Abstract
Peroxiredoxins (PRDXs), a ubiquitous family of redox‐regulating proteins, are reported of potential to eliminate various reactive oxygen species (ROS). As a major member of the antioxidant enzymes, PRDX1 can become easily over‐oxidized on its catalytically active cysteine induced by a variety of stimuli in vitro and in vivo. In nucleus, oligomeric PRDX1 directly associates with p53 or transcription factors such as c‐Myc, NF‐κB and AR, and thus affects their bioactivities upon gene regulation, which in turn induces or suppresses cell death. Additionally, PRDX1 in cytoplasm has anti‐apoptotic potential through direct or indirect interactions with several ROS‐dependent (redox regulation) effectors, including ASK1, p66Shc, GSTpi/JNK and c‐Abl kinase. PRDX1 is proven to be a versatile molecule regulating cell growth, differentiation and apoptosis. Recent studies have found that PRDX1 and/or PRDX1‐regulated ROS‐dependent signalling pathways play an important role in the progression and metastasis of human tumours, particularly in breast, oesophageal and lung cancers. In this paper, we review the structure, effector functions of PRDX1, its role in cancer and the pivotal role of ROS in anticancer treatment.
Keywords: peroxiredoxin 1, reactive oxygen species, cancer
Introduction
Reactive oxygen species (ROS), including superoxide (O2 −), hydrogen peroxide (H2O2) and hydroxyl radical (OH•), are converted directly or indirectly from free oxygen but are more chemically reactive 1, 2. The low‐to‐moderate ROS level is indispensable to normal cellular proliferation, differentiation and survival 3. The net emission of ROS depends upon the balance between free radical production (pro‐oxidative process) and its elimination by antioxidants (antioxidant defense process) 4. Peroxiredoxins (PRDXs), a family of this kind of antioxidants, are classified on the basis of having either one (1‐Cys) or two (2‐Cys) conserved cysteine residues 5. PRDX1 is a member of the 2‐Cys PRDXs subfamily and is present mainly in the cytosol 6. PRDX1 containing a cysteine at the N‐terminal Cys52 detoxifies peroxides at the expense of Cys52 oxidation through intermolecular disulphide formation with the other conserved C‐terminal Cys173 residue 7. Oxidized PRDX1 could be converted to its active form by various mechanisms 7, 8.
PRDX1 was firstly reported as an antioxidant enzyme, but its physiological role in oxidization–reduction balance remains unclear because it is highly susceptible to oxidative stress. Upon peroxidatic Cys oxidation, 2‐Cys PRDX1 is structurally converted from a peroxidase enzyme to a molecular chaperone under stress conditions 9, 10. In addition to its peroxidase and chaperone functions, PRDX1 could also enhance natural killer cell cytotoxicity and suppress oncogenic proteins such as c‐Myc and c‐Abl 11, 12, 13. Recent studies have shown that abnormal expression of PRDX1 has been observed in several human cancers, including breast, oesophageal, lung and prostate cancers 14, 15, 16, 17. Furthermore, PRDX1 also regulates several ROS‐dependent signalling pathways and is thought to be a key intracellular intermediate balancing cell survival and apoptosis 7, 18.
In this review, we provide an overview of the structure, effector functions of PRDX1 and its role in ROS‐dependent signalling pathways. We also summarize the recent advances implicating PRDX1 in cancer development and the importance of targeting ROS‐dependent/redox pathways in anticancer treatment.
Structure, functions of PRDX1 and its role in ROS‐dependent signalling
Mammalian PRDXs are divided into three categories based on their number of conserved Cys residues and catalytic mechanism: 2‐Cys PRDXs (PRDX1–4), atypical 2‐Cys PRDX (PRDX5) and 1‐Cys PRDX (Prx6) 5. All PRDXs contain a thioredoxin fold with a few additional secondary structure elements present as insertions 5. PRDX1 contains a conserved Cys52 at the N‐terminal and a conserved Cys173 at the C‐terminal 7. PRDX1 is domain‐swapped homodimers in which the C terminus of one subunit reaches across the dimer interface to interact with the other subunit 8. In addition, PRDX1 crystallized as toroid‐shaped complexes consisting of a pentameric arrangement of dimers [an (a2)5 decamer], consistent with observations that PRDX1 dimers can form discrete higher‐order oligomers 8. Compared with other antioxidant enzymes, PRDX1 employs a particular mechanism to detoxify peroxide with reducing equivalents provided through the thioredoxin (Trx) system but not from glutaredoxin 19.
Previous studies have indicated that PRDXs or Trx peroxidases are important endogenous antioxidants, protecting cells from oxidative damage by reducing H2O2 and peroxynitrite and scavenging thiyl radicals 20, 21, 22. PRDX1 cooperates with Trx in suppressing H2O2‐induced cell death involving different types of kinases and enzymes, such as apoptosis signal‐regulating kinase 1 (ASK1), p66Shc and glutathione S‐transferase pi (GSTpi)/c‐Jun NH2‐terminal kinase (JNK), which play key roles in the regulation of cell death and/or apoptosis depending on cell type and stimuli 23, 24, 25. Additionally, PRDX1 is known as a physiological inhibitor of c‐Abl tyrosine kinase. It could bind the SH‐3 domain of c‐Abl 13. As c‐Abl plays a crucial role in oxidative stress‐induced cell death and is known to function as an upstream effector of the JNK and p38 mitogen‐activated protein kinase (MAPK) pathways 18, 26, we speculate that PRDX1 may play a role in suppressing stress‐induced cell death through ROS‐dependent signalling pathway (Fig. 1). Notably, PRDX1 prevents Akt‐driven tumourigenesis and then induces cell death through protecting phosphatase and tensin homologue (PTEN) lipid phosphatase activity from oxidation‐induced inactivation 7.
Figure 1.
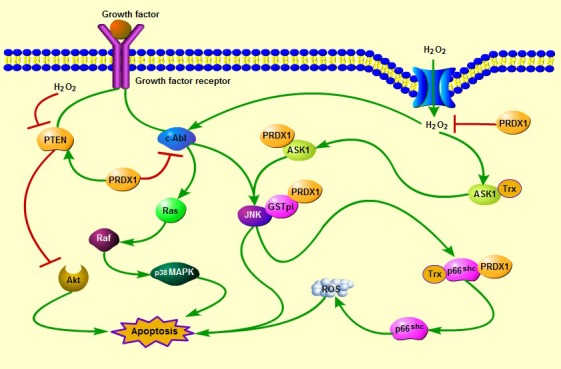
The roles of cytoplasm PRDX1 in the oxidative stress‐induced apoptosis. PRDX1 cooperates with Trx in suppressing H2O2‐induced cell death involving different types of kinases and enzymes, such as ASK1, p66Shc and GSTpi/JNK. In addition, PRDX1 could inhibit JNK and p38 MAPK‐induced cell apoptosis through direct interaction with c‐Abl tyrosine kinase under oxidative stress conditions. Notably, PRDX1 prevents Akt‐driven tumourigenesis and then promotes cell apoptosis through protecting PTEN lipid phosphatase activity from oxidation‐induced inactivation.
