Abstract
Botulinum Toxin A has been the main treatment for spasticity since the beginning of the 1990s. Surprisingly, there is still no consensus regarding injection parameters or, importantly, how to determine which muscles to target to improve specific functions. The aim of this study was to develop a systematic approach to determine this, using the example of the arm flexion pattern. We first determined anatomical landmarks for selective motor block of the brachialis nerve, using 20 forearms from 10 fresh cadavers in Ecole Européenne de Chirurgie and a university‐based dissection centre, Paris, France. We then carried out selective blocks of the motor nerves to the brachialis, brachioradialis and biceps brachii in patients with stroke with an arm flexion pattern, in a University Rehabilitation Hospital, Garches, France. We measured: the resting angle of the elbow angle in standing (manual goniometer), active and passive range of extension, and spasticity using the Held and Tardieu and the Modified Ashworth scales. Range of passive elbow extension was also measured with the shoulder in 90° of flexion. The resting angle of the elbow in standing decreased by 35.0° (from 87.6 ± 23.7 to 52.6 ± 24.2°) with inhibition of brachialis, by a further 3.9° (from 52.6 ± 24.2 to 48.7 ± 23.7°) with inhibition of brachioradialis and a further 14.5° (from 48.7 ± 23.7to 34.2 ± 20.7°) with inhibition of biceps brachii. These results were consistent with the clinical evaluation of passive elbow range of motion with the shoulder at 90°. Sequential blocking of the nerves to the three main elbow flexors revealed that the muscle that limited elbow extension the most, was brachialis. This muscle should be the main target to improve the arm flexion pattern. These results show that it is important not simply to inject the most superficial or powerful muscles to treat a spastic deformity. A comprehensive assessment is required. The strategy proposed in this paper should increase the effectiveness of botulinum toxin injections by ensuring that the relevant muscles are targeted.
Keywords: brachialis muscle, elbow flexion, hemiplegia, motor nerve block, spasticity
Introduction
The management of hypertonia and/or upper limb muscle contractures in patients with stroke is quite challenging (Baguley et al. 2011). Spasticity of the elbow flexor muscles is frequent (Kong & Chua, 1999; van Kuijk et al. 2002). It reduces elbow extension and thus the functional capacity of the upper limb (Keenan, 1988; van Kuijk et al. 2002). This can impact on the patient's quality of life (Bhakta et al. 1996) and may also be painful (Bhakta et al. 1996; Kong & Chua, 1999; van Kuijk et al. 2002). Balance capacity can be reduced, particularly during gait (Kong & Chua, 1999), as walking and fatigue tend to increase hypertonia. Patients also very frequently complain that involuntary elbow flexion during gait or activities is not aesthetic (Esquenazi et al. 2012).
Botulinum Toxin (BoNT) A has been used to treat muscle spasticity in several countries since the 1990s. Surprisingly, more than 20 years later, there are still no robust guidelines regarding injection parameters (i.e. dilution, identification of the muscles to treat, number of injection points, depth of injection, etc.; Kwakkel & Meskers, 2015). The injection parameters used in clinical practice are therefore heterogeneous (Baguley et al. 2011) and evaluations are not standardized. This may be one of the reasons why studies fail to show that BoNT increases range of motion, functional capacity or amount of limb use (Turner‐Stokes et al. 2013b; Kwakkel & Meskers, 2015), particularly in the upper limb. Determining the appropriate muscles to inject is not simple (Baguley et al. 2011; Kwakkel & Meskers, 2015), and many different muscles are injected to treat similar spasticity patterns, with varying results. A study using EMG and electrogoniometers showed that troublesome spasticity and contractures occur essentially in the three main elbow flexors: brachioradialis, biceps brachii and brachialis (Keenan, 1988), but the accessory elbow flexors could also be involved. The muscles targeted to reduce involuntary elbow flexion are often chosen based on how superficial, and therefore how easy to inject, they are. Thus the biceps brachii (Bhakta et al. 1996; Hesse et al. 1998; Bakheit et al. 2000, 2001, 2004; Lagalla et al. 2000; Wang et al. 2002; Chen et al. 2005; Slawek et al. 2005; Suputtitada & Suwanwela, 2005; Caty et al. 2009; Lai et al. 2009; McCrory et al. 2009; Bensmail et al. 2010a,b; Sun et al. 2010; Baguley et al. 2011; Demetrios et al. 2014), brachioradialis (Bhakta et al. 1996; Wang et al. 2002; Slawek et al. 2005; Lai et al. 2009; McCrory et al. 2009; Bensmail et al. 2010a,b; Baguley et al. 2011; Demetrios et al. 2014) and accessory flexor muscles such as flexor digitorum superficialis and pronator teres (Bhakta et al. 1996; Hesse et al. 1998; Bakheit et al. 2000, 2001, 2004; Lagalla et al. 2000; Wang et al. 2002; Slawek et al. 2005; Suputtitada & Suwanwela, 2005; Caty et al. 2009; McCrory et al. 2009; Bensmail et al. 2010a,b; Sun et al. 2010; Baguley et al. 2011; Demetrios et al. 2014) are often injected, whereas the brachialis, which is deeper, is less frequently injected (Hesse et al. 1998; Lagalla et al. 2000; Caty et al. 2009; McCrory et al. 2009; Bensmail et al. 2010a,b; Baguley et al. 2011; Demetrios et al. 2014).
To provide an appropriate treatment for a spasticity pattern, a comprehensive assessment is necessary to determine the muscles involved. A useful adjunct to the usual clinical assessment is selective motor nerve block (SMNB; Elovic et al. 2009). SMNBs are easy to perform, innocuous and the effect only lasts a few hours. The method involves local anaesthesia of a nerve, preventing its conduction and causing an immediate decrease in spasticity and voluntary muscle activation. It is then possible to evaluate passive range of motion and to differentiate between spasticity and contracture, as well as to evaluate the strength of antagonist muscles. Changes in posture and movement can be evaluated and the involvement of the temporarily inhibited muscle determined. Selective block of the nerves that innervate the three main elbow flexors (brachialis, biceps brachii and brachioradialis) has already been described. The brachioradialis muscle can be inhibited by blocking the radialis nerve and the brachialis and the biceps brachii muscles can be inhibited by blocking the musculocutaneous nerve (Yang et al. 1995; Kong & Chua, 1999). However, blocking the musculocutaneous nerve simultaneously inhibits the biceps brachii and the brachialis muscles, thus it is not possible to differentiate them.
We developed a method involving the successive inhibition of each elbow flexor in order to determine the contribution of each to the abnormal movement. The method is based on three successive SMNB and involves a process of deduction to determine the role of each muscle:
Block of the brachialis branch of the musculocutaneous nerve– contribution of the brachialis is determined.
Block of the radial nerve – contribution of the brachioradialis is determined.
Block of the musculocutaneous nerve – contribution of the biceps is determined.
