Abstract
Background and Purpose
There is increasing evidence suggesting that ROS play a major pathological role in bladder dysfunction induced by bladder inflammation and/or obstruction. The aim of this study was to determine the effect of H2O2 on different types of bladder afferents and its mechanism of action on sensory neurons in the guinea pig bladder.
Experimental Approach
‘Close‐to‐target’ single unit extracellular recordings were made from fine branches of pelvic nerves entering the guinea pig bladder, in flat sheet preparations, in vitro.
Key Results
H2O2 (300–1000 μM) preferentially and potently activated capsaicin‐sensitive high threshold afferents but not low threshold stretch‐sensitive afferents, which were only activated by significantly higher concentrations of hydrogen peroxide. The TRPV1 channel agonist, capsaicin, excited 86% of high threshold afferents. The TRPA1 channel agonist, allyl isothiocyanate and the TRPM8 channel agonist, icilin activated 72% and 47% of capsaicin‐sensitive high threshold afferents respectively. The TRPA1 channel antagonist, HC‐030031, but not the TRPV1 channel antagonist, capsazepine or the TRPM8 channel antagonist, N‐(2‐aminoethyl)‐N‐[[3‐methoxy‐4‐(phenylmethoxy)phenyl]methyl]thiophene‐2‐carboxamide, significantly inhibited the H2O2‐induced activation of high threshold afferents. Dimethylthiourea and deferoxamine did not significantly change the effect of H2O2 on high threshold afferents.
Conclusions and Implications
The findings show that H2O2, in the concentration range detected in inflammation or reperfusion after ischaemia, evoked long‐lasting activation of the majority of capsaicin‐sensitive high threshold afferents, but not low threshold stretch‐sensitive afferents. The data suggest that the TRPA1 channels located on these capsaicin‐sensitive afferent fibres are probable targets of ROS released during oxidative stress.
Abbreviations
- AITC
allyl isothiocyanate
- BCTC
4‐(3‐chloro‐2‐pyridinyl)‐N‐[4‐(1,1‐dimethyl)phenyl]‐1‐piperazine carboxamide
- DRG
dorsal root ganglion
- M8‐B
N‐(2‐aminoethyl)‐N‐[[3‐methoxy‐4‐(phenylmethoxy)phenyl]methyl]thiophene‐2‐carboxamide
- NPPB
5‐nitro‐2‐[(3phenylpropyl)amino]benzoic acid
Tables of Links
| LIGANDS | |
|---|---|
| AITC | H2O2 |
| BCTC | Icilin |
| Capsaicin | M8‐B |
| Capsazepine | Nicardipine |
| HC030031 | NPPB |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
It is well established that oxidative stress during tissue injury, ischaemia and inflammation leads to generation of ROS (such as O2 −, OH−, H2O2) that are released by polymorphonuclear neutrophils and macrophages, endothelial cells and smooth muscle cells (Swindle et al., 2002; Yu et al., 2004; Saitoh et al., 2006; Allen and Bayraktutan, 2009). Increased production of ROS may contribute to a variety of pathologies such as bladder outlet obstruction, bladder overactivity and bladder dysfunction developed with age (Brading et al., 2004; Scheepe et al., 2011; Nomiya et al., 2012; Nocchi et al., 2014).
Sensory neurons, specialized for the detection of noxious endogenous stimuli and environmental irritants, employ a variety of specialized ion channels. Among them, the transient receptor potential (TRP) channel family is the largest group, consisting of six subfamilies of non‐selective cation channels in mammals (Yoshida et al., 2006; Miller and Zhang, 2011). A variety of TRP channels may be activated by free radicals. These include TRPA1, TRPV1, TRPV4, TRPC3–5, TRPM2, TRPM7 and TRPM8 channels (Yoshida et al., 2006; Andersson et al., 2008; Miller and Zhang, 2011; Naziroglu et al., 2013; Nocchi et al., 2014). Numerous TRP channels are expressed by sensory neurons innervating the bladder, including TRPV1, TRPA1 and TRPM8 channels (Stein et al., 2004; Avelino and Cruz, 2006; Mukerji et al., 2006; Hayashi et al., 2011; La et al., 2011). TRPA1 channels are polymodal signal detectors that can act as mechanosensors, cool receptors and biosensors for large number of noxious endogenous and exogenous environmental agents (Hinman et al., 2006; Macpherson et al., 2007). In the rat bladder, TRPA1 channels are expressed in the urothelium, muscle layer and in TRPV1‐positive sensory neurons (Du et al., 2007; Streng et al., 2008), while in the mouse and guinea pig bladder, TRPA1 and TRPV1 channels are expressed in dorsal root ganglion (DRG) neurons but not in the urothelium (Everaerts et al., 2010; Skryma et al., 2011). The TRPV1 ion channel is also activated by polymodal stimuli, including capsaicin, noxious heat, low pH and some endogenous ligands, and has been strongly implicated in nociceptive signalling. Both TRPV1 and TRPA1 channels are co‐expressed on DRG sensory neurons (Story et al., 2003). Recently, it has been proposed that TRPM8 channels in the urothelium may also serve as sensors in many conditions associated with high level of ROS (Nocchi et al., 2014). The TRPM8 channel has been identified as a cold receptor since it is activated by both innocuous and noxious cool temperatures, and by compounds that evoke cooling sensations, such as menthol and icilin (Bautista et al., 2007). TRPM8 channels are expressed in Aδ and C fibre DRG neurons and are often co‐expressed with TRPV1 channels (Story et al., 2003; Hayashi et al., 2009). In the bladder, these channels are expressed in both the urothelium and in sensory nerves (Stein et al., 2004; Hayashi et al., 2009; Mukerji et al., 2006).
Oxidative stress produced by application of H2O2 has been widely used to investigate the mechanism of action of ROS on sensory neurons. In the lung and heart, capsaicin‐sensitive fibres were identified as primary targets for H2O2 acting via TRPV1 channels (Schultz and Ustinova, 1998; Ruan et al., 2005). However, in studies on isolated DRG neurons, it was shown that H2O2 acts via TRPA1 channels (Andersson et al., 2008; Sawada et al., 2008). Intravesically applied H2O2 (10–100 mM) evoked bladder overactivity, most likely via an action on capsaicin‐sensitive afferents (Masuda et al., 2007). A novel model of chronic inflammatory and overactive bladder utilizes a single intravesical injection of H2O2 (Homan et al., 2013). It is still unclear which types of bladder sensory neurons are activated by free radicals and what are their major targets. The aim of this study was to determine the mechanism of action of H2O2 on the major types of bladder afferents. We have found that H2O2 was more potent in activating capsaicin‐sensitive high threshold afferents in the bladder than low threshold stretch‐sensitive afferents. H2O2 probably acts mostly via TRPA1, rather than TRPV1 or TRPM8 channels, located on the peripheral endings of capsaicin‐sensitive high threshold afferents.