The function of PRDX1 is not restricted to its antioxidant activities in cancers. It is also known that PRDX1 functions as a chaperone in the form of oligomers, and molecular chaperone activities enhanced under oxidative stress conditions 27, 28. PRDX1 oligomer could interact with the c‐Myc oncogene and suppresses its transcriptional activity, which in turn inhibits tumourigenesis and promotes tumour cell apoptosis 29. However, PRDX1 may act as an oncogene, and suppresses tumour cell death by directly associating with transcription factors such as nuclear factor kappa B (NF‐κB) and androgen receptor (AR) 30, 31. It has been widely recognized that p53‐dependent apoptosis signalling is participated in the oxidative stress‐induced cell death 32. P53 induces the expression of apoptosis factors such as Bak and Bax under oxidative stress conditions, which in turn promotes the activation of caspases, and then activates the mitochondrial apoptotic signalling pathway. Interestingly, oligomeric PRDX1 is an essential intermediate in H2O2‐induced mammalian Ste20‐like kinase‐1 (MST1) activation and cell apoptosis through p53 33. In addition, oxidative stress induces the c‐Abl‐dependent tyrosine phosphorylation of MST1 and increases the interaction between MST1 and FOXO3 (Forkhead box O3), thereby activating the MST1–FOXO signalling pathway and leading to cell death 34. It has been demonstrated that c‐Abl is also known as a modulator of p53 signalling pathway 35.These studies suggest that oligomeric PRDX1 may play a key role in modulating stress‐induced apoptosis through its interaction with p53 or nuclear transcription factors (Fig. 2).
Figure 2.
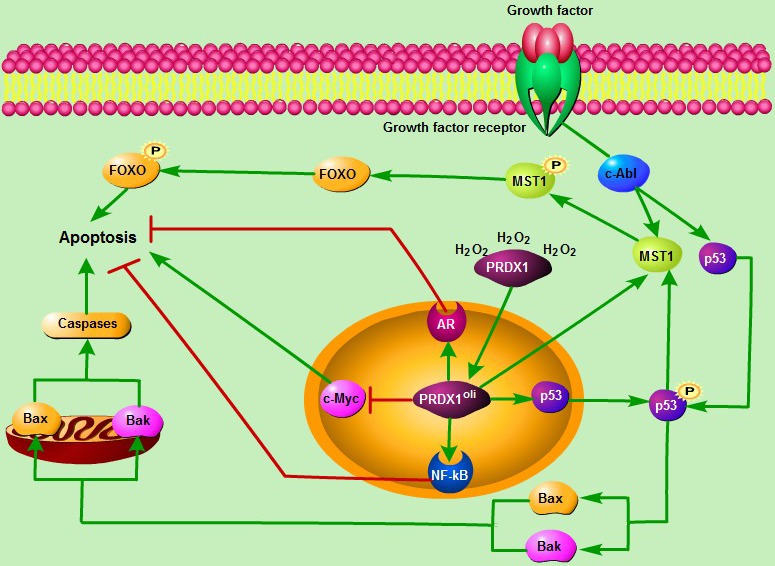
The roles of nucleus PRDX1 in the H2O2‐induced apoptosis. It is also known that PRDX1 functions as a chaperone in the form of oligomers, and molecular chaperone activities enhanced under oxidative stress conditions. PRDX1 oligomer directly interacts with the transcription factors including c‐Myc, NF‐κB and AR, and thus affects their bioactivities upon gene regulation, which in turn induces or suppresses cell death. In addition, p53 induces the expression of apoptosis factors such as Bak and Bax under oxidative stress conditions, and promotes the activation of caspases and p53‐dependent mitochondria apoptotic signalling pathway. Importantly, PRDX1 oligomer is an essential intermediate in H2O2‐induced activation of c‐Abl/MST1/FOXO signalling pathway and cell apoptosis through direct interaction with p53. Furthermore, it has been recognized that c‐Abl acts as a modulator of p53. PRDX1oli, PRDX1 oligomer.
Genomic studies in cancer
DNA amplification or chromosomal losses is a common mechanism leading to oncogenic activation in human cancers. PRDX1 gene is located on chromosomal band 1q34.1. Loss of heterozygosity (LOH) of 1q has been suggested in tumour progression 36, 37, 38. Mice lacking PRDX1 develop severe haemolytic anaemia and various types of malignancies, indicating that PRDX1 functions as a tumour suppressor 39. In the region of 1q33‐34, Sulman E P et al. have identified several tumour suppressor candidates, including MUTYH, PRDX1, FOXD2, FOXE3, PTCH2 and RAD54L genes in meningioma 40. However, PRDX1 gene mutations have not been identified in human tumours. Interestingly, several studies have confirmed that the PRDX1 gene is overexpressed in human malignancies, suggesting that PRDX1 may be a proto‐oncogene.
The identification of PRDX1 as a potential oncogene was reported in the cases of lung adenocarcinomas 41, 42, colorectal cancer 43 and soft tissue sarcomas 44. 1q is amplified in approximately 35.5% of lung cancer 45, and the PRDX1 expression is significantly elevated in lung cancer patients as compared with patients with benign lung disease 46. Li R et al. found that elevated expression of PRDX1 is characterized of both increased DNA copy number and increased transcript levels in lung adenocarcinoma 41. Recurrent gains were found for chromosome 1q in colorectal cancer 47, 48. PRDX1 was obviously amplified in colorectal primary tumours compared with normal colon samples by the significance analysis of microarrays 43. Taken together, these genomic studies validate the presence of PRDX1 amplification in human cancers.
PRDX1 and breast cancer
The specific role for PRDX1 in mammary carcinomas is controversial. PRDX1 protein was found to be overexpressed in breast cancer tissues from most patients compared with normal tissues, but no significant relationship was found between PRDX1 levels and clinicopathological factors, including oestrogen receptor (ER) status 14, 49. Similar to these data, Wang X et al. found that PRDX1 is more abundant in breast cancer cell lines (including ER+ and ER‐) than in normal or pseudonormal breast cell lines, but not found a significant difference in PRDX1 levels between ER+ and ER‐ breast cancer cell lines 50. However, other studies have shown that elevated mRNA expression of PRDX1 in human breast carcinoma is relevant with higher tumour grade 51, and high expression of cytoplasmic PRDX1 correlated with a great risk of local recurrence after radiotherapy 52. PRDX1 could act as a chaperone to enhance the transactivation potential of NF‐κB in ER‐breast cancer cells, and then suppresses tumour cell death 50. Meanwhile, PRDX1 has a protective function in doxorubicin/peroxide‐induced cytotoxicity of breast cancer cells 53, 54. Repression of PRDX1 expression leads to high levels of ROS‐induced phosphorylation of p38 MAPKα, and promotes H2O2‐induced senescence in breast cancer cells 55. It was also found that drug resistance formation is accompanied by a significant increase in the expression of PRDX1 gene in breast cancer cell strains, which confirms the important contribution of redox‐dependent mechanisms to the development of cisplatin resistance of cancer cells 56.