The intrinsic characteristics (viscosity and elasticity) of the accessory flexors are then determined, as they are the only elbow flexors that remain active following the three blocks.
The aim of this study was to develop and test this systematic approach, using the example of the arm flexion pattern. We hypothesized, based on our clinical experience, that the contribution of the brachialis muscle to the loss of elbow extension would be greater than the contributions of the brachioradialis and biceps brachii. We first carried out a cadaver study to determine appropriate anatomical landmarks for the brachialis nerve block. SMNBs of the elbow flexors were then carried out in patients with stroke‐related spasticity to determine the respective contributions of each elbow flexor to the arm flexion pattern (Elovic et al. 2009). The results of this study should help clinicians in the choice of muscles to target with botulinum toxin injections for the treatment of elbow flexor spasticity. The study also provides a methodology for the systematic evaluation of the involvement of spastic muscles in an abnormal pattern or movement in any part of the body.
Methods
Design
This study was carried out in two phases: (i) anatomical study on fresh cadavers [Ecole Européenne de Chirurgie (EEC), ‘Centre du Don de Corps’ and the Université Paris Descartes, Paris, France]; and (ii) observational study of patients with hemiplegia (Department of Physical Medicine and Rehabilitation, Hôpital Poincaré, Garches, France). Patients were included between 27 March 2015 and 3 July 2015 (each patient was included for 1 day).
Determination of anatomical landmarks for brachialis nerve block, using fresh cadavers
We used a previously published method (Yang et al. 1995; Albert et al. 2000) to determine anatomical landmarks for the location of the brachialis nerve branch for selective motor block, using fresh cadavers. The age, sex and arm dominance of the cadavers was noted.
The forearm of the cadaver was positioned in supination. The musculocutaneous nerve was exposed through a linear incision between the deltoid and pectoralis major muscles, with the coracobrachialis muscle deflected laterally. The nerve was traced from the coracoid process (used as the reference point) to the middle of the elbow crease. The biceps brachii was then discarded and the branches of the musculocutaneous nerve that innervated the brachialis were identified. Four measurements [proximal‐distal (mm)] were taken to describe the location of the brachialis motor branch: ‘d0’ = distance from the medial epicondyle to the coracoid process; ‘d1’ = distance from the medial epicondyle to the exit point of the brachialis motor branch from the musculocutaneous trunk; ‘d2’ = distance from the medial epicondyle to the entry point of the brachialis motor branch into the brachialis muscle (Fig. 1), and ‘r’, the depth of the needle inserted transversely from medial to lateral in the arm until it contacted the nerve (mm). The distance between the exit point of the brachial nerve from the musculocutaneous trunk and its entry into the brachialis (d1–d2) was calculated to specify the portion of the branch that could be anaesthetized to ensure a block of only the brachialis. To account for morphological differences, the distance from the medial epicondyle to the entry point of the brachialis motor branch in the muscle was expressed as the ratio between d2 and d0.
Figure 1.
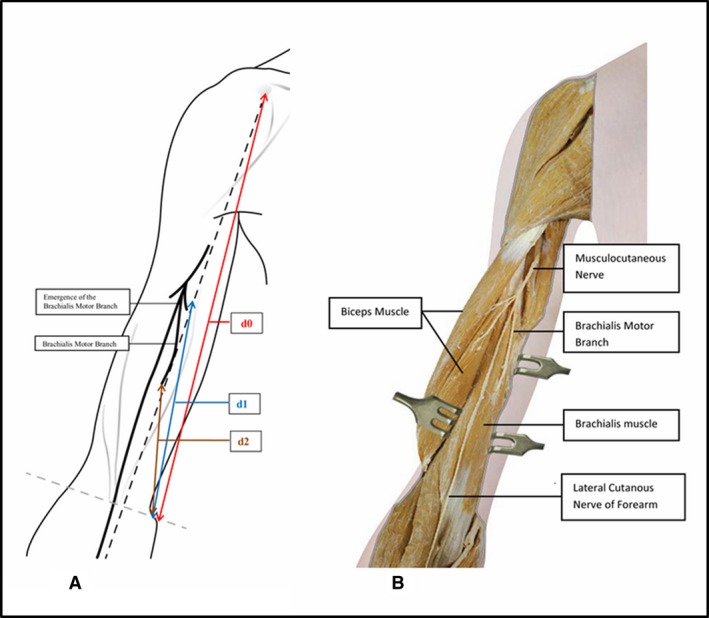
(A) Anterior view of the right upper limb. ‘d0’ = distance from the medial epicondyle to the coracoid process; ‘d1’ = distance from the medial epicondyle to the exit point of the brachialis motor branch from the musculocutaneous trunk; ‘d2’ = distance from the medial epicondyle to the entry point of the brachialis motor branch into the muscle. (B) Medial view of the arm with the biceps muscle removed.
Determination of the role of each flexor muscle in the arm flexion pattern
We then tested the methodology of successive SMNB in patients with stroke. To ensure homogeneity, we chose to test it on the arm flexion pattern, as this is the most common spasticity pattern of the upper limb.
Procedure: 20 patients with stroke‐related spastic hemiparesis were included after they signed the informed consent form. Inclusion criteria: ability to walk indoors, participation in a regular program of botulinum toxin injections, presence of spastic arm flexion pattern (i.e. shoulder adduction and medial rotation, elbow flexion with the forearm in neutral or pronation, and finger and wrist flexion; patterns III and IV according to the classification by Jost et al. 2014), fixed elbow flexion deformity of less than 120° (to avoid confounding factors related to altered biomechanics in the case of large muscle contractures which alter the lever arm). Exclusion criteria: refusal to participate in the study, pregnancy, not affiliated to the public health care regime, patient under guardianship or trusteeship.
First, the brachialis motor branch was blocked with 4 cm3 of Lidocaïne® 1% (located using the anatomical landmarks described above combined with electrical stimulation guidance at 0.8–1 mA). Half an hour later, the radialis motor nerve was blocked (using a medial approach, three finger‐widths above the medial epicondyle) using the same procedure, in order to inhibit the brachioradialis. If the brachialis has a mixed innervation (Mahakkanukrauh & Somsarp, 2002), this SMNB will ensure complete inhibition of this muscle. It does not affect the triceps brachii, which is innervated proximally (close to the shoulder). After another half an hour, the musculocutaneous motor nerve block was performed (using a medial approach, at the base of the inferior limit of the pectoralis major and along the biceps brachii tendon).
Data collection
Age, sex, stroke aetiology, side of hemiparesis, spontaneous elbow angle in standing, active range of elbow extension in the anatomical reference position, spasticity using the Held and Tardieu scale (Tardieu et al. 1954; Gracies et al. 2010; slow velocity V1, high velocity V3 (following the Held and Tardieu anatomical position covenant), spasticity angle (V1–V3), angle of paresis (V1 – active range of motion and Tardieu grade) and the Modified Ashworth scale (Bohannon & Smith, 1987) were recorded before beginning the SMNBs.
