Abstract
The niche theory predicts that environmental heterogeneity and species diversity are positively correlated in tropical forests, whereas the neutral theory suggests that stochastic processes are more important in determining species diversity. This study sought to investigate the effects of soil nutrient (nitrogen and phosphorus) heterogeneity on tree species diversity in the Xishuangbanna tropical seasonal rainforest in southwestern China. Thirty‐nine plots of 400 m2 (20 × 20 m) were randomly located in the Xishuangbanna tropical seasonal rainforest. Within each plot, soil nutrient (nitrogen and phosphorus) availability and heterogeneity, tree species diversity, and community phylogenetic structure were measured. Soil phosphorus heterogeneity and tree species diversity in each plot were positively correlated, while phosphorus availability and tree species diversity were not. The trees in plots with low soil phosphorus heterogeneity were phylogenetically overdispersed, while the phylogenetic structure of trees within the plots became clustered as heterogeneity increased. Neither nitrogen availability nor its heterogeneity was correlated to tree species diversity or the phylogenetic structure of trees within the plots. The interspecific competition in the forest plots with low soil phosphorus heterogeneity could lead to an overdispersed community. However, as heterogeneity increase, more closely related species may be able to coexist together and lead to a clustered community. Our results indicate that soil phosphorus heterogeneity significantly affects tree diversity in the Xishuangbanna tropical seasonal rainforest, suggesting that deterministic processes are dominant in this tropical forest assembly.
Keywords: community phylogenetic structure, heterogeneity, nitrogen, phosphorus, species diversity, tropical forest
1. Introduction
The tropical forests harbor an enormous diversity of plant species and are conservation priorities in a fast‐changing world (Figure S1); however, the mechanisms that determine tropical tree community assembly remain important yet not well‐solved questions in community ecology (Valladares, Bastias, Godoy, Granda, & Escudero, 2015). The classical niche theory predicts that communities with more environmental heterogeneity will have higher species diversity than those with less heterogeneity because more niches can be partitioned in a heterogeneous habitat (Figure 1a; Hutchinson, 1957; Kadmon & Allouche, 2007; Macarthur & Macarthur, 1961; Ricklefs, 1977; Svenning, 2001). Environmental heterogeneity is typically determined to be the universal driver of species diversity in a variety of ecosystems (Stein, Gerstner, & Kreft, 2014), for example, limestone pavement (Lundholm & Larson, 2003), pine forest (Gundale, Metlen, Fiedler, & DeLuca, 2006), and temperate swamp forest (Douda, Doudova‐Kochankova, Boublik, & Drasnarova, 2012) ecosystems. However, in the tropics, the demonstration of a positive correlation between environmental heterogeneity and species diversity is still lacking (Holl, Stout, Reid, & Zahawi, 2013). First, resource heterogeneity usually covaries with average resource supply rate, making the effect of heterogeneity difficult to separate (Lundholm, 2009; Stevens & Carson, 2002). Second, the general unimodal theory predicts a unimodal relationship rather than a positive correlation between environmental heterogeneity and species diversity (Figure 1b) because as heterogeneity increases, the effective area available for individual species decreases, reducing population sizes and increasing the likelihood of stochastic extinctions (Allouche, Kalyuzhny, Moreno‐Rueda, Pizarro, & Kadmon, 2012; Kadmon & Allouche, 2007). Additionally, neutral community ecology theory suggests that stochastic processes (e.g., dispersal limitations or ecological drift) are dominant in regulating plant distributions in the tropics; thus, no correlation between heterogeneity and diversity would be expected within such a community (Figure 1c; Hubbell, 2001; Rosindell, Hubbell, & Etienne, 2011). Although the neutral theory provides new insights into how tropical forests are structured, the strict assumption of ecological equivalence among species has limited empirical support in general (Chave, 2004; Gaston & Chown, 2005). To date, several studies have indicated that deterministic rather than the neutral processes are dominant in tropical forest assembly (Brown et al., 2013; Condit, Engelbrecht, Pino, Perez, & Turner, 2013; John et al., 2007; Kraft, Valencia, & Ackerly, 2008; Yang et al., 2014, 2015).
Figure 1.