Conversely, there are several reports indicated that PRDX1 may act as a tumour suppressor in breast cancer. PRDX1‐deficient mice suffer from shortened lifespan owing to the development of haemolytic anaemia and several malignant cancers including breast carcinomas 39. Simultaneous expression of PRDX1 and c‐Myc in PRDX1‐deficient mice cells apparently attenuates regulation of some c‐Myc target genes and thus inhibits tumour growth, indicating a role for PRDX1 as a tumour suppressor 12, 29. In addition, PRDX1 oligomer mediates cisplatin‐induced MST1 activation and p53‐dependent cell death in breast cancer 33. It has been reported that high PRDX1 expression appears to be associated with less aggressive breast tumours 57. In addition, Cao J et al. have identified that PRDX1 protects the tumour suppressive function of PTEN phosphatase from ROS‐induced inactivation, and inhibits Ras‐driven mammary tumours 58. Notably, biomarker studies have confirmed that PRDX1 is one of the diagnostic biomarkers for invasive ductal carcinoma of breast with human epidermal growth factor receptor‐2 (HER2)‐enriched subtypes 59, and PRDX1 protects ERα from oxidative stress‐induced suppression and is a protein biomarker of favourable prognosis in mammary tumours 60.
miRNAs post‐transcriptionally repress gene expression mostly by binding to the 3′UTR of mRNA transcripts to either cause degradation or prevent translation, depending upon complementarity. It was identified that miR‐510 is elevated in breast tumour samples while absent in the matched non‐tumour breast tissue samples 61. Overexpression of miR‐510 leads to decreased PRDX1, which, in turn, increases the activity of PI3K/Akt pathway and promoted cell and tumour growth in breast cancer 62. This exemplifies yet another mechanism of PRDX1 regulation in breast carcinogenesis.
PRDX1 and oesophageal cancer
Similar to breast cancer, the functions of PRDX1 in oesophageal cancer remain enigmatic. Proteomic study has found that the expression level of PRDX1 is elevated in oesophagus squamous cell carcinoma (ESCC) tissue 63. Further study demonstrated that PRDX1 is significantly increased in ESCC tissues compared with the paired adjacent normal tissues 15. In addition, several studies have identified that PRDX1 is overexpressed in ESCC cells compared with the non‐cancerous oesophageal epithelial cells 64, 65. Elevated PRDX1 promotes tumourigenesis through regulating the activity of mTORp70S6K pathway in ESCC 65. Interestingly, silencing PRDX1 leads to increased levels of tumour suppressor genes LKB1 and p‐AMPK, and decreases the oncogene Aurora A expression, suggesting that PRDX1 may affect carcinogenesis 64. Apart from these, PRDX1 also has a protective function in dioscin/ionizing radiation‐induced ROS accumulation in oesophageal cancer cells 66, 67. These data highlight that PRDX1 promotes tumourigenesis by functioning as “accomplices” of certain oncoproteins or by the activity of its antioxidant enzyme.
However, several lines of evidence suggest that PRDX1 may play a role as a tumour suppressor in oesophageal cancer. p21WAF1, a cyclin‐dependent kinase inhibitor, has been reported to play an important role in the maintenance of cell cycle arrest 68, 69, 70. Histone deacetylase inhibitor FK228 induces growth inhibition and apoptosis in human ESCC cells, at least partially through activating the PRDX1 gene with histones H3 and H4 acetylation of its promoter, resulting in elevated p21WAF1 expression 71. Accordingly, Hoshino I et al. showed that high PRDX1 expression appears to be associated with longer overall survival in ESCC 72.
PRDX1 and lung cancer
Previous studies have shown that PRDX1 is expressed at significantly higher levels in lung cancer tissues compared with normal lung tissues 73, 74, 75, 76, and elevated PRDX1 associated with shorter survival in non‐small cell lung cancer (NSCLC) 77, 78. Furthermore, PRDX1 is up‐regulated in NSCLC tissue interstitial fluid, and high level of PRDX1 expression is related with lymph node metastasis and tumour differentiation, suggesting that PRDX1 may act as a marker of neoplastic progression 46. Knockdown of PRDX1 in lung cancer cells significantly inhibits transforming growth factor β1 (TGF‐β1)‐induced epithelial–mesenchymal transition (EMT) and cell migration, whereas PRDX1 overexpression enhances TGF‐β1‐induced EMT and cell migration 79. In addition, in vivo studies have shown that silencing of PRDX1 leads to tumour suppression of growth and metastases through reducing the activation of c‐Jun 80, 81. However, a recent study suggests that PRDX1 functions as a nuclear erythroid 2‐related factor 2 (Nrf2) dependently inducible tumour suppressant in K‐ras‐driven lung tumourigenesis by prohibiting ROS/ERK/cyclin D1 pathway activation 82.
PRDX1 also suppresses drug/radiation‐induced cytotoxicity in lung cancer, and the related mechanisms are under investigation 25, 83. PRDX1 knockdown evokes an increase in cellular apoptotic potential through activation of the caspase cascade and suppression docetaxel‐induced phosphorylation of Akt and its substrate FOXO1 in A549 xenograft tumours 83, 84. In addition, PRDX1 suppresses JNK activation through an indirect interaction with JNK in a peroxidase‐independent manner 25. GSTpi has been shown to play a great role in suppressing JNK activation by forming the GSTpi‐JNK complex 85, 86, 87. When cells are exposed to ionizing radiation or ROS, GSTpi is oxidized, thereby triggering a dissociation of the GSTpi‐JNK complex and releasing JNK. Interestingly, PRDX1 physically interacts with GSTpi and suppresses JNK release/activation from the GSTpi‐JNK complex after γ‐ray radiation exposure in human lung cancer 1170i cells 25.
As mentioned above, PRDX1 is a potential target for cancer radiotherapy and/or chemotherapy. Several studies have suggested that anticancer molecules/gene reverse radioresistance through targeting PRDX1 on human lung cancer 88, 89, 90. Vitamin K3 (vitK3) is a synthetic naphthoquinone exhibiting significant anticancer activity against multiple human cancers both in vitro and in vivo 91, 92, 93, and its major anticancer mechanism is depended on the generation of ROS 94, 95. Increased ROS accumulation inhibits PRDX1 expression and induces lung cancer cell death after vitK3 treatment 96. In addition, after the treatment of JS‐K, a nitric oxide prodrug, oxidative/nitrosative stress is evoked in lung adenocarcinoma cells with high basal levels of ROS/reactive nitrogen species (RNS), and PRDX1 correlating with drug dose 97. PRDX1 is a useful biomarker for identifying tumours response to therapy as PRDX1 protein can be easily measured in lung cancer 98, 99.
PRDX1 and prostate cancer
PRDX1 overexpression is also found in human prostate cancer specimens and prostate cancer cell lines 17, 100. Prostate cancer risk and prognosis are adversely associated with a number of inflammatory and angiogenic mediators, including Toll‐like receptor 4 (TLR4), NF‐κB and VEGF 101, 102, 103, 104. Elevated PRDX1 increases prostate tumour vasculature, and shows up‐regulation of angiogenic proteins such as VEGF in the tumour region. Conversely, silencing of PRDX1 in prostate cancer cell lines reduces tumour vascular formation, and causes down‐regulation of VEGF 17, 105. PRDX1 mediates these effects by activated TLR4 and NF‐κB, which results in the increased expression of hypoxia inducible factor‐1α (HIF‐1α), a transcription factor involved in angiogenesis 105. This novel PRDX1‐TLR4‐ HIF‐1α pathway can be targeted by TLR4 inhibitors and can perhaps serve as a therapeutic target in the treatment of PRDX1‐driven prostate tumour angiogenesis.