Spontaneous elbow angle and active range of elbow extension (both with the arm by the side) were evaluated while standing with a manual goniometer, before and after each successive SMNB.
Statistical analysis
R 2.14.0 (R Development Core Team, http://www.R-project.org) software was used throughout. Data were expressed as means ± standard deviation (SD), numbers and percentages. Paired tests (Wilcoxon rank tests) were used to compare ranges of motion pre‐ and post‐selective motor nerve block. A P‐value < 0.05 was considered statistically significant throughout.
Ethical considerations
The study was registered on ClinicalTrials.gov (ID: NCT02455232). It was a non‐interventional study with usual procedures and without additional procedures (diagnosis or medical supervision). In France, patient consent is not needed for such an anonymous observational study. The study was approved and piloted by our local scientific committee CIC‐IT 1429, 104 bd Raymond Poincaré, 92380 Garches, France and Comité de Protection des Personnes IDF XI, Saint‐Germain‐en‐Laye, France.
Results
Determination of anatomical landmarks for brachialis nerve block, using fresh cadavers (Table 1, Fig. 1)
Table 1.
Distance from the medial epicondyle to the coracoid process (d0); from the medial epicondyle to the exit point of the brachialis motor branch (d1), and from the medial epicondyle to entry point of the brachialis motor branch (d2, mm); nerve depth (r, mm) and ratio (d2/d0, %)
| Distance (mm) | Medial epicondyle to the middle of the coracoid process (d0) | Medial epicondyle to exit point of the brachialis motor branch (d1) | Medial epicondyle to entry point of the brachialis motor branch (d2) | Nerve depth (r, mm) | Ratio (d2/d0, %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Total | Right | Left | Total | Right | Left | Total | Right | Left | Total | Right | Left | Total | |
| Mean | 296 | 300 | 298 | 28.7 | 28.9 | 28.8 | 35.0 | 33.1 | 34.1 | 153 | 156 | 155 | 104 | 100 | 102 |
| Minimum | 270 | 280 | 270 | 20.0 | 20.0 | 20.0 | 28.6 | 25.0 | 25.0 | 140 | 140 | 140 | 80 | 70 | 70 |
| Maximum | 310 | 340 | 340 | 33.0 | 38.0 | 38.0 | 41.9 | 39.4 | 41.9 | 170 | 170 | 170 | 130 | 130 | 130 |
| SD | 14.3 | 22.6 | 18.5 | 4.47 | 5.43 | 4.84 | 0.04 | 0.05 | 0.05 | 11.6 | 9.7 | 10.5 | 15.1 | 21.1 | 17.9 |
Twenty forearms from 10 fresh cadavers were used for the study (six men, four women, age range 68–84 years).
The brachialis nerve branch exited from the musculocutaneous nerve trunk at a mean distance of 155 ± 10.5 mm (range 140–170) from the medial epicondyle (d1). It entered the brachialis muscle at a mean distance of 102 ± 17.9 mm (range 70–130) from the medial epicondyle (d2) and at a mean depth (r) of 28.8 ± 4.84 mm (range 20–38). The mean ratio from the medial epicondyle was 34.1% ± 0.05% (range 25.0–41.9). The brachialis branch divided from the musculocutaneous trunk 53 ± 13.7 mm [(d1–d2), range 30–80] after entering the brachialis muscle. There was no influence of the side of the dissected arm (left vs. right).
Conclusion. To block the brachialis branch of the musculocutaneous nerve, a medial approach should be used, 1–1.5 hand‐widths above the medial epicondyle, just below the belly of the biceps brachii.
Determination of the role of each flexor muscle in the arm flexion pattern
Twenty patients with stroke‐related spastic hemiparesis were included. Demographic data are shown in Table 2. Mean age was 55.8 years (± 11.7 from 29.4 to 68.7) and mean time since stroke was 48.2 months (± 47.9 from 5.2 to 195.8). The sex ratio was 52.6% (10 males; nine females; Tables 3, 4, 5 Fig. 2).
Table 2.
Demographic data and results of the Modified Ashworth and Tardieu scores (spasticity angles V1–V3), shoulder in anatomical position and 90° flexion before the three motor nerve blocks patients with stroke, and Tardieu score (spasticity angles V1–V3) after the first SMNB
| n | Sex (M/F) | Age (years) | Time since stroke (months) | Etiology | Side of hemiplegia (Right/Left) | Modified Ashworth score | Tardieu score: spasticity angles V1–V3 (°) | ||
|---|---|---|---|---|---|---|---|---|---|
| Shoulder in Anatomical position | Shoulder in Anatomical position | Brachialis spasticity angle T1–T2 | Main cause of flexor pattern | ||||||
| 1 | M | 58.7 | 75.7 | Haemorrhagic | Right | 3 | 40 | 0 | Brachialis |
| 2 | M | 62.4 | 118.3 | Ischaemic | Left | 3 | 40 | 20 | Brachialis |
| 3 | M | 68.2 | 61.5 | Haemorrhagic | Left | 3 | 50 | 30 | Brachialis |
| 4 | F | 55.6 | 57.8 | Ischaemic | Right | 3 | 40 | 20 | Brachialis |
| 5 | F | 38.4 | 203.3 | Haemorrhagic | Right | 2 | 30 | 20 | Brachialis |
| 6 | F | 55.7 | 79.0 | Ischaemic | Left | 3 | 60 | 50 | Brachialis |
| 7 | F | 48.5 | 49.0 | Ischaemic | Left | 3 | 50 | 30 | Brachialis |
| 8 | F | 29.4 | 496.8 | Haemorrhagic | Right | 2 | 40 | 40 | Brachialis |
| 9 | F | 58.6 | 155.3 | Ischaemic | Right | 3 | 30 | 10 | Biceps brachii |
| 10 | M | 60.6 | 16.8 | Haemorrhagic | Right | 2 | 50 | 00 | Brachialis |
| 11 | M | 60.1 | 45.2 | Ischaemic | Left | 3 | 30 | 20 | Brachialis |
| 12 | F | 65.1 | 113.8 | Ischaemic | Left | 3 | 30 | 10 | Brachialis & Biceps brachii |
| 13 | M | 65.0 | 41.0 | Ischaemic | Right | 3 | 50 | 20 | Brachialis |
| 14 | M | 64.0 | 235.1 | Ischaemic | Right | 2 | 10 | 10 | Brachialis |
| 15 | M | 48.4 | 127.5 | Haemorrhagic | Left | 3 | 40 | 20 | Brachialis & Biceps brachii |
| 16 | M | 59.8 | 13.2 | Ischaemic | Right | 2 | 30 | 20 | Brachialis |
| 17 | F | 30.4 | 55.3 | Haemorrhagic | Right | 3 | 70 | 60 | Brachialis |
| 18 | M | 63.1 | 49.8 | Haemorrhagic | Right | 3 | 50 | 30 | Brachialis |
| 19 | F | 68.7 | 329.3 | Ischaemic | Left | 3 | 60 | 50 | Brachialis |
Table 3.