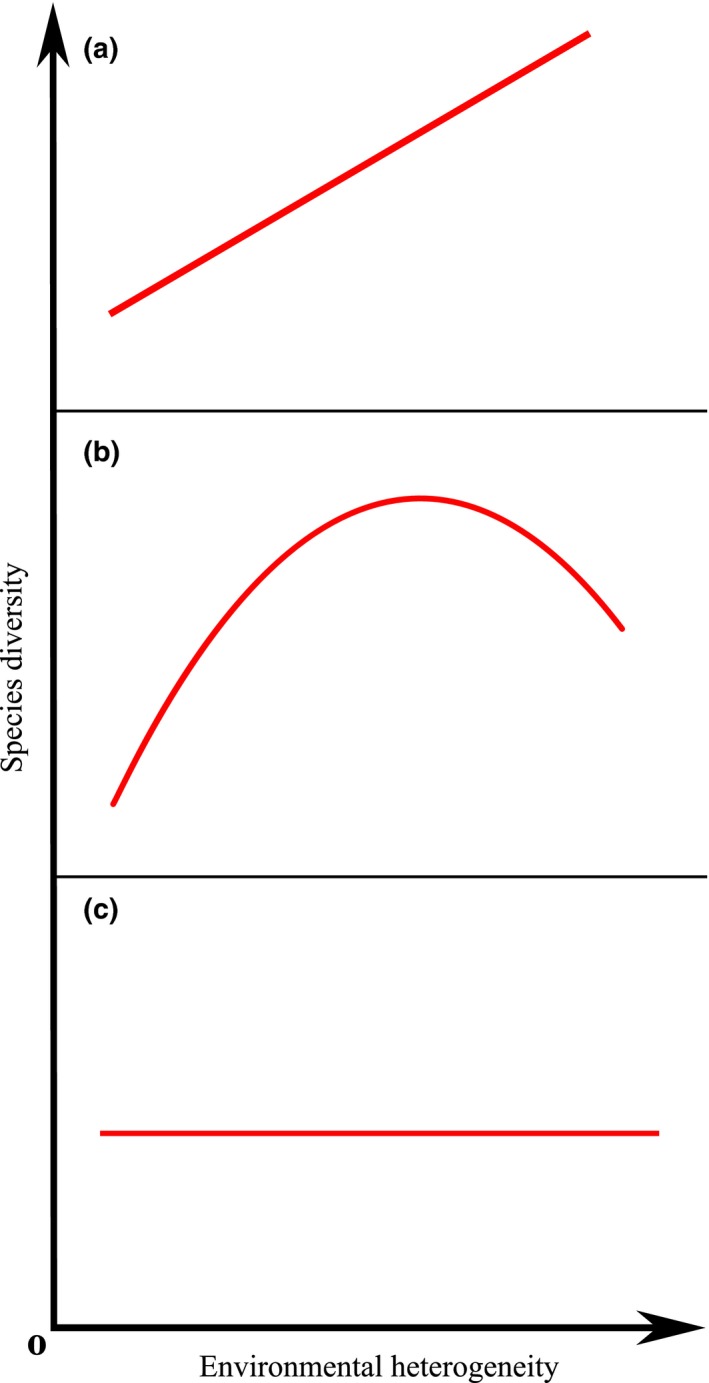
Theoretical predictions between environmental heterogeneity and species diversity. (a) Classical niche theory. Environmental heterogeneity and niche partitioning as the main factors structuring ecological communities and promoting species coexistence. (b) General unimodal theory. Heterogeneity increases, while the amount of effective area available for individual species decreases, reducing population sizes and increasing the likelihood of stochastic extinctions. (c) Neutral community ecology theory. Stochastic processes dominate the community assembly, and the species diversity within the community is not related to environmental heterogeneity
If deterministic processes dominate the assembly of Xishuangbanna tropical seasonal rainforest (Yang et al., 2014, 2015), a positive correlation between environmental heterogeneity and species diversity could be expected. However, direct evidence for a positive heterogeneity–diversity relationship within this area is lacking, and how environmental heterogeneity affects plant diversity within the tropics is largely unknown. Recent studies on phylogenetic community ecology have provided new insights into environment–plant interactions and how communities are assembled (Cavender‐Bares, Kozak, Fine, & Kembel, 2009; Qian & Jiang, 2014; Webb, Ackerly, McPeek, & Donoghue, 2002). For example, Stevens, Gavilanez, Tello, and Ray (2012) have found that an increase in environmental heterogeneity (food heterogeneity) significantly affects rodent species diversity within a desert ecosystem, and on the basis of further community phylogenetic analysis, that the species in the community are phylogenetically overdispersed in environments with low heterogeneity and tend to cluster with an increase in environmental heterogeneity. Therefore, with an increase in environmental heterogeneity, closely related and similar species could coexist within the community.
Nitrogen (N) and phosphorus (P) are generally considered the two most limiting elements to terrestrial vegetation and play an essential role in plant community assembly (Daufresne & Hedin, 2005; Reich & Oleksyn, 2004). In tropical ecosystems, phosphorus is usually suggested as the most limiting soil nutrient (Laliberte et al., 2013; Vitousek, 1984; Vitousek, Porder, Houlton, & Chadwick, 2010; Vitousek & Sanford, 1986). A previous study in Xishuangbanna tropical seasonal rainforest has confirmed that soil phosphorous is very deficient and substantially affects the community assembly (Xu et al., 2016). In this study, we randomly established 39 forest vegetation plots each with 400 m2 (20 × 20 m) in the Xishuangbanna tropical seasonal rainforest in southwestern China (Figure S2). Soil nutrient (N and P) availability and heterogeneity, tree species diversity, and community phylogenetic structure were measured in each plot. We attempted to explore the following two questions: (1) Is the soil N or P heterogeneity correlated with tree species diversity in a community? (2) What is the mechanism underlying the relationship between soil heterogeneity and species diversity within a community?