Methods
Animals
All animal care and experimental procedures were approved by the Animal Welfare Committee of Flinders University and performed in accordance with guidance under the ‘Australian code of practice for the care and use of animals for scientific purposes’ (8th edition, 2013) and the ARRIVE guidelines (Kilkenny et al., 2010), and the editorial on reporting animal studies (McGrath and Lilley, 2015). Adult Dunkin Hartley guinea pigs (N = 123), weighing between 250–350 g, provided by Flinders University School of Medicine Animal Facility were used in the present study. Experiments were performed with animals maintained under 12 h light/dark cycles with free access to food and water. Animals were killed by overdose with isoflurane (5%) followed by cutting through the cervical spinal cord. Guinea pigs were chosen for this study since we had previously characterized in detail the functional properties of different classes of sensory neurons in this species (Zagorodnyuk et al., 2007, 2009, 2010).
Extracellular recording
‘Close‐to‐target’ single unit extracellular recordings were made from fine branches of pelvic nerves entering the guinea pig bladder in flat sheet preparations in vitro as previously described (Zagorodnyuk et al., 2007). Briefly, the bladder was removed and opened into a flat sheet and washed with Krebs solution (mM: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95% O2 – 5% CO2). A full thickness, flat sheet preparation (~15 × 15–20 mm, which represents about a half of the bladder) was superfused at 3 mL·min−1, temperature 34°C and studied with the mucosa uppermost. The preparations were pinned along one edge in a 10 mL organ bath while other edge was attached, via a 15 mm ‘rake’ to an isotonic transducer (Harvard Bioscience 52–9511, S Natick, MA. U.S.A). Increasing counterweights (load range 10–600 mN) could be applied to the bladder so as to distend the preparation isotonically while measuring the resulting changes in length.
Fine nerve fibres, originating from the pelvic ganglia, were dissected free and, together with a separate strand of connective tissue, were pulled into a second small chamber (~1 mL volume) separated by a cover slip and silicon grease barrier (Ajax Chemicals, Australia). The small chamber was filled with paraffin oil and differential extracellular recordings were made via platinum electrodes. Signals were amplified (DAM 80, WPI, USA) and recorded at 20 kHz with a Maclab/8s data acquisition system with Chart 7 software (AD Instruments, Castle Hill, NSW, Australia) using an iMac computer running OSX 10.8.5 (Apple, Cupertino, CA). Single units were discriminated by amplitude and duration using Spike Histogram software (AD Instruments, Sydney, Australia).
To identify different types of bladder mechanoreceptors, the following strategy was used. First, the preparations were stretched by 10–600 mN load and stretch‐sensitive single units (if any) were identified. Stretch‐activated units were considered as low threshold stretch‐sensitive group of afferents if they were activated by low 10–100 mN circumferential loads. Afferents with threshold of ≥200 mN were classified as high threshold. In some preparations, bladder tissue was probed with 3–10 mN von Frey hairs (care was taken not to over‐stimulate afferents). Receptive fields (‘hotspots’) identified in this way were marked with carbon particles on the von Frey hair. In most cases, hot Krebs solution (45–46°C) was applied by direct spraying from a syringe onto the marked receptive field of particular afferent. This procedure was used to activate capsaicin‐sensitive (temperature sensitive) afferents. Spraying Krebs solution at bath temperature on the identified hotspot served as control, as established previously (Zagorodnyuk et al., 2009).
It has been previously reported that the effects of H2O2 were attenuated significantly when applied intravesically compared with serosal applications (Nocchi et al., 2014). To overcome the barrier created by the urothelium, the mechanism of action of H2O2 and effects of TRP channels agonists and antagonists were studied in urothelium‐free bladder preparations. For this, we carefully removed the superficial urothelial layer, under a binocular microscope, in order to expose the sensory endings of bladder afferents to drugs. In the majority of experiments, the superficial urothelial layer was removed from the bladder preparation at the beginning of the experiment. H2O2 and agonists for TRP channels (allyl isothiocyanate, AITC and 5‐nitro‐2‐[(3phenylpropyl)amino]benzoic acid, NPBB) for TRPA1, capsaicin for TRPV1 and icilin for TRPM8, Bautista et al., 2006, 2007; Liu et al., 2010] were added directly into the bath and mixed in continuously perfused Krebs solution (rate: 3 mL·min−1). After 120 s, they were then washed out at a higher (6 mL·min−1) rate flow. All antagonists were superfused in Krebs solution for at least for 30 min before adding H2O2 or agonists. Because the majority of high threshold afferents were spontaneously active (in some preparations firing repetitive single spikes, but in 33% of preparations, firing spontaneous bursts of spikes), the effect of agonists was calculated as a change in firing rate, that is spontaneous firing rate was subtracted from the firing rate recorded during drug application. For H2O2 and most of the agonists, the increase in mean firing rate was calculated during the 2 min of action (spontaneous firing rate from the previous 2 min was subtracted). To determine whether each afferent fibre was capsaicin‐sensitive, a high concentration of capsaicin (3 μM) was applied once at the end of the experiment. As some units were quickly desensitized by capsaicin, only the first 30 s of its action was analysed and maximal firing during 10 s (subtracted by 10 s spontaneous firing, if any) was calculated. All experiments were performed in the presence of nicardipine (4 μM) to minimise smooth muscle contractions (Zagorodnyuk et al., 2007).
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Results are expressed as means ± SEM. The use of ‘n’ numbers in the results section refers to the number of units and N to the number of animals. Statistical analysis was performed using Student's two‐tailed t‐test for paired or unpaired data or by one‐way or two‐way ANOVA using Prism 6 software (GraphPad Software, Inc., San Diego, CA, USA) by using N as independent variables. Differences were considered significant if P < 0.05.
Materials
H2O2, AITC, NPBB, HC‐030031, BCTC (4‐(3‐chloro‐2‐pyridinyl)‐N‐[4‐(1,1‐dimethyl)phenyl]‐1‐piperazinecarboxamide), M8‐B ( N‐(2‐aminoethyl)‐N‐[[3‐methoxy‐4‐(phenylmethoxy)phenyl]methyl]thiophene‐2‐carboxamide), dimethylthiourea, deferoxamine, capsaicin, capsazepine, icilin and nicardipine were obtained from Sigma Chemical Company (St Louis, MO). Stock solutions in ethanol were prepared in advance and stored in the freezer at −20°C for AITC (100 mM), capsaicin and capsazepine (10 mM each). Stock solutions in DMSO were prepared for NPBB (100 mM), icilin, HC‐030031 and BCTC (10 mM, each) while H2O2, M8‐B, dimethythiourea, deferoxamine and nicardipine were dissolved directly in Krebs solution or in isotonic NaCl solution.