Changes in elbow angle following the selective motor nerve blocks
| Patient | Resting elbow angle (°) | Active extension ROM: Slow angle ROM (V1; °) | Tardieu: Fast angle ROM (V3;°) | Tardieu | Tardieu: spasticity angles V1–V3 (°) | Angle of paresis V1 – active extension ROM (°) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n° | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 |
| 1 | 100 | 70 | 40 | 30 | 90 | 70 | 40 | 30 | 130 | 150 | 150 | 150 | 90 | 110 | 140 | 150 | 40 | 40 | 10 | 0 | 40 | 40 | 10 | 0 |
| 2 | 80 | 60 | 50 | 40 | 80 | 60 | 50 | 40 | 140 | 140 | 145 | 150 | 100 | 120 | 130 | 140 | 40 | 20 | 15 | 10 | 40 | 20 | 15 | 10 |
| 3 | 120 | 80 | 80 | 70 | 120 | 80 | 80 | 70 | 120 | 120 | 120 | 125 | 70 | 100 | 100 | 110 | 50 | 20 | 20 | 15 | 60 | 20 | 20 | 15 |
| 4 | 100 | 80 | 75 | 65 | 90 | 70 | 60 | 50 | 130 | 130 | 130 | 130 | 90 | 110 | 115 | 125 | 40 | 20 | 15 | 5 | 40 | 20 | 10 | 0 |
| 5 | 110 | 90 | 90 | 80 | 110 | 70 | 70 | 50 | 110 | 130 | 130 | 130 | 80 | 120 | 120 | 125 | 30 | 10 | 10 | 5 | 40 | 20 | 20 | 0 |
| 6 | 110 | 30 | 20 | 20 | 110 | 30 | 20 | 20 | 140 | 160 | 170 | 180 | 80 | 150 | 160 | 160 | 60 | 10 | 10 | 20 | 55 | 10 | 10 | 0 |
| 7 | 80 | 40 | 40 | 30 | 60 | 30 | 30 | 30 | 170 | 170 | 170 | 170 | 120 | 140 | 140 | 160 | 50 | 30 | 30 | 10 | 70 | 10 | 10 | 20 |
| 8 | 85 | 20 | 20 | 20 | 85 | 20 | 20 | 20 | 170 | 170 | 170 | 170 | 130 | 170 | 170 | 170 | 40 | 0 | 0 | 0 | 50 | 20 | 20 | 20 |
| 9 | 80 | 70 | 70 | 50 | 80 | 70 | 70 | 50 | 130 | 130 | 130 | 130 | 100 | 110 | 120 | 130 | 30 | 20 | 10 | 0 | 75 | 10 | 10 | 10 |
| 10 | 70 | 30 | 30 | 10 | 70 | 30 | 30 | 10 | 180 | 180 | 180 | 180 | 130 | 130 | 160 | 170 | 50 | 50 | 20 | 10 | 30 | 20 | 20 | 0 |
| 11 | 90 | 50 | 50 | 30 | 80 | 40 | 40 | 30 | 150 | 150 | 150 | 150 | 120 | 140 | 140 | 150 | 30 | 10 | 10 | 0 | 70 | 30 | 30 | 10 |
| 12 | 110 | 80 | 80 | 50 | 90 | 70 | 70 | 40 | 140 | 140 | 140 | 140 | 110 | 120 | 120 | 140 | 30 | 20 | 20 | 0 | 50 | 10 | 10 | 0 |
| 13 | 90 | 50 | 50 | 30 | 90 | 50 | 50 | 30 | 160 | 160 | 160 | 160 | 110 | 130 | 140 | 150 | 50 | 30 | 20 | 10 | 50 | 30 | 30 | 0 |
| 14 | 40 | 10 | 10 | 10 | 20 | 10 | 10 | 10 | 170 | 180 | 180 | 180 | 160 | 180 | 180 | 170 | 10 | 0 | 0 | 10 | 70 | 30 | 30 | 10 |
| 15 | 100 | 70 | 70 | 40 | 50 | 40 | 40 | 10 | 150 | 150 | 150 | 170 | 110 | 130 | 130 | 150 | 40 | 20 | 20 | 0 | 10 | 10 | 10 | 10 |
| 16 | 40 | 20 | 20 | 10 | 40 | 20 | 20 | 10 | 180 | 180 | 180 | 180 | 150 | 170 | 170 | 180 | 30 | 10 | 10 | 0 | 20 | 10 | 10 | 0 |
| 17 | 110 | 70 | 50 | 30 | 110 | 70 | 50 | 30 | 140 | 140 | 140 | 160 | 70 | 130 | 130 | 140 | 70 | 10 | 10 | 0 | 40 | 20 | 20 | 10 |
| 18 | 50 | 30 | 30 | 15 | 50 | 30 | 30 | 15 | 180 | 180 | 180 | 180 | 130 | 160 | 160 | 165 | 50 | 20 | 20 | 15 | 70 | 30 | 10 | 10 |
| 19 | 100 | 50 | 50 | 20 | 100 | 50 | 50 | 20 | 150 | 150 | 150 | 160 | 90 | 110 | 110 | 150 | 60 | 40 | 40 | 0 | 50 | 30 | 30 | 15 |
| Mean | 87.6 | 52.6 | 48.7 | 4.2 | 80.3 | 47.9 | 43.7 | 29.7 | 149.5 | 153.2 | 153.9 | 157.8 | 107.4 | 133.2 | 138.7 | 149.2 | 42.1 | 20.0 | 15.3 | 5.8 | 49.7 | 21.1 | 17.6 | 7.4 |
| Min | 40.0 | 10.0 | 10.0 | 10.0 | 20.0 | 10.0 | 10.0 | 10.0 | 110.0 | 120.0 | 120.0 | 125.0 | 70.0 | 100.0 | 100.0 | 110.0 | 10.0 | 0.0 | 0.0 | 0.0 | 10.0 | 10.0 | 10.0 | 0.0 |
| Max | 120.0 | 90.0 | 90.0 | 80.0 | 120.0 | 80.0 | 80.0 | 70.0 | 180.0 | 180.0 | 180.0 | 180.0 | 160.0 | 180.0 | 180.0 | 180.0 | 70.0 | 50.0 | 40.0 | 20.0 | 75.0 | 40.0 | 30.0 | 20.0 |
| SD | 23.7 | 24.2 | 23.7 | 20.7 | 26.5 | 21.8 | 20.1 | 16.9 | 21.2 | 19.5 | 19.6 | 19.0 | 25.6 | 23.3 | 22.6 | 18.4 | 14.0 | 13.3 | 9.5 | 6.5 | 18.4 | 8.8 | 7.9 | 7.1 |
T1 before SMNB. T2 after the brachialis SMNB. T3 after the radialis SMNB. T4 after the biceps brachii SMNB.