In addition, AR signalling is integral to the development and progression of prostate cancer 106. Hypoxia/reoxygenation activates the AR in cultured prostate cancer cells, and PRDX1 acts as a key mediator as monitored with an AR‐dependent luciferase reporter assay 107. PRDX1 overexpression enhances the AR activity in response to hypoxia/reoxygenation, whereas PRDX1 knockdown has the opposite effect and reduces the growth rate of the androgen‐dependent cancer cells 107. Interestingly, the AR stimulatory function of PRDX1 is independent of its antioxidant activity 31, 107. PRDX1 enhances AR function in prostate cancer cells through increasing affinity of AR to dihydrotestosterone (DHT) 31. In PRDX1‐rich LNCap cells, the combination of PRDX1 knockdown and finasteride, a structural analogue of DHT, was found to produce a greater inhibitory effect on AR activity and cell growth than either treatment alone 108. The above findings suggest that PRDX1 may play a critical role in the context of developing strategies to improve the outcome of androgen deprivation therapy in prostate cancer. Furthermore, a train of evidence indicates that PRDX1 and other ROS‐regulated enzymes are targets for modulating intracellular redox status in therapeutic strategies for prostate cancer 109, 110, 111.
PRDX1 and other types of malignancy
The levels of PRDX1 expression are significantly increased in pancreatic cancer compared to normal tissues, and this overexpression is closely related to tumour angiogenesis 112. In addition, PRDX1 associates with the formation of membrane protrusions through modulation of the activity of p38MAPK, which in turn promotes pancreatic cancer cell invasion 113. Although the expression of PRDX1 is weak in cervical cancer 114, PRDX1 knockdown significantly enhances HeLa cell sensitivity to ROS‐generating drugs 115. A previous study has reported that PRDX1 suppresses proteasome inhibitor‐mediated cell death through the influence on ASK1 activation in human thyroid cancer 116. However, Nicolussi A et al. have demonstrated that PRDX1 and PRDX6 are reduced in papillary thyroid carcinomas by BRAF V600E‐dependent and ‐independent mechanisms 117. Additionally, down‐regulation of PRDX1 suppresses growth and promotes apoptosis in bladder cancer cells through NF‐kB signalling pathway after Bifidobacterium infantis thymidine kinase/nucleoside analogue ganciclovir (BI‐TK/GCV) treatment 118. Overexpression of PRDX1 has also been observed in gallbladder cancer 119, cholangiocarcinoma 120, 121, liver cancer 122 and abdominal aortic aneurysm 123. Recently, reduced expression of PRDX1 is frequent in oligodendroglial tumours with 1p/19q deletion and likely contributes to radio/chemosensitivity of these tumours 124, 125. Subsequently, the decreased expression of PRDX1 and PRDX2 has also been identified in melanomas 126, and PRDX2 represses melanoma metastasis by increasing E‐cadherin/β‐catenin complexes in the plasma membrane 127. Considering that PRDX1 and PRDX2 belong to typical 2‐Cys PRDXs, and have similar active sites 8, we therefore hypothesize that PRDX1 may also play important roles in the suppression of melanoma development. Interestingly, the expression and role of PRDX1 in B cell‐derived malignancies is controversial. PRDX1 expression is high in multiple myeloma, but absent in plasmablastic lymphoma 128. However, Trzeciecka A et al. have demonstrated that PRDX1 and PRDX2 are up‐regulated in Burkitt lymphoma, and lymphoma cells treated with SK053 trigger the formation of covalent PRDX dimers, accumulation of intracellular reactive oxygen species, phosphorylation of ERK1/2 and AKT and attenuate the cells growth rate 129. By contrary, high total PRDXs is correlated with favourable disease‐specific survival and overall survival in patients with follicular lymphoma 130.
Conclusion and future perspectives
As discussed above, the expression and functional roles of PRDX1 in many human tumours remain controversial (summarized in Table 1), and further researches need to be done to reveal its underlying role in the progression and metastasis of cancer. In spite of the various functions of PRDX1 in cancers, we could acutely discover that when PRDX1 acts as antioxidant enzyme but not chaperone, it plays a positive role in radio/chemoresistance. The current PRDX1‐based anticancer studies are mainly based on its antioxidant activity. Although results from preclinical trial, both in vivo and in vitro, for PRDX1‐based anticancer are promising, there is a long path from laboratory to clinical trial. Another important concern is off‐target effect. Is the drug targeting PRDX1 sufficient for controlling the final anticancer effects when other PRDXs and/or ROS‐independent tyrosine kinase are present? If the answer is positive, then what is the underlying mechanism? Only these events are fully explored, can the treatment targeting PRDX1 be used in clinical application.
Table 1.
Expression and functional characterization of PRDX1 in cancer
| Name | Expression | Role | Target gene | References |
|---|---|---|---|---|
| Breast cancer | Up‐regulated | Oncogene | NF‐κB | 50 |
| Up‐regulated | Tumour suppressor | c‐Myc, p53 | 12, 29, 33, 39 | |
| Oesophageal cancer | Up‐regulated | Oncogene | mTOR | 15, 65 |
| Up‐regulated | Tumour suppressor | Not mentioned | 71 | |
| Lung cancer | Up‐regulated | Oncogene | GSTpi‐JNK, c‐Jun | 25, 80, 81 |
| Prostate cancer | Up‐regulated | Oncogene | AR | 31, 107 |
| Pancreatic cancer | Up‐regulated | Oncogene | p38 MAPK | 112, 113 |
| Cervical cancer | Weak | Chemoresistance | Not mentioned | 114, 115 |
| Thyroid cancer | Up‐regulated | Oncogene | ASK1 | 116 |
| Down‐regulated | Tumour suppressor | BRAF | 117 | |
| Oligodendroglial tumour | Down‐regulated | Radio/chemosensitivity | Not mentioned | 124, 125 |
| Melanoma | Down‐regulated | Not mentioned | Not mentioned | 126 |
| B cell‐derived lymphoma | Up‐regulated | Oncogene | SK053 | 129 |
| Down‐regulated | Not mentioned | Not mentioned | 128 |
Moreover, several important scientific issues remain to be addressed. The basal levels of ROS are higher in PRDX1‐/‐ murine embryonic fibroblasts and erythrocytes, whereas no significant differences are observed in either splenocytes or hepatocytes, indicating that PRDX1 controls ROS levels in certain tissues 29. Interestingly, Demasi AP et al. have demonstrated that PRDX1 is expressed in plasma cells but not in B lymphocytes, suggesting that its expression is development‐associated 128. Notably, PRDX1 is highly susceptible to oxidative stress and its molecular chaperone activities enhanced under oxidative stress conditions. In addition, PRDX1 often cooperates with other PRDXs and/or redox‐regulating proteins such as Trx in suppressing H2O2‐induced cell death. According to these data, we speculate that the plausible reasons for the ambiguous expression and function of PRDX1 in tumours are listed as following: (i) PRDX1 expression is development‐associated in some tissues; (ii) Redox activity and chaperone activity, which play a dominant role decided the role of PRDX1 in promoting or suppressing oncogenesis for certain types of cancer; (iii) PRDX1 may have specificity in some tissues or populations; (iv) The final functional role of PRDX1 is influenced by the synergy or suppression of other PRDXs and/or redox‐regulating molecules.