Results
Low threshold stretch‐sensitive and high threshold groups of bladder afferents
Based on sensitivity to stretch, two major groups of afferents were distinguished in this study – low threshold stretch‐sensitive afferents and high threshold afferents (see Methods) (Figure 1). Previous studies have shown that low threshold afferents could be subdivided by using their responses to mucosal stroking with a light von Frey hair (0.1–1 mN). The low threshold units that were also activated by mucosal stroking corresponded to muscular‐urothelial (=muscular‐mucosal) afferents as reported previously (Figure 1B) (Zagorodnyuk et al., 2007; Xu and Gebhart, 2008). Another class of low threshold afferents did not respond to mucosal stroking and are referred to as muscular afferents (Figure 1A) (Zagorodnyuk et al., 2007; Xu and Gebhart, 2008). In the present study, these two classes were grouped together as low threshold stretch‐sensitive afferents, as experiments were mostly performed in urothelium‐free preparations where the stroking response could not be measured. In urothelium‐free preparations, 41% (66 units out of 162, N = 51) of stretch‐sensitive units were identified as low threshold afferents with a mean threshold of 43.8 ± 3.7 mN (n = 66, N = 51). In cases where a stretch/response (10 to 600 mN load stimuli) curve was calculated, saturation of firing was usually observed at 200–400 mN load stimuli (Figure 1E). High threshold afferents could be divided into two subtypes. One type responded to mucosal stroking with a light von Frey hair (0.1–1 mN) but not to high intensity stretch (200–600 mN) (Figure 1C). This class has been previously identified as urothelial (=mucosal) afferents (Zagorodnyuk et al., 2007; Xu and Gebhart, 2008). The second type was insensitive to stroking of the mucosa (Figure 1D), but was activated by high intensity stretch (threshold varied in most cases between 200–400 mN). These probably include several classes of high threshold mechanoreceptors (Zagorodnyuk et al., 2007, 2010; Xu and Gebhart, 2008; Song et al., 2009). As we have studied the effect of H2O2 on sensory neurons in urothelium‐free preparations, all high threshold afferents were grouped together in the present study. Firing rate of afferents of the high threshold afferents did not saturate with 600 mN load stimuli, and was significantly smaller than that of low threshold afferents (Figure 1E).
Figure 1.
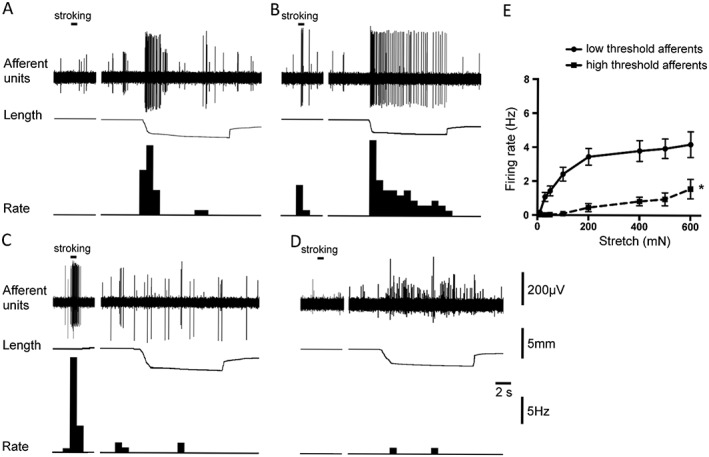
Typical tracings representing responses of two groups of afferents: low threshold stretch‐sensitive afferents (A, B) and high threshold afferents (C, D) to stretch and mucosal stroking. (A) Muscular afferent (large amplitude unit) responded to small stretch (50 mN load) but not to light (0.1 mN) von Frey hair stroking of its receptive field. (B) Muscular‐urothelial afferent (large amplitude unit) was activated by stroking of its receptive field with a 0.1 mN von Frey hair and by small stretch (50 mN load). (C) Urothelial (mucosal) afferent (large amplitude unit) was activated by a light (0.1 mN) von Frey hair stroking of its receptive field area, but not by high intensity stretch (200 mN load). (D) High threshold afferent (large amplitude unit) was slightly activated by a high intensity stretch (200 mN load) but not by a light (0.1 mN) von Frey hair stroking of its receptive field. Note in each trace the largest amplitude units were discriminated and used to calculate firing rate as shown in each panel. (E) Averaged data of the effects of stretch (10–600 mN) on the low threshold stretch sensitive (shown by solid line, n = 18, N = 14) and high threshold (shown by dashed line, n = 21, N = 15) afferents. *P < 0.05, significantly different from low threshold values.
Effects of H2O2 and TRPV1 channel agonist, capsaicin on bladder afferents
H2O2 in the low concentration range (300–1000 μM) activated the majority (73%, 63 out of 86 units, N = 51) of high threshold afferents, inducing long‐lasting regular bursting, with mean firing rate of 0.62 ± 0.08 Hz (n = 86, N = 51). Capsaicin (3 μM) evoked robust excitation in the majority (86%, 127 units out of 148, N = 69) of high threshold afferents, with mean maximal firing of 5.26 ± 0.30 Hz (n = 127, N = 69) (Figure 2B). When applied to the same high threshold afferents, 65% (56 units out of 86, N = 51) were activated by both drugs. H2O2 induced a concentration‐dependent activation of capsaicin‐sensitive high threshold afferents with EC50 of 1250 μM (95% confidence intervals = 320–4890 μM, n = 9, N = 6) (Figure 2C).
Figure 2.