2. Methods
2.1. Study site
Thirty‐nine plots each with 400 m2 (20 × 20 m) were randomly established in an area of approximately 100 km2 in the Xishuangbanna tropical seasonal rainforest in southwestern China (Figures S1 and S2). This region has an average annual rainfall of 1,493 mm, and laterite soils developed from siliceous rocks (Cao, Zou, Warren, & Zhu, 2006). The region is part of the Indo‐Burma biodiversity hotspot (Myers, Mittermeier, Mittermeier, da Fonseca, & Kent, 2000). The dominant tree species in the plots included Pittosporopsis kerrii Craib (Icacinaceae), Parashorea chinensis H. Wang (Dipterocarpaceae), and Garcinia cowa Roxburgh (Clusiaceae).
2.2. Plot survey and soil nutrients (N and P) analysis
Within each plot, all trees with a diameter at breast height (DBH, 1.3 m above the ground) greater than 1 cm were recorded and identified in July 2013. Tree richness (number of tree species within a plot) and effective number of species (calculated as e H′, with , where f i is the proportion of stems in a plot belonging to the ith species; Chao, Chiu, & Jost, 2010; Hill, 1973) were determined for each plot. As the tree abundance varies between plots, the tree richness of each plot was rarefied (rarefied tree richness, R TR) to the smallest sample size using the community ecology R package “vegan” (Oksanen et al., 2015; R Core Team, 2015). In each plot, we sampled 500 g of soil from each of the four corners from the 1 to 10 cm depth below the litter layer in May 2013. A microdiffusion method was used to determine alkali‐hydrolyzable nitrogen (AN) in the soil, and extractable phosphorus (EP) was extracted with solution containing 0.03 mol/L NH4F and 0.025 mol/L HCl and estimated colorimetrically following the protocol as described in Hu et al. (2012).
2.3. Community phylogenetic reconstruction and phylogenetic diversity
We used the molecular phylogeny of 156 taxa recorded within our plots for community phylogenetic structure analysis. The species recorded are listed in Appendix S1. The phylogeny was assembled using RAxML (Stamatakis, 2006) based on the DNA barcodes rbcL, matK, trnH–psbA, and ITS (the original sequences have been reported in Huang, Ci, Conran, & Li, 2015). A semiparametric method based on a penalized likelihood in the R statistical software package “ape” was used to generate an ultrametric phylogenetic tree (Paradis, Claude, & Strimmer, 2004; R Core Team, 2015; Figure 2, Appendix S2). The mean pairwise phylogenetic distances (MPD) among individual tree species within each plot (400 m2; n = 39) were calculated using the R package “picante” (Kembel et al., 2010; R Core Team, 2015). The MPD was assumed to reflect the phylogenetic structure across the entire phylogeny (Webb, 2000). For the comparison of communities in the plots, the observed value of the MPD was standardized as follows: standardized effect size (SES) = (observed value − mean of 9,999 randomized values)/standard deviation, where the randomized value was calculated using the null model “taxa.labels” (Kembel et al., 2010) and the MPD was not weighted by species abundance (Stevens et al., 2012). The net relatedness index (NRI) was calculated by multiplying SES by negative one; a positive NRI value for a particular community indicated phylogenetic clustering, whereas a negative value indicated phylogenetic overdispersion (Kembel et al., 2010; Webb, 2000).
Figure 2.
The community phylogeny of 156 tree species recorded in the 39 plots. The community phylogeny was constructed based on a maximum likelihood analysis of rbcL, matK, psbA‐trnH, and ITS sequence data with APG III as a constraint tree
Based on the molecular phylogeny constructed, the rarefied Faith's phylogenetic diversity (PD, rarefied to the smallest sample size of 84; Faith, 1992; Nipperess & Matsen, 2013; R Core Team, 2015) and the phylogenetic diversity based on Hill numbers (q = 1; Chao et al., 2010; Marcon & Herault, 2015; R Core Team, 2015) were calculated for each plot.
2.4. Statistical analyses
The mean and coefficient of variation (CV = SD/M, SD = standard deviation, M = mean) of AN and EP were calculated to represent the availability and heterogeneity of these nutrients within each plot (Baer, Blair, Collins, & Knapp, 2004; Douda et al., 2012; Holl et al., 2013). The Shapiro–Wilk test was first implemented to evaluate the normal distribution of all variables; all the soil variables within each plot were log‐transformed to promote normality. Pearson correlations were used to explore the relations among soil nutrient (N and P) availability and heterogeneity, tree species diversity, and NRI across the plots. Both Shapiro–Wilk tests and Pearson correlations were implemented using the SPSS 16.0 statistical software package (SPSS Inc, Chicago, IL, USA). The significance was determined at p < .05.
3. Results
3.1. Plot characteristics
The content and CV of AN and EP in the 39 plots was highly variable with AN varying between 123.25 and 240.25 mg/kg (CV: 0.02–0.35), and EP ranging from 2.43 to 23.4 mg/kg (CV: 0.16–1.13). The content and CV of EP was significantly correlated within the plots (r = .525, p = .001) while the content and CV of AN was not (r = −.214, p = .190; Figure S3). A total of 167 tree species (with DBH > 1 cm) were identified from the 39 plots (Appendix S1) with an average of 36 tree species in each plot, ranging from 25 to 47. The rarefied tree richness ranged from 19 to 34 in the plots. The NRI in the 39 plots ranged from −1.49 (overdispersed) to 2.75 (clustered), with an average of −0.075.