Conflicts of interest
No conflict of interest.
Author contribution
Chenbo Ding was responsible for the conception and design of the manuscript. Chenbo Ding participated in drafting the manuscript. Xiaobo Fan and Guoqiu Wu were responsible for the review and/or revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30970809, 81271636), the Natural Science Foundation of Jiangsu Province (No. BK2009274) and the Special Fund of Clinical Medicine of Jiangsu Province (No. BL2012063).
References
- 1. Kobayashi CI, Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2012; 227: 421–30. [DOI] [PubMed] [Google Scholar]
- 2. Gupta SC, Hevia D, Patchva S, et al Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012; 16: 1295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giannoni E, Buricchi F, Raugei G, et al Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage‐dependent cell growth. Mol Cell Biol. 2005; 25: 6391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iwabuchi T, Yoshimoto C, Shigetomi H, et al Oxidative stress and antioxidant defense in endometriosis and its malignant transformation. Oxid Med Cell Longev. 2015; 2015: 848595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhee SG, Kang SW, Chang TS, et al Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001; 52: 35–41. [DOI] [PubMed] [Google Scholar]
- 6. Hirotsu S, Abe Y, Okada K, et al Crystal structure of a multifunctional 2‐Cys peroxiredoxin heme‐binding protein 23 kDa/ proliferation‐associated gene product. Proc Natl Acad Sci USA. 1999; 96: 12333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009; 8: 4072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wood ZA, Schroder E, Robin Harris J, et al Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003; 28: 32–40. [DOI] [PubMed] [Google Scholar]
- 9. Jang HH, Lee KO, Chi YH, et al Two enzymes in one; two yeast peroxiredoxins display oxidative stress‐dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004; 117: 625–35. [DOI] [PubMed] [Google Scholar]
- 10. Moon JC, Hah YS, Kim WY, et al Oxidative stress‐dependent structural and functional switching of a human 2‐Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2‐induced cell death. J Biol Chem. 2005; 280: 28775–84. [DOI] [PubMed] [Google Scholar]
- 11. Shau H, Gupta RK, Golub SH. Identification of a natural killer enhancing factor (NKEF) from human erythroid cells. Cell Immunol. 1993; 147: 1–11. [DOI] [PubMed] [Google Scholar]
- 12. Mu ZM, Yin XY, Prochownik EV. Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c‐Myc and selectively alters its biological function and target gene expression. J Biol Chem. 2002; 277: 43175–84. [DOI] [PubMed] [Google Scholar]
- 13. Wen ST, Van Etten RA. The PAG gene product, a stress‐induced protein with antioxidant properties, is an Abl SH3‐binding protein and a physiological inhibitor of c‐Abl tyrosine kinase activity. Genes Dev. 1997; 11: 2456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karihtala P, Mantyniemi A, Kang SW, et al Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003; 9: 3418–24. [PubMed] [Google Scholar]
- 15. Ren P, Ye H, Dai L, et al Peroxiredoxin 1 is a tumor‐associated antigen in esophageal squamous cell carcinoma. Oncol Rep. 2013; 30: 2297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deng B, Ye N, Luo G, et al Proteomics analysis of stage‐specific proteins expressed in human squamous cell lung carcinoma tissues. Cancer Biomark. 2005; 1: 279–86. [DOI] [PubMed] [Google Scholar]
- 17. Riddell JR, Bshara W, Moser MT, et al Peroxiredoxin 1 controls prostate cancer growth through Toll‐like receptor 4‐dependent regulation of tumor vasculature. Cancer Res. 2011; 71: 1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishii T, Warabi E, Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr. 2012; 50: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang B, Wang Y, Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009; 286: 154–60. [DOI] [PubMed] [Google Scholar]
- 20. Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005; 38: 1543–52. [DOI] [PubMed] [Google Scholar]
- 21. Netto LE, Antunes F. The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol Cells. 2016; 39: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujii J, Ikeda Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002; 7: 123–30. [DOI] [PubMed] [Google Scholar]
- 23. Kim SY, Kim TJ, Lee KY. A novel function of peroxiredoxin 1 (Prx‐1) in apoptosis signal‐regulating kinase 1 (ASK1)‐mediated signaling pathway. FEBS Lett. 2008; 582: 1913–8. [DOI] [PubMed] [Google Scholar]
- 24. Gertz M, Fischer F, Leipelt M, et al Identification of Peroxiredoxin 1 as a novel interaction partner for the lifespan regulator protein p66Shc. Aging (Albany NY). 2009; 1: 254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YJ, Lee WS, Ip C, et al Prx1 suppresses radiation‐induced c‐Jun NH2‐terminal kinase signaling in lung cancer cells through interaction with the glutathione S‐transferase Pi/c‐Jun NH2‐terminal kinase complex. Cancer Res. 2006; 66: 7136–42. [DOI] [PubMed] [Google Scholar]
- 26. Neumann CA, Fang Q. Are peroxiredoxins tumor suppressors? Curr Opin Pharmacol. 2007; 7: 375–80. [DOI] [PubMed] [Google Scholar]
- 27. Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signal. 2011; 15: 781–94. [DOI] [PubMed] [Google Scholar]
- 28. Lee W, Choi KS, Riddell J, et al Human peroxiredoxin 1 and 2 are not duplicate proteins: the unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J Biol Chem. 2007; 282: 22011–22. [DOI] [PubMed] [Google Scholar]
- 29. Egler RA, Fernandes E, Rothermund K, et al Regulation of reactive oxygen species, DNA damage, and c‐Myc function by peroxiredoxin 1. Oncogene. 2005; 24: 8038–50. [DOI] [PubMed] [Google Scholar]
- 30. Hansen JM, Moriarty‐Craige S, Jones DP. Nuclear and cytoplasmic peroxiredoxin‐1 differentially regulate NF‐kappaB activities. Free Radic Biol Med. 2007; 43: 282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chhipa RR, Lee KS, Onate S, et al Prx1 enhances androgen receptor function in prostate cancer cells by increasing receptor affinity to dihydrotestosterone. Mol Cancer Res. 2009; 7: 1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Budanov AV. The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell Biochem. 2014; 85: 337–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morinaka A, Funato Y, Uesugi K, et al Oligomeric peroxiredoxin‐I is an essential intermediate for p53 to activate MST1 kinase and apoptosis. Oncogene. 2011; 30: 4208–18. [DOI] [PubMed] [Google Scholar]
- 34. Xiao L, Chen D, Hu P, et al The c‐Abl‐MST1 signaling pathway mediates oxidative stress‐induced neuronal cell death. J Neurosci. 2011; 31: 9611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levav‐Cohen Y, Goldberg Z, Zuckerman V, et al C‐Abl as a modulator of p53. Biochem Biophys Res Commun. 2005; 331: 737–49. [DOI] [PubMed] [Google Scholar]