Table 4.
Results of the selective motor nerve blocks for Ashworth Scale and Tardieu degree of spasticity (Y)
| Patient | Ashworth | Tardieu: degree of spasticity Y | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 |
| 1 | 3 | 2 | 2 | 1 | 3 | 2 | 0 | 0 |
| 2 | 3 | 2 | 1+ | 1 | 3 | 2 | 2 | 1 |
| 3 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 2 |
| 4 | 3 | 1+ | 1+ | 1 | 3 | 3 | 3 | 1 |
| 5 | 2 | 1+ | 1+ | 1 | 3 | 2 | 2 | 1 |
| 6 | 3 | 2 | 1+ | 1+ | 3 | 2 | 2 | 2 |
| 7 | 3 | 2 | 2 | 1 | 3 | 2 | 2 | 1 |
| 8 | 2 | 1+ | 1+ | 1 | 3 | 1 | 1 | 0 |
| 9 | 3 | 2 | 2 | 1+ | 4 | 3 | 3 | 0 |
| 10 | 2 | 1 | 1 | 1 | 3 | 2 | 1 | 1 |
| 11 | 3 | 2 | 2 | 2 | 4 | 3 | 2 | 0 |
| 12 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 0 |
| 13 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 1 |
| 14 | 2 | 1 | 1+ | 1 | 2 | 1 | 1 | 1 |
| 15 | 3 | 2 | 2 | 1 | 3 | 2 | 2 | 0 |
| 16 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 0 |
| 17 | 3 | 2 | 1+ | 1 | 3 | 2 | 2 | 0 |
| 18 | 3 | 2 | 2 | 1+ | 3 | 2 | 2 | 1 |
| 19 | 3 | 2 | 2 | 1+ | 3 | 2 | 2 | 0 |
T1 before SMNB. T2 after the brachialis SMNB. T3 after the radialis SMNB. T4 after the biceps brachii SMNB.
Table 5.
Comparison of spontaneous elbow angle in standing, active range of extension in the anatomical position, range of motion at slow velocity V1 and high velocity V3 according to the Held and Tardieu scale, and spasticity angle (V1–V3) between each successive pre‐ and post‐selective motor nerve block
| Between T1 and T2, P | Between T2 and T3, P | Between T3 and T4, P | Between T1 and T3, P | |
|---|---|---|---|---|
| Spontaneous elbow angle while standing | < 0.01* | 0.03* | < 0.01* | < 0.01* |
| Active range of extension in the anatomical position | < 0.01* | 0.03* | < 0.01* | < 0.01* |
| Held and Tardieu scale (slow velocity V1) | 0.13 | 0.25 | 0.25 | 0.03* |
| Held and Tardieu scale (high velocity V3) | < 0.01* | 0.01* | < 0.01* | < 0.01* |
| Spasticity angle (V1–V3) | < 0.01* | 0.03* | < 0.01* | < 0.01* |
| Angle of paresis (V1 –active ROM) | < 0.01* | 0.10 | < 0.01* | < 0.01* |
T1 before SMNB, T2 after the brachialis SMNB, T3 after the radialis SMNB, T4 after the biceps brachii SMNB. Wilcoxon rank test (P). For all tests, P‐values < 0.05 were considered statistically significant (*).
Figure 2.
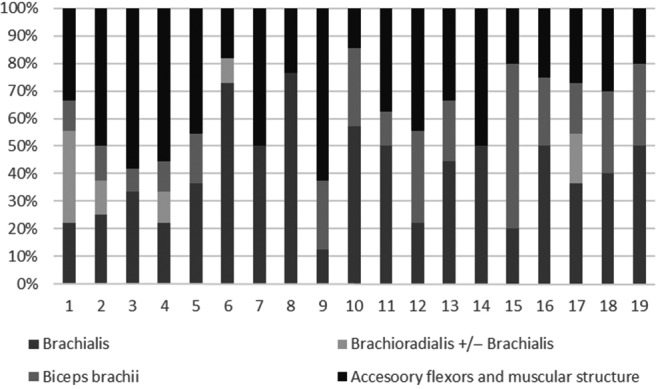
Respective increase in resting elbow angle in standing after each motor nerve block (%): involvement of each elbow flexor (brachialis, brachioradialis, biceps brachii and accessory flexors and intrinsic characteristics) in the flexion pattern.
Inhibition of the brachialis muscle reduced the resting angle of the elbow in standing by a mean 35.0° [41.7%; from 87.6 ± 23.7° (min. 40.0, max. 120.0) to 52.6 ± 24.2° (min. 10.0, max. 90.0)]. Subsequent inhibition of the brachioradialis muscle resulted in a further mean decrease of 3.9° [3.9%; from 52.6 ± 24.2° (min. 10.0, max. 90.0) to 48.7 ± 23.7° (min. 10.0, max. 90.0)] and inhibition of the biceps brachii by a further mean 14.5° [16.9%; from 48.7 ± 23.7 (min. 10.0, max. 90.0) to 34.2 ± 20.7° (min. 10.0, max. 80.0)].
Inhibition of the brachialis muscle reduced the spasticity angle by a mean 22.1° [from 42.1 ± 14.0° (min. 10.0, max. 70.0) to 20.0 ± 13.3° (min. 0.0, max. 50.0)]. Subsequent inhibition of the brachioradialis muscle resulted in a further mean decrease of 4.7° [from 20.0 ± 13.3° (min. 0.0, max. 50.0) to 15.3 ± 9.5° (min. 0.0, max. 40.0)] and inhibition of the biceps brachii by a further mean 9.5° [from 15.3 ± 9.5 (min. 0.0, max. 40.0) to 5.8 ± 6.5° (min. 0.0, max. 20.0)].
Inhibition of the brachialis muscle reduced the angle of paresis by a mean 28.6° [from 49.7 ± 18.4° (min. 10, max. 75) to 21.1 ± 8.8 (min. 10, max. 40)]. Subsequent inhibition of the brachioradialis muscle resulted in a further mean decrease of 3.5° [21.1 ± 8.8 (min. 10, max. 40) to 17.6 ± 7.9° (min. 10.0, max. 30.0)] and inhibition of the biceps brachii by a further mean 10.2° [from 17.6 ± 7.9° (min. 10.0, max. 30.0) to 7.4 ± 7.1° (min. 0.0, max. 20.0)].
All changes in angle with each SMNB were significant, except for V1 (measured at low speed; Table 5).
Discussion
Statement of principal findings
The cadaver study determined that a medial approach, 1–1.5 hand‐widths above the medial epicondyle, just below the belly of the biceps brachii should be used for SMNB of the brachialis muscle. The study in stroke patients demonstrated the usefulness of three sequential SMNB to determine the contribution of different muscles in a spasticity pattern. The results revealed that, in the arm flexion pattern, the muscle that reduced elbow extension to the greatest extent was brachialis (Fig. 3).