3.2. Soil nutrient (N and P) heterogeneity and tree species diversity
Both AN and EP were not correlated with tree species diversity within the plots (AN and R TR: r = .087, p = .597; AN and e H′: r = −.072, p = .665; EP and R TR: r = .086, p = .604; EP and e H′: r = .049, p = .767; Figure S4). The CV of EP and tree species diversity within the plots was positively correlated (CV of EP and R TR: r = .534, p < .001; CV of EP and e H′: r = .475, p = .002; Figure 3a,b). However, there was no correlation between CV of AN and tree species diversity (CV of AN and R TR: r = .137, p = .405; CV of AN and e H′: r = .228, p = .162; Figure S5a,b). Soil phosphorus heterogeneity and the phylogenetic diversity within the plots were significantly correlated (Figure 4), while there was no correlation between the soil nitrogen heterogeneity and the phylogenetic diversity within the plots (Figure S6).
Figure 3.
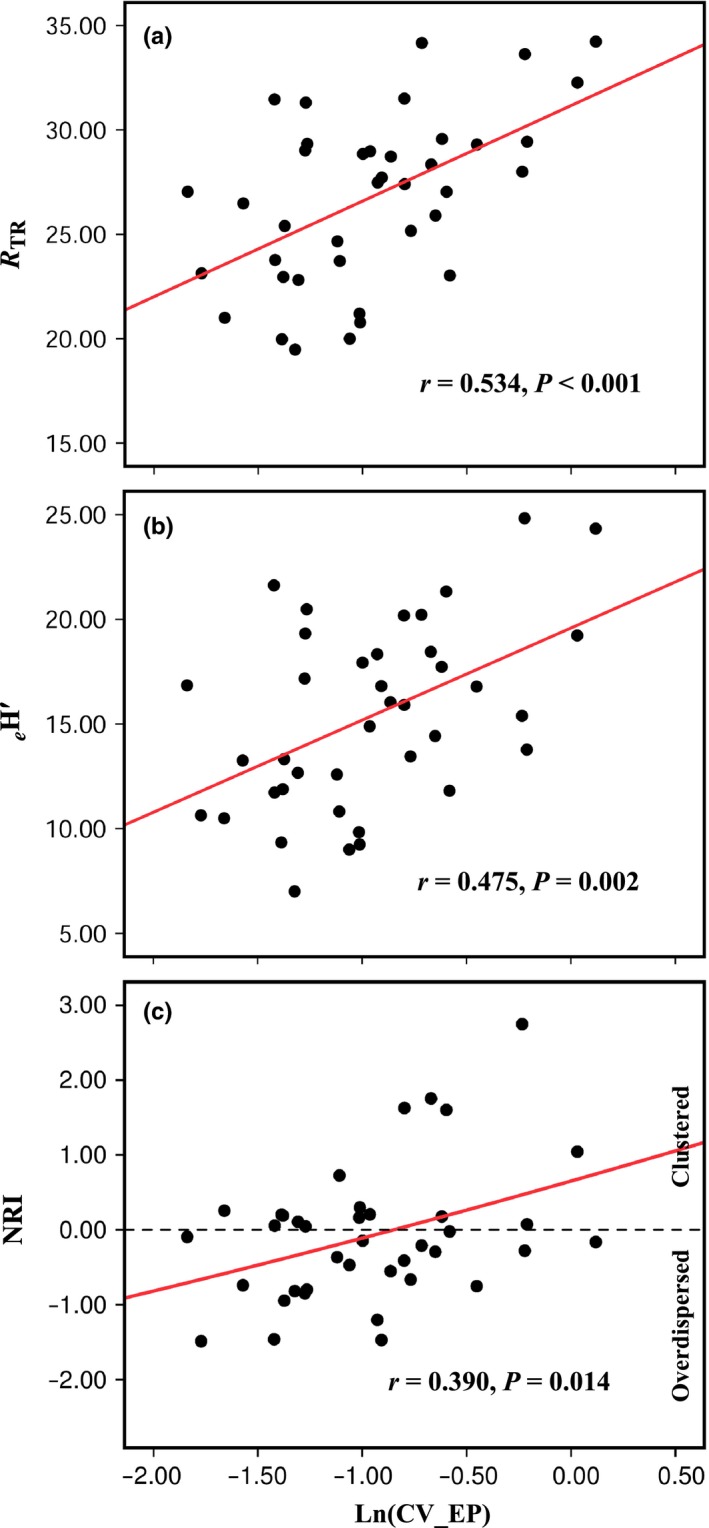
Effects of soil phosphorus heterogeneity on tree species diversity and community phylogenetic structure in the Xishuangbanna tropical seasonal rainforest in southwestern China. RTR, rarefied tree richness, rarefied to the smallest sample size of 84; eH′, effective number of species, with H′ as the Shannon–Wiener index; NRI, net relatedness index; CV_EP, the coefficient of variation of EP, Ln‐transformed; see section 2
Figure 4.