- 36. Leone PE, Bello MJ, de Campos JM, et al NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999; 18: 2231–9. [DOI] [PubMed] [Google Scholar]
- 37. Müller P, Henn W, Niedermayer I, et al Deletion of chromosome 1p and loss of expression of alkaline phosphatase indicate progression of meningiomas. Clin Cancer Res. 1999; 5: 3569–77. [PubMed] [Google Scholar]
- 38. Lamszus K, Vahldiek F, Mautner VF, et al Allelic losses in neurofibromatosis 2‐associated meningiomas. J Neuropathol Exp Neurol. 2000; 59: 504–12. [DOI] [PubMed] [Google Scholar]
- 39. Neumann CA, Krause DS, Carman CV, et al Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003; 424: 561–5. [DOI] [PubMed] [Google Scholar]
- 40. Sulman EP, White PS, Brodeur GM. Genomic annotation of the meningioma tumor suppressor locus on chromosome 1p34. Oncogene. 2004; 23: 1014–20. [DOI] [PubMed] [Google Scholar]
- 41. Li R, Wang H, Bekele BN, et al Identification of putative oncogenes in lung adenocarcinoma by a comprehensive functional genomic approach. Oncogene. 2006; 25: 2628–35. [DOI] [PubMed] [Google Scholar]
- 42. Wright CM, Larsen JE, Hayward NK, et al ADAM28: a potential oncogene involved in asbestos‐related lung adenocarcinomas. Genes Chromosom Cancer. 2010; 49: 688–98. [DOI] [PubMed] [Google Scholar]
- 43. Lin HM, Chatterjee A, Lin YH, et al Genome wide expression profiling identifies genes associated with colorectal liver metastasis. Oncol Rep. 2007; 17: 1541–9. [DOI] [PubMed] [Google Scholar]
- 44. Takahashi A, Nakayama R, Ishibashi N, et al Analysis of gene expression profiles of soft tissue sarcoma using a combination of knowledge‐based filtering with integration of multiple statistics. PLoS ONE. 2014; 9: e106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yen CC, Liang SC, Jong YJ, et al Chromosomal aberrations of malignant pleural effusions of lung adenocarcinoma: different cytogenetic changes are correlated with genders and smoking habits. Lung Cancer. 2007; 57: 292–301. [DOI] [PubMed] [Google Scholar]
- 46. Li S, Wang R, Zhang M, et al Proteomic analysis of non‐small cell lung cancer tissue interstitial fluids. World J Surg Oncol. 2013; 11: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mampaey E, Fieuw A, Van Laethem T, et al Focus on 16p13.3 locus in colon cancer. PLoS ONE. 2015; 10: e0131421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loo LW, Tiirikainen M, Cheng I, et al Integrated analysis of genome‐wide copy number alterations and gene expression in microsatellite stable, CpG island methylator phenotype‐negative colon cancer. Genes Chromosom Cancer. 2013; 52: 450–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noh DY, Ahn SJ, Lee RA, et al Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001; 21: 2085–90. [PubMed] [Google Scholar]
- 50. Wang X, He S, Sun JM, et al Selective association of peroxiredoxin 1 with genomic DNA and COX‐2 upstream promoter elements in estrogen receptor negative breast cancer cells. Mol Biol Cell. 2010; 21: 2987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009; 28: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woolston CM, Storr SJ, Ellis IO, et al Expression of thioredoxin system and related peroxiredoxin proteins is associated with clinical outcome in radiotherapy treated early stage breast cancer. Radiother Oncol. 2011; 100: 308–13. [DOI] [PubMed] [Google Scholar]
- 53. Goncalves K, Sullivan K, Phelan S. Differential expression and function of peroxiredoxin 1 and peroxiredoxin 6 in cancerous MCF‐7 and noncancerous MCF‐10A breast epithelial cells. Cancer Invest. 2012; 30: 38–47. [DOI] [PubMed] [Google Scholar]
- 54. McDonald C, Muhlbauer J, Perlmutter G, et al Peroxiredoxin proteins protect MCF‐7 breast cancer cells from doxorubicin‐induced toxicity. Int J Oncol. 2014; 45: 219–26. [DOI] [PubMed] [Google Scholar]
- 55. Turner‐Ivey B, Manevich Y, Schulte J, et al Role for Prdx1 as a specific sensor in redox‐regulated senescence in breast cancer. Oncogene. 2013; 32: 5302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalinina EV, Berezov TT, Shtil' AA, et al Expression of peroxiredoxin 1, 2, 3, and 6 genes in cancer cells during drug resistance formation. Bull Exp Biol Med. 2012; 153: 878–81. [DOI] [PubMed] [Google Scholar]
- 57. Pei‐Jou ChuaE‐HL, Chunhua G, George W‐CY, et al Clinicopathological correlation of peroxiredoxin I in breast cancer In: Proceedings of the World Medical Conference. 2011, Prague, Czech Republic. [Google Scholar]
- 58. Cao J, Schulte J, Knight A, et al Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009; 28: 1505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pendharkar N, Gajbhiye A, Taunk K, et al Quantitative tissue proteomic investigation of invasive ductal carcinoma of breast with luminal B HER2 positive and HER2 enriched subtypes towards potential diagnostic and therapeutic biomarkers. J Proteomics. 2016; 132: 112–30. [DOI] [PubMed] [Google Scholar]
- 60. O'Leary PC, Terrile M, Bajor M, et al Peroxiredoxin‐1 protects estrogen receptor α from oxidative stress‐induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014; 16: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Findlay VJ, Turner DP, Moussa O, et al MicroRNA‐mediated inhibition of prostate‐derived Ets factor messenger RNA translation affects prostate‐derived Ets factor regulatory networks in human breast cancer. Cancer Res. 2008; 68: 8499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo QJ, Mills JN, Bandurraga SG, et al MicroRNA‐510 promotes cell and tumor growth by targeting peroxiredoxin1 in breast cancer. Breast Cancer Res. 2013; 15: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang J, Wang K, Zhang J, et al Using proteomic approach to identify tumor‐associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011; 10: 2863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gong F, Liu H, Li J, et al Peroxiredoxin 1 is involved in disassembly of flagella and cilia. Biochem Biophys Res Commun. 2014; 444: 420–6. [DOI] [PubMed] [Google Scholar]
- 65. Gong F, Hou G, Liu H, et al Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med Oncol. 2015; 32: 455. [DOI] [PubMed] [Google Scholar]
- 66. Wang Z, Cheng Y, Wang N, et al Dioscin induces cancer cell apoptosis through elevated oxidative stress mediated by downregulation of peroxiredoxins. Cancer Biol Ther. 2012; 13: 138–47. [DOI] [PubMed] [Google Scholar]