Figure 3.
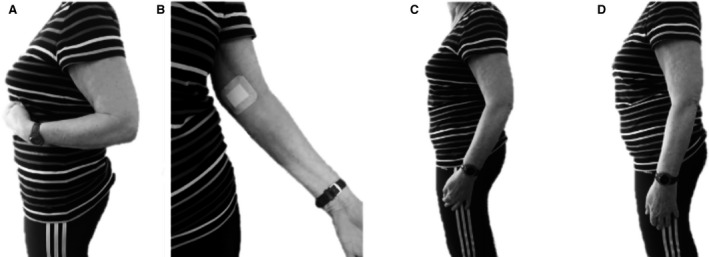
Change in elbow angle: (A) before any motor nerve block (SMNB); (B) injection point for SMNB of the Brachialis; (C) after the selective MNB of the brachialis; (D) after the three SMNBs (brachialis, brachioradialis and biceps brachii).
Determination of anatomical landmarks
Although the course of the musculocutaneous nerve has been described in many studies (de Moura, 1985; Flatow et al. 1989; Yang et al. 1995; Mahakkanukrauh & Somsarp, 2002), and the course and variation of the brachialis branch are well known (de Moura, 1985; Flatow et al. 1989; Yang et al. 1995; Mahakkanukrauh & Somsarp, 2002), these descriptions are only useful for surgical procedures (muscle transfer or neurotization in the case of peripheral nerve lesions; Dahlin, 2008; Songcharoen, 2008). Until now, there have been no descriptions of surface landmarks for SMNB.
During the dissections, the motor branch of the brachialis was easily identified. Discussions mainly concerned the selection of landmarks. The elbow crease and the coracoid process were chosen as reference points because they are easily palpable and have been used in previous studies (Buchanan & Erickson, 1996; Park et al. 2007). The depth of insertion of the needle is also very important but depends on morphological characteristics (gender, muscle size, dominance, etc.). These results are consistent with previous studies that assessed the location of the motor point of the brachialis muscle (Buchanan & Erickson, 1996; Park et al. 2007).
Current management of elbow flexor spasticity
The Upper Limb International Spasticity (ULIS) Survey (Aymard, 2009; Bakheit et al. 2010) was a large, prospective study that investigated practice in 122 centres around the world (76% in Europe). A total of 974 patients were included. The results showed that the injection frequency for brachioradialis, brachialis and biceps brachii was, respectively, 19, 26 and 61% in the UK, 43, 22 and 68% in Germany and 27, 46 and 31% in France. A more recent study in Australia also found that the biceps brachii was most frequently injected (46% in patients with stroke), followed by brachioradialis (29%) and brachialis (21%; Nott et al. 2014). The ULIS II study confirmed these results, showing that of 456 adults with hemiplegia (84 centres, 22 countries), 270 underwent BoNT injection in the biceps brachii (59.2%) and 156 in the brachioradialis (34.2%), whereas only 130 patients (28.5%) were injected in the brachialis (Turner‐Stokes et al. 2013a). In 2010, the results from the BoTULS program showed that in the UK, only the biceps brachii and the brachioradialis muscles were injected to improve the arm flexion pattern (170 patients; Shaw et al. 2010). In 2012, results from the patient registry of outcomes in spasticity care in the USA, which included 487 patients (with stroke or traumatic brain injury), showed that 190 injections were carried out in the biceps, 159 in the brachioradialis and only 142 in the brachialis (Esquenazi et al. 2012).
It is thus very evident from the literature that the muscle that is the most frequently injected with botulinum toxin to reduce elbow flexor spasticity, is the biceps brachii. The results of the present study are therefore very important, as they suggest this is inappropriate. There are likely several reasons for the choice of muscles to inject to treat an arm flexion pattern. First, the heterogeneity of marketing authorizations between different commercialized BoNTA products in different countries strongly impacts the choice of muscles injected. Secondly, muscle function is often examined in static positions, which does not provide an indication of muscle activity in dynamic situations, including functional upper limb activities and gait. Thirdly, the biceps brachii is a superficial muscle, making it much easier to inject than the brachialis.
The results of our study show that the biceps brachii may not always play an important role in the arm flexion pattern. The biceps brachii is not a strong elbow flexor (compared with brachialis). It is a multi‐joint muscle that requires good proximal stability around the scapula to function optimally, which is frequently not the case in patients with hemiplegia. The fact that it is fusiform and digastric, with its fibres terminating on a common distal tendon, means that it is less able to bear stress, and some power is lost. Moreover, the biceps brachii is a forearm supinator (along with brachioradialis when the forearm is pronated) and is therefore unlikely to generate a flexion–pronation pattern. In contrast, the brachialis is a single‐joint, pennate muscle and is consequently more powerful. The direct insertion of its fibres in the fascia increases its resistance to stress and its capacity to transmit power (reflexion pulley). Therefore, based on its anatomy, the brachialis muscle is likely to play a greater role in the arm flexion pattern than the biceps brachii does.
Determination of the role of each flexor muscle in the arm flexion pattern
This study demonstrated that using a methodology of three sequential SMNBs, it was easy to differentiate between hypertonia of the brachioradialis, biceps brachii and brachialis muscles. Sequential SMNB is useful to determine appropriate treatment strategies. For example, while the block is active, muscle length can be fully examined to determine whether contractures are present. If this is the case, botulinum toxin injection is unlikely to help and surgery may be considered. The distribution of muscle involvement in the flexion pattern will also affect treatment. If the biceps brachii is found to be only slightly involved, it may be decided not to treat it to conserve its action of supination. Another flexor muscle could be injected with botulinum toxin to improve the flexion pattern.
Despite the three SMNB, full passive elbow extension was not achieved in all patients, and some spasticity persisted. This is probably because of overactivity of the accessory elbow flexors that were not blocked (flexor digitorum superficialis, pronator teres, etc.). It was likely also due to changes in the intrinsic characteristics of the muscles (internal viscosity and elasticity, clinically termed contracture). A median nerve block may be useful to differentiate between contracture and spasticity of the accessory flexor muscles.
The results showed that there was no increase in active range of elbow extension after the successive SMNBs. This was somewhat disappointing; however, it is in line with the literature that shows that motor capacity is more reduced by the loss of muscle strength than by spasticity. This should be considered in the treatment plan and goals should perhaps be orientated more towards improving passive functions than active functions. However, the short duration of action of the SMNB may have affected this result because the patients did not have time to become accustomed to the new state of their arm muscles.
Further studies are now required to compare the effects of botulinum toxin injection in the brachialis, biceps brachii and brachioradialis muscles, on active and passive range of elbow extension in patients with spastic hemiparesis. The results could then be used to determine a consensual strategy for botulinum toxin injections (sites and doses).