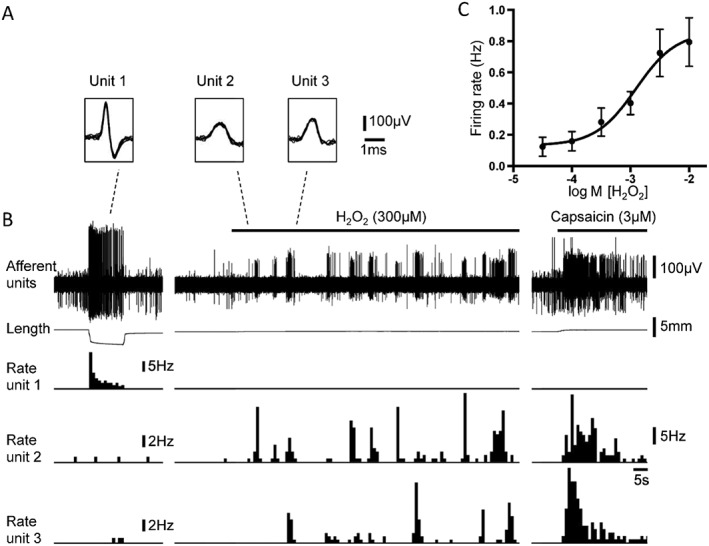
Responses of low threshold stretch‐sensitive and high threshold afferents to low concentration of H2O2 and capsaicin. (A) The shape of seven superimposed action potentials for each of the three discriminated units from tracing in B: unit 1 – low threshold stretch‐sensitive afferent; unit 2 and unit 3 – high threshold afferents. (B) Typical traces showing bursting firing of high threshold afferents (unit 2 and unit 3) evoked by H2O2 (300 μM) and capsaicin (3 μM) but not low threshold stretch‐sensitive afferent (unit 1). Note that low threshold stretch‐sensitive afferent, but not high threshold afferents, was strongly activated by stretch with 400 mN load. (C) Concentration‐response curve for activation of high threshold afferents (n = 9, N = 6) by H2O2.
Low threshold stretch‐sensitive afferents (97%, 57 units out of 59, N = 33) were not generally activated by H2O2 in the low concentration range (300–1000 μM) (Figure 2 and Figure 3). However, about half of low threshold afferents (51%, 39 units out of 76, N = 34) were excited by a significantly higher (10 mM) concentration of H2O2. In low threshold afferents that did respond to H2O2, bath application induced concentration‐dependent activation with EC50 of 5.8 mM (95% confidence intervals = 2.9–11.3 mM, n = 7, N = 6) (Figure 3A, C). The effect of a high concentration of H2O2 (10 mM) was not repeatable on subsequent application: 0.52 ± 0.16 Hz (n = 9, N = 8) for first application and 0.28 ± 0.09 Hz (n = 9, N = 8) for second application. Stretch‐sensitivity of these afferents was significantly reduced by this high concentration of H2O2 (10 mM): 4.24 ± 0.66 Hz firing induced by 100 mN load before and 2.88 ± 0.44 Hz, 2–3 min after H2O2 application (n = 15, N = 11, paired t‐test). These results suggest that at high concentrations (≥10 mM), H2O2 may have damaged the sensory nerve endings of the low threshold afferents, including the mechanism underlying their mechano‐sensitivity. The TRPV1 channel agonist, capsaicin (3 μM) activated only a small proportion (7%, 8 units out of 111, N = 49) of low threshold afferents, producing a mean firing rate of 5.25 ± 0.98 Hz (n = 8, N = 7).
Figure 3.
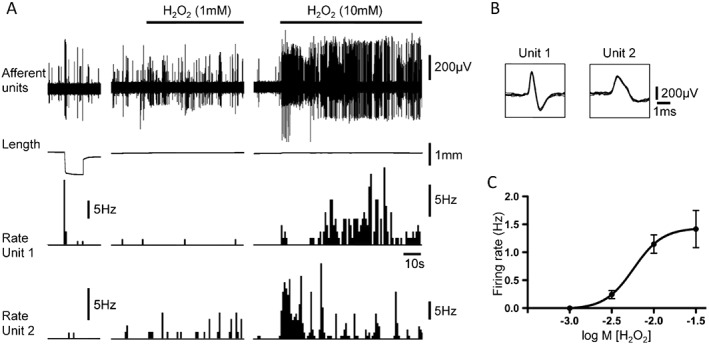
Responses of low threshold stretch‐sensitive and high threshold afferents to a high concentration of H2O2 (10 mM). (A) Typical traces showing activation of low threshold stretch‐sensitive afferents (unit 1) and high threshold afferents (unit 2) by H2O2 (10 mM). In contrast to the high threshold afferent, the low threshold stretch‐sensitive afferent was not activated by 1 mM H2O2. Note that the low threshold stretch‐sensitive afferent, but not the high threshold afferent, was strongly activated by stretch with 50 mN load. (B) The shape of seven superimposed action potentials for each of the two discriminated units from tracings in A: unit 1 – low threshold stretch‐sensitive afferent; unit 2 – high threshold afferent. (C) Concentration‐response curve for activation of low threshold stretch‐sensitive afferents (n = 7, N = 6) by H2O2.
Effects of TRPA1 and TRPM8 channel agonists on bladder afferents
The TRPA1 channel agonist, AITC, at a low concentration (10 μM) activated 54% (7 out of 13 units, N = 9) of high threshold afferents. At a higher concentration range (100–300 μM), it excited 72% (52 out of 72 units, N = 33) of high threshold capsaicin‐sensitive afferents. Two high threshold units (n = 2) were activated by AITC (300 μM) but not by capsaicin (3 μM). The effects of AITC on high threshold afferents were concentration‐dependent (Figure 4A, C), but an EC50 could not be calculated because the effect did not reach a maximum at 300 μM. There was a positive significant correlation (Pearson r = 0.61, n = 13, N = 7) between the amplitude of responses to AITC (300 μM) and responses to H2O2 (300 μM), when applied to the same high threshold afferents. Another, TRPA1 channel agonist, NPPB, (300 μM) activated 71% (10 out of 14 units, N = 8) of capsaicin‐sensitive high threshold afferents. The effect of NPPB on these afferents was also concentration‐dependent (Figure 4D). The TRPM8 agonist, icilin (5–10 μM) activated 47% (18 units out of 38, N = 19) of capsaicin‐sensitive high threshold afferents (Figure 4B). Icilin induced a mean increase in firing rate of 0.35 ± 0.1 Hz (n = 12, N = 11) and 0.38 ± 0.16 Hz (n = 11, N = 10), at 5 and 10 μM respectively. Only 1 out of 8 (n = 6) capsaicin‐insensitive high threshold units was activated by icilin (5 μM). There was no correlation between the size of effects of H2O2 (1 mM) and icilin (5 μM), when both drugs were applied to the same bladder afferents (Pearson r = −0.28, n = 14, N = 11, NS).
Figure 4.