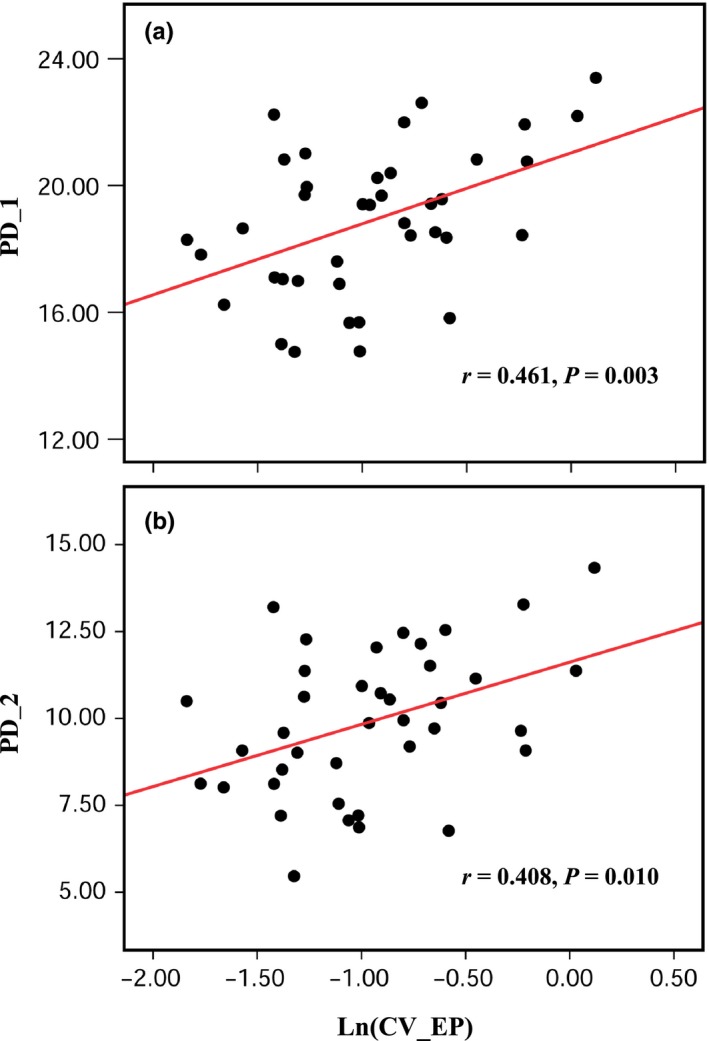
The increase in soil phosphorus heterogeneity promotes phylogenetic diversity of tree species within the community. PD_1, Faith's phylogenetic diversity, rarefied to the smallest sample size of 84; PD_2, phylogenetic diversity based on Hill numbers (q = 1); see section 2
3.3. Soil nutrient (N and P) heterogeneity and community phylogenetic structure
The CV of EP and NRI was positively correlated (r = .390; p = .014; Figure 3c) while the CV of AN and NRI was not (r = .212; p = .194; Figure S5c). NRI and tree species richness within the plots was not correlated (r = .006; p = .970).
4. Discussion
4.1. Soil nutrient (N and P) heterogeneity and tree species diversity
It has long been hypothesized that local environmental heterogeneity significantly affects the distribution and diversity of plants in the tropics (Ricklefs, 1977), while a direct positive correlation between environmental heterogeneity and plant diversity in the tropics has yet to be demonstrated (Holl et al., 2013). On the basis of the analysis from 39 plots within the Xishuangbanna tropical seasonal rainforest, we revealed that soil phosphorus heterogeneity significantly promoted tree species diversity which provided evidence that deterministic and not neutral processes dominated in the assembly of the Xishuangbanna tropical seasonal rainforest (Yang et al., 2014, 2015).
Resource availability and heterogeneity usually covary and confound the effects of resource heterogeneity on species diversity (Lundholm, 2009; Stevens & Carson, 2002). In this study, we revealed that soil phosphorus heterogeneity but not the availability significantly affects tree species diversity within community although the soil phosphorus heterogeneity and availability are also correlated (Figure S3b). Our previous study suggested that phosphorus availability significantly affects tree species diversity in the Xishuangbanna tropical seasonal rainforest (Xu et al., 2016). Compared with 40 × 40 m plot in the previous study, 20 × 20 m plot was set in the current study. It has been suggested that competitive exclusion is more apparent at smaller spatial scales while environmental filtering is more apparent at medium‐to‐large scales (Swenson, Enquist, Thompson, & Zimmerman, 2007). It is likely that increasing resource heterogeneity at small scales moderates the competitive exclusion among species and promotes species coexistence. With the increases in scale, competitive exclusion among species could be relaxed and increasing resource availability may facilitate tree species passing through the environmental filters (e.g., low phosphorus availability) and promote species diversity (Xu et al., 2016).
Neither soil nitrogen availability nor its heterogeneity was correlated with tree species diversity within the plots. It has been suggested that nitrogen levels in the tropical soils are relatively high (Huston, 1980). When the availability of a particular resource within the community is high, the effect of heterogeneity of such resource on species diversity is usually relatively low, as predicted in resource competition theory (Tilman, 1982). Therefore, an increase in soil nitrogen availability or heterogeneity may not affect tree species diversity because it is not a limiting nutrient.