Limitations of the study
This study is the first to provide a systematic methodology for the accurate selection of muscles to treat to improve a spasticity pattern. The method is based on a combination of clinical experience, knowledge of muscle anatomy, and results of selective motor nerve blocks. However, the study is limited by the fact that the order of the motor nerve blocks was not randomized. Mahakkanukrauh & Somsarp (2002) found that in approximately 81.6% of cases, the brachialis muscle has a double innervation: it always receives a branch from the musculocutaneous nerve and often also from the radial nerve. This anatomical variation is one of the reasons why it was not possible to randomize the order of the motor nerve blocks. Indeed, to be sure that the brachialis nerve is blocked (to assess its impact on passive and active elbow extension), the radial nerve block must be carried out just after the brachialis nerve block. However, the results showed only a slight improvement in the angle of paresis following the radial SMNB, suggesting that few patients in the sample had a double innervation of the brachialis muscle. The other reason was that beginning distally ensures the selectivity of the motor nerve blocks; a proximal block may inhibit other muscles.
Finally, the results of the SMNBs were assessed manually. It is therefore possible that the muscles were not totally paralyzed, although the blocks were performed by experienced teams.
Generalization of the procedure
The procedure described in this study could be used for the assessment of any spasticity pattern. The systematic approach allows the extent of involvement of each muscle in a spasticity pattern or abnormal movement to be assessed fully. Treatment such as botulinum toxin or other focal treatments can therefore be targeted at the appropriate muscle, improving effectiveness (Fig. 4).
Figure 4.
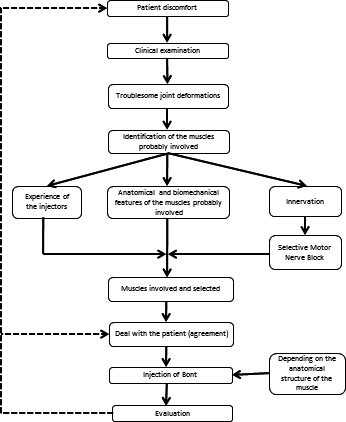
Flow chart summary of the systematic procedure to determine the muscles involved in a spasticity pattern.
Conclusion
We determined anatomical landmarks for the performance of a motor block of the brachialis nerve. Successive SMNBs of the brachialis, brachioradialis and biceps brachii muscles in patients with stroke revealed that the brachialis muscle was the most involved in the flexion pattern, consistently with its anatomical function. We therefore suggest that the brachialis muscle should be the first target of treatment to improve an arm flexion pattern. However, our findings should be confirmed by randomized trials to evaluate the effects of botulinum toxin injection in the different flexor muscles. This systematic approach for the assessment of the muscles involved in a spasticity pattern should be further developed to determine accurately the muscles to treat with botulinum toxin injections, as well as functional surgery. The results demonstrate the importance of carrying out a comprehensive assessment of the muscles potentially involved, and not simply injecting superficial muscles or muscles that appear to be the most powerful.
Clinical message
Procedures for botulinum toxin injections must be homogenized. The hierarchy of involvement of different muscles in each spasticity pattern should be determined. The brachialis muscle appears to be the most involved in the arm flexion pattern and should be the first target for botulinum toxin injection.
Funding
Allergan SA supported the dissections of fresh cadaver arms.
Author contributions
F.G. provided the design of the study, the analysis and the presentation of the data and draft most sections of the manuscript. A.S., L.T., M.S., F.D.B., B.P. made substantial contributions to the recovery and analysis of the data. The orthopaedic expertise was provided by P.D., C.D. and B.P. A.S., P.D., C.D. and B.P. made substantial contributions to the anatomical dissections of cadavers. L.T., A.S., M.S. and F.D.B. made substantial contributions to the writing and revising of the manuscript). The manuscript was approved by all the authors.
Conflict of interest
F.G., A.S., P.D., B.P. and L.T. are consultants for Allergan, Ipsen and Merz, from whom they received research grant support. The other authors declare no conflict of interest in this study.
Acknowledgements
The authors wish to thank Allergan SA and, for their help with the organization of the fresh cadaver dissections and scientific support, the ‘Centre du Don de Corps’ and the Université Paris Descartes. The authors also wish to thank G. Genêt, E. Vlachos and R. Vigne (CRAs) for their assistance in this study.
References
- Albert T, Yelnik A, Colle F, et al. (2000) Anatomic motor point localization for partial quadriceps block in spasticity. Arch Phys Med Rehabil 81, 285–287. [DOI] [PubMed] [Google Scholar]
- Aymard C (2009) ULIS : Des Données Originales Pour Un Tour Du Monde Des Pratiques Actuelles. Symposium IPSEN; SOFMER Lyon, France.
- Baguley IJ, Nott MT, Turner‐Stokes L, et al. (2011) Investigating muscle selection for botulinum toxin‐A injections in adults with post‐stroke upper limb spasticity. J Rehabil Med 43, 1032–1037. [DOI] [PubMed] [Google Scholar]
- Bakheit AM, Thilmann AF, Ward AB, et al. (2000) A randomized, double‐blind, placebo‐controlled, dose‐ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke 31, 2402–2406. [DOI] [PubMed] [Google Scholar]
- Bakheit AM, Pittock S, Moore AP, et al. (2001) A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur J Neurol 8, 559–565. [DOI] [PubMed] [Google Scholar]
- Bakheit AMO, Fedorova NV, Skoromets AA, et al. (2004) The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles. J Neurol Neurosurg Psychiatry 75, 1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakheit AM, Zakine B, Maisonobe P, et al. (2010) The profile of patients and current practice of treatment of upper limb muscle spasticity with botulinum toxin type A: an international survey. Int J Rehabil Res 33, 199–204. [DOI] [PubMed] [Google Scholar]
- Bensmail D, Robertson J, Fermanian C, et al. (2010a) Botulinum toxin to treat upper‐limb spasticity in hemiparetic patients: grasp strategies and kinematics of reach‐to‐grasp movements. Neurorehabil Neural Repair 24, 141–151. [DOI] [PubMed] [Google Scholar]
- Bensmail D, Robertson JVG, Fermanian C, et al. (2010b) Botulinum toxin to treat upper‐limb spasticity in hemiparetic patients: analysis of function and kinematics of reaching movements. Neurorehabil Neural Repair 24, 273–281. [DOI] [PubMed] [Google Scholar]