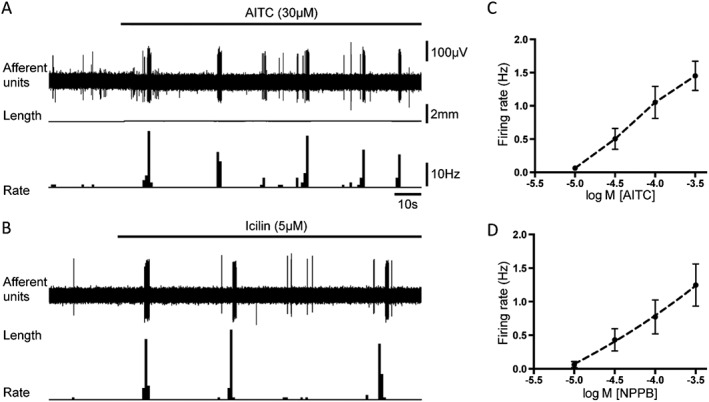
Typical responses of high threshold afferents to AITC, NPPB and icilin. (A) Typical traces showing activation of high threshold afferent by the TRPA1 channel agonist, AITC (30 μM). (B) Typical traces showing activation of high threshold afferent by the TRPM8 channel agonist, icilin (5 μM). (C) and (D) – average data for concentration‐dependent activation of high threshold afferents by AITC (n = 14, N = 9) and NPPB (n = 10, N = 7) respectively.
AITC at a low concentration (10 μM) did not activate any of the low threshold afferents tested (n = 23, N = 14). AITC at a high concentration (300 μM) activated 58% (18 units out of 31, N = 20) of low threshold stretch‐sensitive afferents, increasing firing of responsive units of 1.04 ± 0.22 Hz (n = 18, N = 12). Only a small proportion of these responding units (11%, 2 out of 18 units, N = 12) were activated by capsaicin (3 μM) when both drugs were applied on the same afferents. AITC concentration dependently activated low threshold afferents with EC50 = 52.3 μM (95% confidence intervals = 17.1–160 μM, n = 12, N = 8) (Figure 5). There was a positive correlation between the amplitude of the responses to AITC (300 μM) and H2O2 (10 mM) (Pearson r = 0.53, n = 22, N = 12), when they were applied to the same afferents. NPPB concentration‐dependently activated 70% (7 units out of 10, N = 7) of low threshold stretch‐sensitive afferents (Figure 5 D). No activation of low threshold afferents was seen at 10 and 30 μM NPPB. The TRPM8 channel agonist, icilin (5 μM) activated 29% (11 out of 38 units, N = 19) of low threshold afferents. The mean increase (where present) was very small (0.18 ± 0.05 Hz, n = 11, N = 8). This may reflect a low density of these channels on low threshold afferents in the guinea pig bladder. No correlation was found between the effects of H2O2 (10 mM) and icilin (5 μM), when they were applied to the same low threshold afferents (Pearson r = −0.17, n = 17, N = 8, NS).
Figure 5.
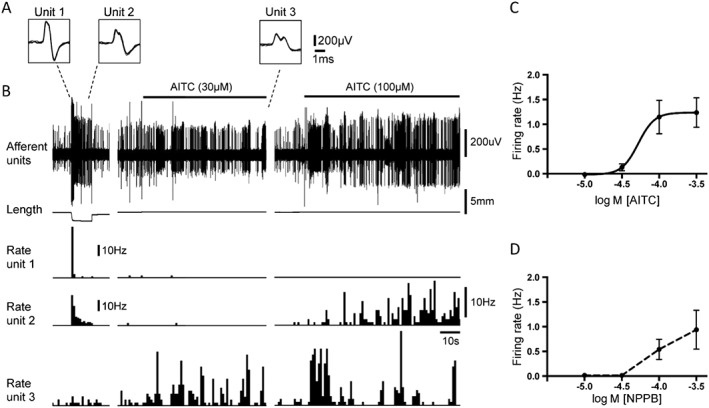
Responses of low threshold stretch‐sensitive afferents to AITC and NPPB. (A) The shape of seven superimposed action potentials for each of the three discriminated units from tracings in B: unit 1 and unit 2 – low threshold stretch‐sensitive afferents; unit 3 – high threshold afferent. (B) Typical traces showing activation of one of the low threshold stretch‐sensitive afferents (unit 2) by 100 μM but not 30 μM AITC. The high threshold afferent (unit 3) was activated by both 30 and 100 μM AITC. Note that the low threshold stretch‐sensitive afferents, but not the high threshold afferent, were strongly activated by stretch with 100 mN load. (C) and (D) – average data for concentration‐dependent activation of low threshold stretch‐sensitive afferents by AITC (n = 12, N = 8) and NPPB (n = 7, N = 7) respectively.
Effects of TRP channel antagonists on H2O2‐induced responses of high threshold afferents
The mechanisms underlying the effects of H2O2 on afferents were investigated in high threshold afferents only, since low threshold afferents required higher concentrations, which caused impaired functions and non‐repeatable effects. Repeated application of H2O2 (300 μM, 30–35 min apart) on the same capsaicin‐sensitive high threshold afferents produced a non‐significant reduction in the responses (NS, one‐way ANOVA, Tukey's post test) (Figure 6B). Bath application of the TRPA1 channel antagonist, HC‐030031 (10 μM) prior to the second exposure significantly inhibited H2O2 (300 μM)‐induced activation of high threshold afferents by 65 ± 8% (n = 11, N = 7, paired t‐test) (Figure 6A, C). HC‐030031 (10 μM) also significantly reduced the effect of the TRPA1 channel agonist, AITC (30 μM) on high threshold afferents by 70 ± 8% (n = 11, N = 5, paired t‐test) (Figure 7C). A second application of AITC (30 μM after 35–45 min wash) on its own evoked a slightly smaller increase in firing in high threshold afferents (−16 ± 5%, n = 7, N = 5). Adding HC‐030031 prior to the second application of AITC caused a significantly greater reduction in AITC's effect (unpaired t‐test). The TRPV1 channel antagonist, capsazepine (10 μM) did not affect the action of H2O2 (300 μM) on high threshold afferents (−2 ± 24%, n = 8, N = 5, NS, paired t‐test) (Figure 7A). However, application of capsazepine (10 μM) abolished the effect of capsaicin (0.5 μM) (by 99 ± 1%, n = 10, N = 5, paired t‐test) (Figure 7B). Capsaicin (0.5 μM) activated high threshold afferents with a mean firing rate of 3.16 ± 0.50 Hz (n = 17, N = 10). When capsaicin (0.5 μM) was applied twice on the same afferents, response to the consequent second application of capsaicin (after 45 min of washing) did not significantly change compared to the first application (−9 ± 11%, n = 6, N = 5, NS, paired t‐test). The TRPM8 channel antagonist M8‐B (3 μM) (Almeida et al., 2012) did not affect the H2O2 (1 mM)‐induced excitation in high threshold afferents (−5 ± 26%, n = 7, N = 5, NS, paired t‐test) (Figure 7D). However, application of M8‐B (3 μM) significantly inhibited (by 90 ± 3%, n = 6, N = 5, paired t‐test) the effect of icilin (10 μM) on the high threshold afferents (Figure 7E). The mixed TRPV1 and TRPM8 channel antagonist, BCTC (10 μM) (Behrendt et al., 2004; Benko et al., 2012), did not significantly alter the effects of H2O2 (1 mM) on high threshold afferents (−33 ± 14%, n = 8, N = 5, NS, paired t‐test) (Figure 7F).