- 67. Gao MC, Jia XD, Wu QF, et al Silencing Prx1 and/or Prx5 sensitizes human esophageal cancer cells to ionizing radiation and increases apoptosis via intracellular ROS accumulation. Acta Pharmacol Sin. 2011; 32: 528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Niculescu AB 3rd, Chen X, Smeets M, et al Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998; 18: 629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li G, Zhang Z, Wang R, et al Suppression of STIM1 inhibits human glioblastoma cell proliferation and induces G0/G1 phase arrest. J Exp Clin Cancer Res. 2013; 32: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ananda Sadagopan SK, Mohebali N, Looi CY, et al Forkhead Box Transcription Factor (FOXO3a) mediates the cytotoxic effect of vernodalin in vitro and inhibits the breast tumor growth in vivo. J Exp Clin Cancer Res. 2015; 34: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoshino I, Matsubara H, Hanari N, et al Histone deacetylase inhibitor FK228 activates tumor suppressor Prdx1 with apoptosis induction in esophageal cancer cells. Clin Cancer Res. 2005; 11: 7945–52. [DOI] [PubMed] [Google Scholar]
- 72. Hoshino I, Matsubara H, Akutsu Y, et al Tumor suppressor Prdx1 is a prognostic factor in esophageal squamous cell carcinoma patients. Oncol Rep. 2007; 18: 867–71. [PubMed] [Google Scholar]
- 73. Kim HJ, Chae HZ, Kim YJ, et al Preferential elevation of Prx I and Trx expression in lung cancer cells following hypoxia and in human lung cancer tissues. Cell Biol Toxicol. 2003; 19: 285–98. [DOI] [PubMed] [Google Scholar]
- 74. Lehtonen ST, Svensk AM, Soini Y, et al Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer. 2004; 111: 514–21. [DOI] [PubMed] [Google Scholar]
- 75. Park JH, Kim YS, Lee HL, et al Expression of peroxiredoxin and thioredoxin in human lung cancer and paired normal lung. Respirology. 2006; 11: 269–75. [DOI] [PubMed] [Google Scholar]
- 76. Chang JW, Jeon HB, Lee JH, et al Augmented expression of peroxiredoxin I in lung cancer. Biochem Biophys Res Commun. 2001; 289: 507–12. [DOI] [PubMed] [Google Scholar]
- 77. Kim JH, Bogner PN, Ramnath N, et al Elevated peroxiredoxin 1, but not NF‐E2‐related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non‐small cell lung cancer. Clin Cancer Res. 2007; 13: 3875–82. [DOI] [PubMed] [Google Scholar]
- 78. Kim JH, Bogner PN, Baek SH, et al Up‐regulation of peroxiredoxin 1 in lung cancer and its implication as a prognostic and therapeutic target. Clin Cancer Res. 2008; 14: 2326–33. [DOI] [PubMed] [Google Scholar]
- 79. Ha B, Kim EK, Kim JH, et al Human peroxiredoxin 1 modulates TGF‐β1‐induced epithelial‐mesenchymal transition through its peroxidase activity. Biochem Biophys Res Commun. 2012; 421: 33–7. [DOI] [PubMed] [Google Scholar]
- 80. Chen MF, Keng PC, Shau H, et al Inhibition of lung tumor growth and augmentation of radiosensitivity by decreasing peroxiredoxin I expression. Int J Radiat Oncol Biol Phys. 2006; 64: 581–91. [DOI] [PubMed] [Google Scholar]
- 81. Jiang H, Wu L, Mishra M, et al Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. Am J Cancer Res. 2014; 4: 445–60. [PMC free article] [PubMed] [Google Scholar]
- 82. Park YH, Kim SU, Lee BK, et al Prx I suppresses K‐ras‐driven lung tumorigenesis by opposing redox‐sensitive ERK/cyclin D1 pathway. Antioxid Redox Signal. 2013; 19: 482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hwang KE, Park DS, Kim YS, et al Prx1 modulates the chemosensitivity of lung cancer to docetaxel through suppression of FOXO1‐induced apoptosis. Int J Oncol. 2013; 43: 72–8. [DOI] [PubMed] [Google Scholar]
- 84. Hwang KE, Park C, Seol CH, et al Elevated prx1 provides resistance to docetaxel, but is not associated with predictive significance in lung cancer. Tuberc Respir Dis (Seoul). 2013; 75: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Adler V, Yin Z, Fuchs SY, et al Regulation of JNK signaling by GSTp. EMBO J. 1999; 18: 1321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yin Z, Ivanov VN, Habelhah H, et al Glutathione S‐transferase p elicits protection against H2O2‐induced cell death via coordinated regulation of stress kinases. Cancer Res. 2000; 60: 4053–7. [PubMed] [Google Scholar]
- 87. Wang T, Arifoglu P, Ronai Z, et al Glutathione S‐transferase P1‐1 (GSTP1‐1) inhibits c‐Jun N‐terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001; 276: 20999–1003. [DOI] [PubMed] [Google Scholar]
- 88. Chen MF, Chen WC, Wu CT, et al p53 status is a major determinant of effects of decreasing peroxiredoxin I expression on tumor growth and response of lung cancer cells to treatment. Int J Radiat Oncol Biol Phys. 2006; 66: 1461–72. [DOI] [PubMed] [Google Scholar]
- 89. Guo Q, Huang X, Zhang J, et al Downregulation of peroxiredoxin I by a novel fully human phage display recombinant antibody induces apoptosis and enhances radiation sensitization in A549 lung carcinoma cells. Cancer Biother Radiopharm. 2012; 27: 307–16. [DOI] [PubMed] [Google Scholar]
- 90. Li G, Xie B, Li X, et al Downregulation of peroxiredoxin‐1 by β‐elemene enhances the radiosensitivity of lung adenocarcinoma xenografts. Oncol Rep. 2015; 33: 1427–33. [DOI] [PubMed] [Google Scholar]
- 91. Nakaoka E, Tanaka S, Onda K, et al Effects of vitamin K3 and K5 on daunorubicin‐resistant Human T lymphoblastoid leukemia cells. Anticancer Res. 2015; 35: 6041–8. [PubMed] [Google Scholar]
- 92. Suresh S, Raghu D, Karunagaran D. Menadione (Vitamin K3) induces apoptosis of human oral cancer cells and reduces their metastatic potential by modulating the expression of epithelial to mesenchymal transition markers and inhibiting migration. Asian Pac J Cancer Prev. 2013; 14: 5461–5. [DOI] [PubMed] [Google Scholar]
- 93. Chen MF, Yang CM, Su CM, et al Inhibitory effect of vitamin C in combination with vitamin K3 on tumor growth and metastasis of Lewis lung carcinoma xenografted in C57BL/6 mice. Nutr Cancer. 2011; 63: 1036–43. [DOI] [PubMed] [Google Scholar]
- 94. Yang CR, Liao WS, Wu YH, et al CR108, a novel vitamin K3 derivative induces apoptosis and breast tumor inhibition by reactive oxygen species and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2013; 273: 611–22. [DOI] [PubMed] [Google Scholar]
- 95. Bonilla‐Porras AR, Jimenez‐Del‐Rio M, Velez‐Pardo C. Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signalling mechanism. Cancer Cell Int. 2011; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. He T, Hatem E, Vernis L, et al PRX1 knockdown potentiates vitamin K3 toxicity in cancer cells: a potential new therapeutic perspective for an old drug. J Exp Clin Cancer Res. 2015; 34: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maciag AE, Chakrapani H, Saavedra JE, et al The nitric oxide prodrug JS‐K is effective against non‐small‐cell lung cancer cells in vitro and in vivo: involvement of reactive oxygen species. J Pharmacol Exp Ther. 2011; 336: 313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chang JW, Lee SH, Jeong JY, et al Peroxiredoxin‐I is an autoimmunogenic tumor antigen in non‐small cell lung cancer. FEBS Lett. 2005; 579: 2873–7. [DOI] [PubMed] [Google Scholar]