4.2. Soil nutrient (N and P) heterogeneity and community phylogenetic structure
Many studies, including the current one, revealed a positive correlation between heterogeneity and diversity (e.g., Stein et al., 2014), while the mechanism underlying the influence of heterogeneity on diversity is unclear. Community phylogenetic analysis provides opportunities to examine possible mechanisms (Brown, 2012; Cavender‐Bares et al., 2009; Joly et al., 2014; Swenson et al., 2007; Willis et al., 2010). Stevens et al. (2012) provided the first example on the study of how environmental heterogeneity affects species diversity within the community. These authors have found a significant positive correlation between rodent species diversity and food heterogeneity within a community. Their further community phylogenetic analyses have revealed that increased food heterogeneity promoted phylogenetic clustering as more closely related species coexist within the community. In our study, the soil nitrogen heterogeneity within the plots was neither related to the tree species diversity nor to community phylogenetic structure (Figure S5). However, we identified a significant positive correlation between soil phosphorus heterogeneity and tree species diversity in the Xishuangbanna tropical seasonal rainforest (Figure 3a,b), and further community phylogenetic analysis indicated that the trees in plots with low soil phosphorus heterogeneity were phylogenetically overdispersed, whereas those in plots with high heterogeneity were clustered (Figure 3c).
The competition among trees in a homogeneous environment may lead to a more dispersed community because closely related trees, sharing similar ecological requirements, would be eliminated from the community; however, recent studies have indicated that increased competitive exclusion among species may also eliminate the less related species and lead to a clustered community if competitive ability differences among species is more important than niche differences and positively correlated with phylogenetic distances (Godoy, Kraft, & Levine, 2014; Mayfield & Levine, 2010). In our study, an increase in soil phosphorus heterogeneity significantly promoted tree species diversity within the plots and the phylogenetic structure of trees in plots with low soil phosphorus heterogeneity was phylogenetically overdispersed, but tended to be clustered with the increase in soil phosphorus heterogeneity. Therefore, in the less heterogeneous communities, interspecific competition could eliminate the more closely related tree species, which led to a phylogenetically overdispersed community; however, with an increase in soil phosphorus heterogeneity, the more closely related tree species could coexist within the community because the interspecific competition was moderated (Stevens et al., 2012). It is worth noting that we only collected four soil samples in each plot, while the results of the significant correlation between phosphorus heterogeneity and tree diversity indicate that tree species diversity within the plots increased substantially along the gradient of phosphorus heterogeneity. Future research would benefit from more extensive sampling within the plots.
Conflict of Interests
The authors declare no conflict of interests.
Supporting information
Acknowledgments
The National Natural Science Foundation of China (Grant nos. 31370267; 31500454) and the CAS 135 program (XTBG‐T01) supported this study. The authors would like to thank the Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies (XSTRE) for logistics assistance during the fieldwork and Prof. Sha Liqing for advice on soil nutrient analysis.
Xu, W. , Ci, X. , Song, C. , He, T. , Zhang, W. , Li, Q. and Li, J. (2016), Soil phosphorus heterogeneity promotes tree species diversity and phylogenetic clustering in a tropical seasonal rainforest. Ecology and Evolution, 6: 8719–8726. doi: 10.1002/ece3.2529
Contributor Information
Qiaoming Li, Email: lqm@xtbg.ac.cn.
Jie Li, Email: jieli@xtbg.ac.cn.
References
- Allouche, O. , Kalyuzhny, M. , Moreno‐Rueda, G. , Pizarro, M. , & Kadmon, R. (2012). Area–heterogeneity tradeoff and the diversity of ecological communities. Proceedings of the National Academy of Sciences of the United States of America, 109, 17495–17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer, S. G. , Blair, J. M. , Collins, S. L. , & Knapp, A. K. (2004). Plant community responses to resource availability and heterogeneity during restoration. Oecologia, 139, 617–629. [DOI] [PubMed] [Google Scholar]
- Brown, J. H. (2012). The role of phylogeny in desert rodent community assembly. Journal of Animal Ecology, 81, 307–309. [DOI] [PubMed] [Google Scholar]
- Brown, C. , Burslem, D. F. R. P. , Illian, J. B. , Bao, L. , Brockelman, W. , Cao, M. , Law, R. (2013). Multispecies coexistence of trees in tropical forests: Spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proceedings of the Royal Society of London B: Biological Sciences, 280, 20130502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, M. , Zou, X. M. , Warren, M. , & Zhu, H. (2006). Tropical forests of Xishuangbanna, China. Biotropica, 38, 306–309. [Google Scholar]