Figure 6.
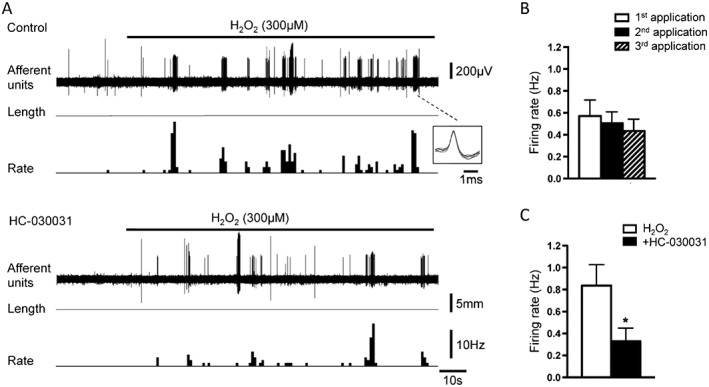
Effects of the TRPA1 channel antagonist, HC‐030031 on H2O2‐induced activation of high threshold afferents. (A) Typical traces showing activation of high threshold afferents by H2O2 (300 μM) in control and after 30 min of application of HC‐030031 (10 μM). Insert shows the shape of seven superimposed action potentials for discriminated high threshold single unit. (B) Average data of the effects of repeated application of H2O2 (300 μM) on the same high threshold afferents (n = 7, N = 5). (C) Average data of the effect of HC‐030031 (10 μM) on the H2O2 (300 μM)‐induced activation of high threshold afferents (n = 11, N = 7). *P < 0.05, significant effect of HC‐030031.
Figure 7.
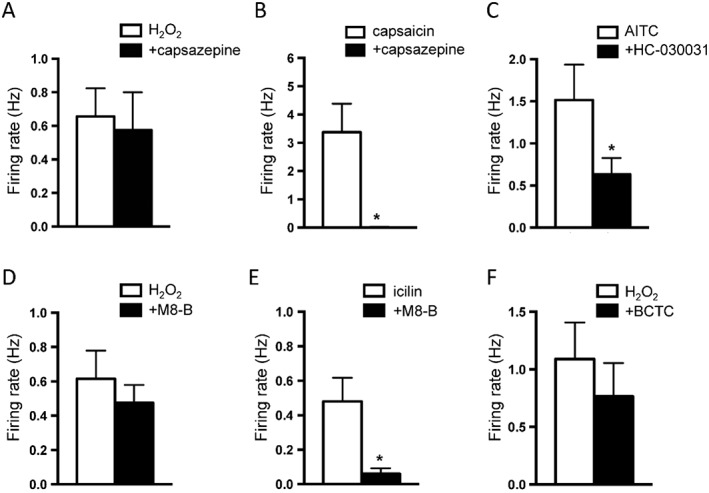
Effects of TRP channel antagonists, capsazepine, HC‐030031, M8‐B and BCTC on the activation of high threshold afferents induced by H2O2 and by corresponding agonists. (A) Average data of the effect of capsazepine (10 μM) on H2O2 (300 μM)‐induced activation of high threshold afferents (n = 8, N = 5). (B) Average data of the effects of capsazepine (10 μM) on capsaicin (0.5 μM)‐induced activation of high threshold afferents (n = 10, N = 5). (C) Average data of the effect of HC‐030031 (10 μM) on AITC (30 μM)‐induced activation of high threshold afferents (n = 11, N = 5). (D) Average data of the effect of M8‐B (3 μM) on H2O2 (1 mM)‐induced activation of high threshold afferents (n = 7, N = 5). (E) Average data of the effect of M8‐B (3 μM) on icilin (10 μM)‐induced activation of high threshold afferents (n = 6, N = 5). (F) Average data of the effect of BCTC (10 μM) on H2O2 (1 mM)‐induced activation of high threshold afferents (n = 8, N = 5). *P < 0.05, significant effect of channel antagonist. Note that repeated application of H2O2 (300 μM) on the same capsaicin‐sensitive high threshold afferents without antagonists produced a non‐significant decay in its responses (see Figure 6B).
Effects of a hydroxyl radical scavenger and a iron chelator on the H2O2 ‐induced responses of high threshold afferents
The role of hydroxyl radicals in the activation of high threshold afferents caused by H2O2‐induced oxidative stress was determined by using dimethylthiourea and deferoxamine. Unexpectedly, dimethylthiourea (10 mM) itself evoked activation of the majority of capsaicin‐sensitive high threshold afferents, with mean increase in firing rate of 0.84 ± 0.23 Hz (n = 13, N = 8). In 7 out 10 units, dimethylthiourea reduced H2O2 (300 μM)‐induced activation; however, overall this was not significant (before 0.74 ± 0.27 and after 0.28 ± 0.16 Hz n = 10, N = 7, NS, paired t‐test). It is worth mentioning that the excitatory effect of dimethylthiourea on high threshold afferents was similar to effects of H2O2, AITC and icilin. All drugs evoked bursting activity or increased both the frequency and duration of bursts in spontaneously bursting units. Deferoxamine (1 mM) did not alter firing of high threshold afferents. In 5 out 12 units, it slightly reduced H2O2 (300 μM)‐induced activation of high threshold afferents; however, overall, its effect was not significant (before 0.70 ± 0.15 Hz and after 0.49 ± 0.12 Hz, n = 12, N = 7, NS, paired t‐test).
Discussion
The current study revealed that H2O2 (300–1000 μM), which is within the range that has been detected in inflammation or reperfusion after ischaemia (Sprong et al., 1997; Stone and Yang, 2006; Schroder and Eaton, 2008), strongly activates the majority of capsaicin‐sensitive high threshold afferents in the guinea pig bladder but not low threshold stretch‐sensitive afferents. The data also suggest that activation of high threshold group of afferents by H2O2 is mediated substantially via TRPA1, rather than TRPV1 or TRPM8 channels.