- 99. Rostila A, Puustinen A, Toljamo T, et al Peroxiredoxins and tropomyosins as plasma biomarkers for lung cancer and asbestos exposure. Lung Cancer. 2012; 77: 450–9. [DOI] [PubMed] [Google Scholar]
- 100. Basu A, Banerjee H, Rojas H, et al Differential expression of peroxiredoxins in prostate cancer: consistent upregulation of PRDX3 and PRDX4. Prostate. 2011; 71: 755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hua D, Liu MY, Cheng ZD, et al Small interfering RNA‐directed targeting of Toll‐like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicity. Mol Immunol. 2009; 46: 2876–84. [DOI] [PubMed] [Google Scholar]
- 102. Li J, Xiang S, Zhang Q, et al Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen‐independent prostate cancer cells through SAPK/JNK and MEK/ERK1/2‐mediated targeting NF‐κB/p65 and MUC1‐C. J Exp Clin Cancer Res. 2015; 34: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huss WJ, Hanrahan CF, Barrios RJ, et al Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res. 2001; 61: 2736–43. [PubMed] [Google Scholar]
- 104. Yang L, You S, Kumar V, et al In vitro the behaviors of metastasis with suppression of VEGF in human bone metastatic LNCaP‐derivative C4‐2B prostate cancer cell line. J Exp Clin Cancer Res. 2012; 31: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Riddell JR, Maier P, Sass SN, et al Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF‐1α. PLoS ONE. 2012; 7: e50394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Brinkmann AO, Trapman J. Genetic analysis of androgen receptors in development and disease. Adv Pharmacol. 2000; 47: 317–41. [DOI] [PubMed] [Google Scholar]
- 107. Park SY, Yu X, Ip C, et al Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res. 2007; 67: 9294–303. [DOI] [PubMed] [Google Scholar]
- 108. Wu Y, Chhipa RR, Zhang H, et al The antiandrogenic effect of finasteride against a mutant androgen receptor. Cancer Biol Ther. 2011; 11: 902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shiota M, Izumi H, Miyamoto N, et al Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high‐mobility group protein B1. Cancer Sci. 2008; 99: 1950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Parmigiani RB, Xu WS, Venta‐Perez G, et al HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc Natl Acad Sci U S A. 2008; 105: 9633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schultz MA, Abdel‐Mageed AB, Mondal D. The nrf1 and nrf2 balance in oxidative stress regulation and androgen signaling in prostate cancer cells. Cancers (Basel). 2010; 2: 1354–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cai CY, Zhai LL, Wu Y, et al Expression and clinical value of peroxiredoxin‐1 in patients with pancreatic cancer. Eur J Surg Oncol. 2015; 41: 228–35. [DOI] [PubMed] [Google Scholar]
- 113. Taniuchi K, Furihata M, Hanazaki K, et al Peroxiredoxin 1 promotes pancreatic cancer cell invasion by modulating p38 MAPK activity. Pancreas. 2015; 44: 331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim K, Yu M, Han S, et al Expression of human peroxiredoxin isoforms in response to cervical carcinogenesis. Oncol Rep. 2009; 21: 1391–6. [PubMed] [Google Scholar]
- 115. He T, Hatem E, Vernis L, et al Peroxiredoxin 1 knockdown sensitizes cancer cells to reactive oxygen species‐generating drugs ‐ an alternative approach for chemotherapy. Free Radic Biol Med. 2014; 75: S13. [DOI] [PubMed] [Google Scholar]
- 116. Du ZX, Yan Y, Zhang HY, et al Suppression of MG132‐mediated cell death by peroxiredoxin 1 through influence on ASK1 activation in human thyroid cancer cells. Endocr Relat Cancer. 2010; 17: 553–60. [DOI] [PubMed] [Google Scholar]
- 117. Nicolussi A, D'Inzeo S, Mincione G, et al PRDX1 and PRDX6 are repressed in papillary thyroid carcinomas via BRAF V600E‐dependent and ‐independent mechanisms. Int J Oncol. 2014; 44: 548–56. [DOI] [PubMed] [Google Scholar]
- 118. Jiang L, Xiao X, Ren J, et al Proteomic analysis of bladder cancer indicates Prx‐I as a key molecule in BI‐TK/GCV treatment system. PLoS ONE. 2014; 9: e98764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Li J, Yang ZL, Ren X, et al ILK and PRDX1 are prognostic markers in squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Tumour Biol. 2013; 34: 359–68. [DOI] [PubMed] [Google Scholar]
- 120. Zhou J, Shen W, He X, et al Overexpression of Prdx1 in hilar cholangiocarcinoma: a predictor for recurrence and prognosis. Int J Clin Exp Pathol. 2015; 8: 9863–74. [PMC free article] [PubMed] [Google Scholar]
- 121. Yonglitthipagon P, Pairojkul C, Chamgramol Y, et al Prognostic significance of peroxiredoxin 1 and ezrin‐radixin‐moesin‐binding phosphoprotein 50 in cholangiocarcinoma. Hum Pathol. 2012; 43: 1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sun YL, Cai JQ, Liu F, et al Aberrant expression of peroxiredoxin 1 and its clinical implications in liver cancer. World J Gastroenterol. 2015; 21: 10840–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martinez‐Pinna R, Ramos‐Mozo P, Madrigal‐Matute J, et al Identification of peroxiredoxin‐1 as a novel biomarker of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2011; 31: 935–43. [DOI] [PubMed] [Google Scholar]
- 124. Dittmann LM, Danner A, Gronych J, et al Downregulation of PRDX1 by promoter hypermethylation is frequent in 1p/19q‐deleted oligodendroglial tumours and increases radio‐ and chemosensitivity of Hs683 glioma cells in vitro. Oncogene. 2012; 31: 3409–18. [DOI] [PubMed] [Google Scholar]
- 125. Poschmann G, Grzendowski M, Stefanski A, et al Redox proteomics reveal stress responsive proteins linking peroxiredoxin‐1 status in glioma to chemosensitivity and oxidative stress. Biochim Biophys Acta. 2015; 1854: 624–31. [DOI] [PubMed] [Google Scholar]
- 126. Hintsala HR, Soini Y, Haapasaari KM, et al Dysregulation of redox‐state‐regulating enzymes in melanocytic skin tumours and the surrounding microenvironment. Histopathology. 2015; 67: 348–57. [DOI] [PubMed] [Google Scholar]
- 127. Lee DJ, Kang DH, Choi M, et al Peroxiredoxin‐2 represses melanoma metastasis by increasing E‐Cadherin/β‐Catenin complexes in adherens junctions. Cancer Res. 2013; 73: 4744–57. [DOI] [PubMed] [Google Scholar]
- 128. Demasi AP, Magalhães MH, Furuse C, et al Peroxiredoxin I is differentially expressed in multiple myelomas and in plasmablastic lymphomas. Oral Dis. 2008; 14: 741–6. [DOI] [PubMed] [Google Scholar]
- 129. Trzeciecka A, Klossowski S, Bajor M, et al Dimeric peroxiredoxins are druggable targets in human Burkitt lymphoma. Oncotarget. 2016; 7: 1717–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Peroja P, Haapasaari KM, Mannisto S, et al Total peroxiredoxin expression is associated with survival in patients with follicular lymphoma. Virchows Arch. 2016; 468: 623–30. [DOI] [PubMed] [Google Scholar]