- Cavender‐Bares, J. , Kozak, K. H. , Fine, P. V. A. , & Kembel, S. W. (2009). The merging of community ecology and phylogenetic biology. Ecology Letters, 12, 693–715. [DOI] [PubMed] [Google Scholar]
- Chao, A. , Chiu, C. H. , & Jost, L. (2010). Phylogenetic diversity measures based on Hill numbers. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 365, 3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chave, J. (2004). Neutral theory and community ecology. Ecology Letters, 7, 241–253. [Google Scholar]
- Condit, R. , Engelbrecht, B. M. J. , Pino, D. , Perez, R. , & Turner, B. L. (2013). Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proceedings of the National Academy of Sciences of the United States of America, 110, 5064–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daufresne, T. , & Hedin, L. O. (2005). Plant coexistence depends on ecosystem nutrient cycles: Extension of the resource‐ratio theory. Proceedings of the National Academy of Sciences of the United States of America, 102, 9212–9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douda, J. , Doudova‐Kochankova, J. , Boublik, K. , & Drasnarova, A. (2012). Plant species coexistence at local scale in temperate swamp forest: Test of habitat heterogeneity hypothesis. Oecologia, 169, 523–534. [DOI] [PubMed] [Google Scholar]
- Faith, D. P. (1992). Conservation evaluation and phylogenetic diversity. Biological Conservation, 61, 1–10. [Google Scholar]
- Gaston, K. J. , & Chown, S. L. (2005). Neutrality and the niche. Functional Ecology, 19, 1–6. [Google Scholar]
- Godoy, O. , Kraft, N. J. B. , & Levine, J. M. (2014). Phylogenetic relatedness and the determinants of competitive outcomes. Ecology Letters, 17, 836–844. [DOI] [PubMed] [Google Scholar]
- Gundale, M. J. , Metlen, K. L. , Fiedler, C. E. , & DeLuca, T. H. (2006). Nitrogen spatial heterogeneity influences diversity following restoration in a Ponderosa pine forest, Montana. Ecological Applications, 16, 479–489. [DOI] [PubMed] [Google Scholar]
- Hill, M. O. (1973). Diversity and evenness: A unifying notation and its consequences. Ecology, 54, 427–432. [Google Scholar]
- Holl, K. D. , Stout, V. M. , Reid, J. L. , & Zahawi, R. A. (2013). Testing heterogeneity–diversity relationships in tropical forest restoration. Oecologia, 173, 569–578. [DOI] [PubMed] [Google Scholar]
- Hu, Y. H. , Sha, L. Q. , Blanchet, F. G. , Zhang, J. L. , Tang, Y. , Lan, G. Y. , & Cao, M. (2012). Dominant species and dispersal limitation regulate tree species distributions in a 20‐ha plot in Xishuangbanna, southwest China. Oikos, 121, 952–960. [Google Scholar]
- Huang, X. C. , Ci, X. Q. , Conran, J. G. , & Li, J. (2015). Application of DNA barcodes in Asian tropical trees—A case study from Xishuangbanna Nature Reserve, Southwest China. PLoS One, 10, e0129295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell, S. P. (2001). A unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- Huston, M. (1980). Soil nutrients and tree species richness in costa‐rican forests. Journal of Biogeography, 7, 147–157. [Google Scholar]
- Hutchinson, G. E. (1957). Concluding remarks. Cold Spring Harbor Symposium on Quantitative Biology, 22, 415–427. [Google Scholar]
- John, R. , Dalling, J. W. , Harms, K. E. , Yavitt, J. B. , Stallard, R. F. , Mirabello, M. , et al. (2007). Soil nutrients influence spatial distributions of tropical tree species. Proceedings of the National Academy of Sciences of the United States of America, 104, 864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly, S. , Davies, T. J. , Archambault, A. , Bruneau, A. , Derry, A. , Kembel, S. W. , et al. (2014). Ecology in the age of DNA barcoding: The resource, the promise and the challenges ahead. Molecular Ecology Resources, 14, 221–232. [DOI] [PubMed] [Google Scholar]
- Kadmon, R. , & Allouche, O. (2007). Integrating the effects of area, isolation, and habitat heterogeneity on species diversity: A unification of island biogeography and niche theory. The American Naturalist, 170, 443–454. [DOI] [PubMed] [Google Scholar]
- Kembel, S. W. , Cowan, P. D. , Helmus, M. R. , Cornwell, W. K. , Morlon, H. , Ackerly, D. D. , et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26, 1463–1464. [DOI] [PubMed] [Google Scholar]
- Kraft, N. J. B. , Valencia, R. , & Ackerly, D. D. (2008). Functional traits and niche‐based tree community assembly in an amazonian forest. Science, 322, 580–582. [DOI] [PubMed] [Google Scholar]
- Laliberte, E. , Grace, J. B. , Huston, M. A. , Lambers, H. , Teste, F. P. , Turner, B. L. , et al. (2013). How does pedogenesis drive plant diversity? Trends in Ecology and Evolution, 28, 331–340. [DOI] [PubMed] [Google Scholar]
- Lundholm, J. T. (2009). Plant species diversity and environmental heterogeneity: Spatial scale and competing hypotheses. Journal of Vegetation Science, 20, 377–391. [Google Scholar]
- Lundholm, J. T. , & Larson, D. W. (2003). Relationships between spatial environmental heterogeneity and plant species diversity on a limestone pavement. Ecography, 26, 715–722. [Google Scholar]
- Macarthur, R. , & Macarthur, J. W. (1961). On bird species diversity. Ecology, 42, 594–598. [Google Scholar]