- Bhakta BB, Cozens JA, Bamford JM, et al. (1996) Use of botulinum toxin in stroke patients with severe upper limb spasticity. J Neurol Neurosurg Psychiatry 61, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB (1987) Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther 67, 206–207. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Erickson JC (1996) Selective block of the brachialis motor point. An anatomic investigation of musculocutaneous nerve branching. Reg Anesth 21, 89–92. [PubMed] [Google Scholar]
- Caty GD, Detrembleur C, Bleyenheuft C, et al. (2009) Effect of upper limb botulinum toxin injections on impairment, activity, participation, and quality of life among stroke patients. Stroke 40, 2589–2591. [DOI] [PubMed] [Google Scholar]
- Chen J‐JJ, Yi‐Ning W, Huang S‐C, et al. (2005) The use of a portable muscle tone measurement device to measure the effects of botulinum toxin type A on elbow flexor spasticity. Arch Phys Med Rehabil 86, 1655–1660. [DOI] [PubMed] [Google Scholar]
- Dahlin LB (2008) Techniques of peripheral nerve repair. Scand J Surg 97, 310–316. [DOI] [PubMed] [Google Scholar]
- Demetrios M, Gorelik A, Louie J, et al. (2014) Outcomes of ambulatory rehabilitation programmes following botulinum toxin for spasticity in adults with stroke. J Rehabil Med 46, 730–737. [DOI] [PubMed] [Google Scholar]
- Elovic EP, Esquenazi A, Alter KE, et al. (2009) Chemodenervation and nerve blocks in the diagnosis and management of spasticity and muscle overactivity. PM R 1, 842–851. [DOI] [PubMed] [Google Scholar]
- Esquenazi A, Mayer N, Lee S, et al. (2012) Patient registry of outcomes in spasticity care. Am J Phys Med Rehabil 91, 729–746. [DOI] [PubMed] [Google Scholar]
- Flatow EL, Bigliani LU, April EW (1989) An anatomic study of the musculocutaneous nerve and its relationship to the coracoid process. Clin Orthop Relat Res 244, 166–171. [PubMed] [Google Scholar]
- Gracies J‐M, Bayle N, Vinti M, et al. (2010) Five‐step clinical assessment in spastic paresis. Eur J Phys Rehabil Med 46, 411–421. [PubMed] [Google Scholar]
- Hesse S, Reiter F, Konrad M, et al. (1998) Botulinum toxin type A and short‐term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double‐blind, placebo‐controlled trial. Clin Rehabil 12, 381–388. [DOI] [PubMed] [Google Scholar]
- Jost WH, Hefter H, Reissig A, et al. (2014) Efficacy and safety of botulinum toxin type A (Dysport) for the treatment of post‐stroke arm spasticity: results of the German‐Austrian open‐label post‐marketing surveillance prospective study. J Neurol Sci 337, 86–90. [DOI] [PubMed] [Google Scholar]
- Keenan MA (1988) Management of the spastic upper extremity in the neurologically impaired adult. Clin Orthop Relat Res 233, 116–125. [PubMed] [Google Scholar]
- Kong KH, Chua KS (1999) Neurolysis of the musculocutaneous nerve with alcohol to treat poststroke elbow flexor spasticity. Arch Phys Med Rehabil 80, 1234–1236. [DOI] [PubMed] [Google Scholar]
- van Kuijk AA, Geurts ACH, Bevaart BJW, et al. (2002) Treatment of upper extremity spasticity in stroke patients by focal neuronal or neuromuscular blockade: a systematic review of the literature. J Rehabil Med 34, 51–61. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Meskers CGM (2015) Botulinum toxin A for upper limb spasticity. Lancet Neurol 14, 969–971. [DOI] [PubMed] [Google Scholar]
- Lagalla G, Danni M, Reiter F, et al. (2000) Post‐stroke spasticity management with repeated botulinum toxin injections in the upper limb. Am J Phys Med Rehabil 79, 377–384; quiz 391‐4. [DOI] [PubMed] [Google Scholar]
- Lai JM, Francisco GE, Buck Willis F (2009) Dynamic splinting after treatment with botulinum toxin type‐A: a randomized controlled pilot study. Adv Ther 26, 241–248. [DOI] [PubMed] [Google Scholar]
- Mahakkanukrauh P, Somsarp V (2002) Dual innervation of the brachialis muscle. Clin Anat 15, 206–209. [DOI] [PubMed] [Google Scholar]
- McCrory P, Turner‐Stokes L, Baguley IJ, et al. (2009) Botulinum toxin A for treatment of upper limb spasticity following stroke: a multi‐centre randomized placebo‐controlled study of the effects on quality of life and other person‐centred outcomes. J Rehabil Med 41, 536–544. [DOI] [PubMed] [Google Scholar]
- de Moura WG (1985) Surgical anatomy of the musculocutaneous nerve: a photographic essay. J Reconstr Microsurg 1, 291–297. [DOI] [PubMed] [Google Scholar]
- Nott MT, Barden HLH, Baguley IJ (2014) Goal attainment following upper‐limb botulinum toxin‐A injections: are we facilitating achievement of client‐centred goals? J Rehabil Med 46, 864–868. [DOI] [PubMed] [Google Scholar]
- Park BK, Shin YB, Ko H‐Y, et al. (2007) Anatomic motor point localization of the biceps brachii and brachialis muscles. J Korean Med Sci 22, 459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Rodgers H, Price C, et al. (2010) BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost‐effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol Assess 14, 1–113, iii–iv. [DOI] [PubMed] [Google Scholar]
- Slawek J, Bogucki A, Reclawowicz D (2005) Botulinum toxin type A for upper limb spasticity following stroke: an open‐label study with individualised, flexible injection regimens. Neurol Sci 26, 32–39. [DOI] [PubMed] [Google Scholar]
- Songcharoen P (2008) Management of brachial plexus injury in adults. Scand J Surg 97, 317–323. [DOI] [PubMed] [Google Scholar]
- Sun S‐F, Hsu C‐W, Sun H‐P, et al. (2010) Combined botulinum toxin type A with modified constraint‐induced movement therapy for chronic stroke patients with upper extremity spasticity: a randomized controlled study. Neurorehabil Neural Repair 24, 34–41. [DOI] [PubMed] [Google Scholar]
- Suputtitada A, Suwanwela NC (2005) The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil 27, 176–184. [DOI] [PubMed] [Google Scholar]
- Tardieu G, Shentoub S, Delarue R (1954) Research on a technic for measurement of spasticity. Rev Neurol (Paris) 91, 143–144. [PubMed] [Google Scholar]
- Turner‐Stokes L, Fheodoroff K, Jacinto J, et al. (2013a) Results from the Upper Limb International Spasticity Study‐II (ULISII):a large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real‐life clinical management. BMJ Open 3, pii: e002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner‐Stokes L, Fheodoroff K, Jacinto J, et al. (2013b) Upper limb international spasticity study: rationale and protocol for a large, international, multicentre prospective cohort study investigating management and goal attainment following treatment with botulinum toxin A in real‐life clinical practice. BMJ Open 3, pii: e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H‐C, Hsieh L‐F, Chi W‐C, et al. (2002) Effect of intramuscular botulinum toxin injection on upper limb spasticity in stroke patients. Am J Phys Med Rehabil 81, 272–278. [DOI] [PubMed] [Google Scholar]
- Yang ZX, Pho RW, Kour AK, et al. (1995) The musculocutaneous nerve and its branches to the biceps and brachialis muscles. J Hand Surg Am 20, 671–675. [DOI] [PubMed] [Google Scholar]


