrick J. Nerve growth factor mediates phosphorylation of specific proteins. Cell. 1980 Nov;22(2 Pt 2):571–581. doi: 10.1016/0092-8674(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Haycock J. W. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990 Jul 15;265(20):11682–11691. [PubMed] [Google Scholar]
- Jessell T. M. Adhesion molecules and the hierarchy of neural development. Neuron. 1988 Mar;1(1):3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R., Skaper S. D., Varon S. Interaction of GM1 ganglioside with PC12 pheochromocytoma cells: serum- and NGF-dependent effects on neuritic growth (and proliferation). J Neurosci Res. 1984;12(2-3):299–310. doi: 10.1002/jnr.490120217. [DOI] [PubMed] [Google Scholar]
- Kreutter D., Kim J. Y., Goldenring J. R., Rasmussen H., Ukomadu C., DeLorenzo R. J., Yu R. K. Regulation of protein kinase C activity by gangliosides. J Biol Chem. 1987 Feb 5;262(4):1633–1637. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lankford K. L., Letourneau P. C. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J Cell Biol. 1989 Sep;109(3):1229–1243. doi: 10.1083/jcb.109.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovici P., Levi B. Z., Lelkes P. I., Koizumi S., Fujita K., Matsuda Y., Ozato K., Guroff G. K-252a inhibits the increase in c-fos transcription and the increase in intracellular calcium produced by nerve growth factor in PC12 cells. J Neurosci Res. 1989 May;23(1):1–8. doi: 10.1002/jnr.490230102. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Seeley P. J., Müller T. H., Helmer-Matyjek E., Sabban E., Goldstein M., Greene L. A. Regulation of tyrosine hydroxylase phosphorylation in PC12 pheochromocytoma cells by elevated K+ and nerve growth factor. Evidence for different mechanisms of action. Mol Pharmacol. 1985 Aug;28(2):220–228. [PubMed] [Google Scholar]
- Leon A., Benvegnù D., Dal Toso R., Presti D., Facci L., Giorgi O., Toffano G. Dorsal root ganglia and nerve growth factor: a model for understanding the mechanism of GM1 effects on neuronal repair. J Neurosci Res. 1984;12(2-3):277–287. doi: 10.1002/jnr.490120215. [DOI] [PubMed] [Google Scholar]
- Leon A., Facci L., Benvegnù D., Toffano G. Morphological and biochemical effects of gangliosides in neuroblastoma cells. Dev Neurosci. 1982;5(1):108–114. doi: 10.1159/000112666. [DOI] [PubMed] [Google Scholar]
- Maher P. A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta S. G., Yorke G., Roisen F. J. Neuritogenic and metabolic effects of individual gangliosides and their interaction with nerve growth factor in cultures of neuroblastoma and pheochromocytoma. Brain Res. 1986 Jun;392(1-2):243–252. doi: 10.1016/0165-3806(86)90250-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- McTigue M., Cremins J., Halegoua S. Nerve growth factor and other agents mediate phosphorylation and activation of tyrosine hydroxylase. A convergence of multiple kinase activities. J Biol Chem. 1985 Jul 25;260(15):9047–9056. [PubMed] [Google Scholar]
- Meldolesi J., Huttner W. B., Tsien R. Y., Pozzan T. Free cytoplasmic Ca2+ and neurotransmitter release: studies on PC12 cells and synaptosomes exposed to alpha-latrotoxin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):620–624. doi: 10.1073/pnas.81.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella-Alonso A., Malgaroli A., Vicentini L. M., Meldolesi J. Early rise of cytosolic Ca2+ induced by NGF in PC12 and chromaffin cells. FEBS Lett. 1986 Nov 10;208(1):48–51. doi: 10.1016/0014-5793(86)81529-0. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Whitlock C., Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978 Jun 29;273(5665):718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Adv Second Messenger Phosphoprotein Res. 1988;22:39–112. [PubMed] [Google Scholar]
- Skaper S. D., Katoh-Semba R., Varon S. GM1 ganglioside accelerates neurite outgrowth from primary peripheral and central neurons under selected culture conditions. Brain Res. 1985 Nov;355(1):19–26. doi: 10.1016/0165-3806(85)90003-3. [DOI] [PubMed] [Google Scholar]
- Vulliet P. R., Woodgett J. R., Cohen P. Phosphorylation of tyrosine hydroxylase by calmodulin-dependent multiprotein kinase. J Biol Chem. 1984 Nov 25;259(22):13680–13683. [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]