- Marcon, E. , & Herault, B. (2015). entropart: An R package to measure and partition diversity. Journal of Statistical Software, 67, 1–26. [Google Scholar]
- Mayfield, M. M. , & Levine, J. M. (2010). Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecology Letters, 13, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , da Fonseca, G. A. B. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. [DOI] [PubMed] [Google Scholar]
- Nipperess, D. A. , & Matsen, F. A. (2013). The mean and variance of phylogenetic diversity under rarefaction. Methods in Ecology and Evolution, 4, 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen, J. , Blanchet, G. , Kindt, R. , Legendre, P. , Minchin, P. R. , & O'Hara, R. B. , et al. (2015). vegan: Community ecology package. R package version 2.3‐0. Retrieved from http://CRAN.R-project.org/package=vegan
- Paradis, E. , Claude, J. , & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. [DOI] [PubMed] [Google Scholar]
- Qian, H. , & Jiang, L. (2014). Phylogenetic community ecology: Integrating community ecology and evolutionary biology. Journal of Plant Ecology, 7, 97–100. [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Retrieved from http://www.R-project.org/ [Google Scholar]
- Reich, P. B. , & Oleksyn, J. (2004). Global patterns of plant leaf N and P in relation to temperature and latitude. Proceedings of the National Academy of Sciences of the United States of America, 101, 11001–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs, R. E. (1977). Environmental heterogeneity and plant species diversity: A hypothesis. The American Naturalist, 111, 376–381. [Google Scholar]
- Rosindell, J. , Hubbell, S. P. , & Etienne, R. S. (2011). The unified neutral theory of biodiversity and biogeography at age ten. Trends in Ecology and Evolution, 26, 340–348. [DOI] [PubMed] [Google Scholar]
- SPSS Inc . Released (2007). SPSS for windows, version 16.0. Chicago, IL: Author. [Google Scholar]
- Stamatakis, A. (2006). RAxML‐VI‐HPC: Maximum likelihood‐based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stein, A. , Gerstner, K. , & Kreft, H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters, 17, 866–880. [DOI] [PubMed] [Google Scholar]
- Stevens, M. H. H. , & Carson, W. P. (2002). Resource quantity, not resource heterogeneity, maintains plant diversity. Ecology Letters, 5, 420–426. [Google Scholar]
- Stevens, R. D. , Gavilanez, M. M. , Tello, J. S. , & Ray, D. A. (2012). Phylogenetic structure illuminates the mechanistic role of environmental heterogeneity in community organization. Journal of Animal Ecology, 81, 455–462. [DOI] [PubMed] [Google Scholar]
- Svenning, J. C. (2001). On the role of microenvironmental heterogeneity in the ecology and diversification of neotropical rain‐forest palms (Arecaceae). The Botanical Review, 67, 1–53. [Google Scholar]
- Swenson, N. G. , Enquist, B. J. , Thompson, J. , & Zimmerman, J. K. (2007). The influence of spatial and size scale on phylogenetic relatedness in tropical forest communities. Ecology, 88, 1770–1780. [DOI] [PubMed] [Google Scholar]
- Tilman, D. (1982). Resource competition and community structure. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- Valladares, F. , Bastias, C. C. , Godoy, O. , Granda, E. , & Escudero, A. (2015). Species coexistence in a changing world. Frontiers in Plant Science, 6, 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek, P. M. (1984). Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology, 65, 285–298. [Google Scholar]
- Vitousek, P. M. , Porder, S. , Houlton, B. Z. , & Chadwick, O. A. (2010). Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen‐phosphorus interactions. Ecological Applications, 20, 5–15. [DOI] [PubMed] [Google Scholar]
- Vitousek, P. M. , & Sanford, R. L. (1986). Nutrient cycling in moist tropical forest. Annual Review of Ecology, Evolution, and Systematics, 17, 137–167. [Google Scholar]
- Webb, C. O. (2000). Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. The American Naturalist, 156, 145–155. [DOI] [PubMed] [Google Scholar]
- Webb, C. O. , Ackerly, D. D. , McPeek, M. A. , & Donoghue, M. J. (2002). Phylogenies and community ecology. Annual Review of Ecology, Evolution, and Systematics, 33, 475–505. [Google Scholar]
- Willis, C. G. , Halina, M. , Lehman, C. , Reich, P. B. , Keen, A. , McCarthy, S. , et al. (2010). Phylogenetic community structure in Minnesota oak savanna is influenced by spatial extent and environmental variation. Ecography, 33, 565–577. [Google Scholar]
- Xu, W. M. , Liu, L. , He, T. , Cao, M. , Sha, L. Q. , Hu, Y. H. , et al. (2016). Soil properties drive a negative correlation between species diversity and genetic diversity in a tropical seasonal rainforest. Scientific Reports, 6, 20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Swenson, N. G. , Zhang, G. C. , Ci, X. Q. , Cao, M. , Sha, L. Q. , et al. (2015). Local‐scale partitioning of functional and phylogenetic beta diversity in a tropical tree assemblage. Scientific Reports, 5, 12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Zhang, G. C. , Ci, X. Q. , Swenson, N. G. , Cao, M. , Sha, L. Q. , et al. (2014). Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Functional Ecology, 28, 520–529. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