Excessive ROS production occurs in many pathological conditions including inflammation and reperfusion after ischaemia, contributing to the pathogenesis of a variety of human diseases (Comhair and Erzurum, 2002). In the bladder, excess production of ROS is strongly implicated in overactive bladder syndrome, bladder obstruction and pathological conditions developed with age (Brading et al., 2004; Nomiya et al., 2012; Nocchi et al., 2014). Excessive chronic generation of ROS during ischaemia‐reperfusion causes suppression of detrusor muscle function, inhibition of bladder afferents and efferents, and patchy nerve degeneration in the detrusor in patients with bladder obstruction and in animal models of obstruction and over‐distension (Brading et al., 2004; Yu et al., 2004; Scheepe et al., 2011).
Several types of TRP channels can be activated by free radicals during oxidative stress (Yoshida et al., 2006; Andersson et al., 2008; Miller and Zhang, 2011; Naziroglu et al., 2013; Nocchi et al., 2014). In the rat lung and heart, evidence suggested that H2O2 excites capsaicin‐sensitive cardiac afferents and vagal lung afferents via TRPV1 channels (Ustinova and Schultz, 1994; Schultz and Ustinova, 1998; Ruan et al., 2005). TRPV1 channels in HEK cells and in the rat DRG neurons were also reported to be activated by H2O2 (Yoshida et al., 2006; Naziroglu et al., 2013).
It is well known that TRP channels from different species can exhibit variations in functional properties (Chen et al., 2013; Zheng, 2013). There are no reports of the properties of TRPV1, TRPA1 and TRPM8 channels in the guinea pigs and only few studies reporting the effectiveness of their antagonists in this species (Skryma et al., 2011; Benko et al., 2012). This was a major impetus for us to investigate the effectiveness of TRP channel antagonists (used in this study to evaluate the possible ion channel targets of H2O2) in inhibiting the effect of corresponding TRP channel agonists in the sensory neurons innervating the guinea pig bladder. Our data indicate that H2O2 preferentially activates capsaicin‐sensitive high threshold afferents and has much less effect on the low threshold stretch‐sensitive afferents, which are mostly capsaicin‐insensitive. However, our data also suggest TRPV1 channels expressed on sensory neurons are not the targets of H2O2 in the guinea pig bladder. The TRPV1 channel antagonist capsazepine in a concentration that effectively abolished the responses induced by capsaicin, did not affect the responses induced by H2O2 in high threshold afferents.
ROS and other noxious compounds, released during tissue damage, oxidize cysteine residues in proteins to form either cysteine sulfenic acids or disulfides (Poole et al., 2004). This may occur in the intracellular N‐terminal domain of TRPA1 channels, representing a likely mechanism for activation of these channels in DRG neurons (Andersson et al., 2008; Sawada et al., 2008). In the rat bladder, TRPA1channels are expressed in the urothelium, muscle layer and in TRPV1‐positive sensory neurons (Du et al., 2007; Streng et al., 2008). Interestingly, in the mouse and guinea pig bladder, TRPA1 and TRPV1 channelsare expressed in DRG neurons but not in the urothelium (Everaerts et al., 2010; Skryma et al., 2011). The present data indicated that TRPA1 channels, located on the capsaicin‐sensitive high threshold afferents, are a likely targets for H2O2 for several reasons: (i) the TRPA1channel antagonist (HC‐030031), used at concentration that effectively inhibited the action of the TRPA1 channel agonist (AITC), significantly attenuated the effect of H2O2 on these afferents; (ii) there was a strong correlation between the magnitude of effects of H2O2 and AITC, when applied to the same afferent fibres; (iii) similar proportions of capsaicin‐sensitive high threshold afferents were activated by H2O2 and TRPA1 channel agonists; and (iv) the effects of H2O2 were not affected by either TRPV1 or TRPM8 channel antagonists (capsazepine, M8‐B and BCTC). In DRG, the majority of TRPA1‐expressing neurons also expressed TRPV1 channels, and around 30% of TRPV1‐expressing neurons express TRPA1 channels (Story et al., 2003). The present data indicate that TRPA1 channel agonists activate the majority of capsaicin‐sensitive high threshold bladder afferents, demonstrating an even higher overlap between TRPA1 and TRPV1 channels in this group of sensory neurons. In contrast, the overlap between TRPA1 and TRPV1 channel responsiveness was much smaller in low threshold afferents. We found that 7% and 58% of the total number of low threshold afferents were activated by capsaicin and AITC respectively. It has been recently shown that while AITC activates TRPA1 channels, at concentrations above 100 μM, it can also act as a weak agonist of TRPV1 channels expressed in HEK293 cells or in mouse and porcine DRG neurons (Ohta et al., 2007; Everaerts et al., 2011). In the sensory neurons of the guinea pig bladder, AITC at low concentrations (10–30 μM) probably acted selectively via TRPA1 channels, in agreement with previous findings obtained in DRG neurons (Jordt et al., 2004; Bautista et al., 2006; Everaerts et al., 2011). However, we cannot exclude the possibility that in high threshold afferents, a small part of the responses evoked at the highest concentration tested (300 μM AITC) was due to activation of TRPV1 channels. This may be why AITC did not reach a maximal effect at 300 μM in high threshold capsaicin‐sensitive afferents, in contrast to low threshold capsaicin‐insensitive fibres.
TRPA1 channel agonists activated similar proportions of low threshold and high threshold afferents; however, they were less potent in low threshold afferents than in high threshold afferents, suggesting a lower density of TRPA1 channels on low threshold afferents. Our data also revealed a weak correlation between the effects of H2O2 and AITC in low threshold afferents. Put together, this suggests that H2O2 may act on TRPA1 channels on the low threshold afferents, although a much higher concentration was needed to induce a significant increase in their firing. We cannot exclude the possibility that other TRP channels are also involved in the effects of high concentrations of H2O2 (10–100 mM) on the low threshold afferents.
TRPM8 channels are expressed in small to medium Aδ and C fibre DRG neurons and can be co‐expressed with TRPV1 channels (McKemy et al., 2002; Story et al., 2003). In the bladder, TRPM8 channels are expressed in the urothelium and in sensory nerve fibres (Stein et al., 2004; Mukerji et al., 2006; Hayashi et al., 2009). In the urothelium, TRPM8 channels may respond to the increased levels of ROS associated with ageing (Nocchi et al., 2014). We found that 47% of capsaicin‐sensitive high threshold afferents were activated by the TRPM8 channel agonist icilin. These data are in agreement with immunohistochemical findings showing 33% co‐expression of TRPM8 and TRPV1 channels in the rat bladder afferent neurons (Hayashi et al., 2009). The present data indicate that TRPM8 channels expressed on sensory neurons are not the targets of H2O2 in urothelium‐free bladder preparations because (i) the TRPM8 channel antagonist, M8‐B, inhibited the effect of icilin, but did not affect the H2O2‐induced activation of high threshold afferents, and (ii) there was no correlation between the effects of icilin and H2O2 when applied to the same afferent fibres. TRPM8 channels are frequently co‐expressed with NF200, a marker of myelinated Aδ fibres in rat bladder sensory neurons (Hayashi et al., 2009). Our data indicate that icilin very mildly activates a small (29%) proportion of low threshold afferents, some of which may well be Aδ fibres innervating the bladder.
It is worth mentioning that H2O2 and the TRP channels agonists, capsaicin, AITC and icilin all evoked bursting activity de novo, or enhanced existing spontaneous bursting activity in the high threshold afferents (by increasing both the frequency and duration of bursts). We have observed this phenomenon previously with other excitatory stimuli such as hot (45–46°C) Krebs solution, low pH solutions, 5‐HT and ATP (Zagorodnyuk et al., 2007; Zagorodnyuk et al., 2009). Capsaicin‐sensitive high threshold afferents identified in vitro are likely to be nociceptive fibres in the bladder (Zagorodnyuk et al., 2009). Similarly, chemoreceptive units were previously reported to show bursting in the rat bladder in vivo, where the authors suggested that this pattern of activity, which can be induced by filling the bladder with high K+ solution, was a characteristic feature of nociceptive signalling from the bladder (Moss et al., 1997).
It is well established that in the presence of iron, H2O2 produces hydroxyl radicals, OH− via the Fenton reaction and it has been suggested that OH− contributes to the effects of H2O2 on sensory neurons (Ustinova and Schultz, 1994; Andersson et al., 2008), as H2O2 effects were reduced by dimethylthiourea (a hydroxyl radical scavenger) (Sprong et al., 1997) and by deferoxamine (an iron chelator that reduces formation of hydroxyl radical) (Comhair and Erzurum, 2002). In the present study, neither dimethylthiourea nor deferoxamine significantly affected H2O2‐induced excitation of high threshold afferents. It is not clear why these results differ from previous studies. One possibility is that we directly applied H2O2 onto the nerve endings, while in other studies H2O2 was applied on the epithelial tissues of the bladder and lung (Ruan et al., 2005; Masuda et al., 2007; Nocchi et al., 2014), where it may have acted on other structures.
In conclusion, the present study provides evidence that H2O2, in a concentration range that has been detected under pathological conditions of inflammation or ischaemia/reperfusion, evoked a long‐lasting activation of most capsaicin‐sensitive high threshold afferents, but not low threshold stretch‐sensitive afferents. The data demonstrate that the TRPA1 channels present on the endings of capsaicin‐sensitive high threshold afferents are probable targets of H2O2 in the bladder.
Author contributions
S.N. and S.Y.Y. performed experiments. S.N. and V.P.Z. analysed the data. V.P.Z. conceived and designed the experiments and wrote the manuscript. S.J.H.B. and N.J.S. critically revised the article.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This study has been supported by the National Health and Medical Research Council of Australia grant #1046881.
Nicholas, S. , Yuan, S. Y. , Brookes, S. J. H. , Spencer, N. J. , and Zagorodnyuk, V. P. (2017) Hydrogen peroxide preferentially activates capsaicin‐sensitive high threshold afferents via TRPA1 channels in the guinea pig bladder. British Journal of Pharmacology, 174: 126–138. doi: 10.1111/bph.13661.
References
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CL, Bayraktutan U (2009). Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4: 461–470. [DOI] [PubMed] [Google Scholar]
- Almeida C, Hew‐Butler T, Soriano R, Rao S, Wang W, Wang J et al. (2012). Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioural cold defences and decreases deep body temperature. J Neurosci 32: 2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S (2008). Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28: 2485–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelino A, Cruz F (2006). TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedebergs Arch Pharmacol 373: 287–299. [DOI] [PubMed] [Google Scholar]
- Bautista D, Jordt SE, Nikai T, Tsuruda P, Read A, Poblete J et al. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282. [DOI] [PubMed] [Google Scholar]
- Bautista D, Siemens J, Glazer J, Tsuruda P, Basbaum A, Stuchy C et al. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–209. [DOI] [PubMed] [Google Scholar]
- Behrendt H, Germann T, Gillen C, Hatt H, Jostock R (2004). Characterization of the mouse cold‐menthol receptor TRPM8 and vanilloid receptor type‐1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko R, Illenyi L, Kelemen D, Papp R, Papp A, Bartho L (2012). Use and limitations of three TRPV‐1 receptor antagonists on smooth muscles of animals and man: a vote for BCTC. Eur J Pharmacol 674: 44–50. [DOI] [PubMed] [Google Scholar]
- Brading A, Pessina F, Esposito L, Symes SE (2004). Effects of metabolic stress and ischemia on the bladder, and the relationship with bladder overactivity. Scand J Urol Nephrol Suppl 38: 84–92. [DOI] [PubMed] [Google Scholar]
- Chen J, Kang D, Xu J, Lake M, Hogan J, Sun C et al. (2013). Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4: 2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair S, Erzurum S (2002). Antioxidant responses to oxidant‐mediated lung diseases. Am J Physiol Lung Cell Mol Physiol 283: L246–L255. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al. (2015). Experimental design and analysis and ther reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Araki I, Yoshiyama M, Nomura T, Takeda M (2007). Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C‐fiber pathway. Urology 70: 826–831. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetreu A et al. (2011). The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 21: 316–321. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D et al. (2010). Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol 298: F692–F701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kondo T, Ishimatsu M, Takeya M, Igata S, Nakamura K et al. (2011). Function and expression pattern of TRPM8 in bladder afferent neurons associated with bladder outlet obstruction in rats. Auton Neurosci 164: 27–33. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kondo T, Ishimatsu M, Yamada S, Nakamura K, Matsuoka K et al. (2009). Expression of the TRPM8‐immunoreactivity in dorsal root ganglion neurons innervating the rat urinary bladder. Neurosci Res 65: 245–251. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D (2006). TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A 103: 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan T, Tsuzuki T, Dogishi K, Shirakawa H, Oyama T, Nakagawa T et al. (2013). A novel model of chronic inflammatory and overactive bladder by a single intravesical injection of hydrogen peroxide. J Pharmacol Sci 121: 327–337. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED et al. (2004). Mustard oils and canabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La JH, Schwartz ES, Gebhart GF (2011). Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore‐domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience 186: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Samuel M, Ho M, Harrison R, Paslay J (2010). NPPB structure‐specifically activates TRPA channels. Biochem Pharmacol 80: 113–121. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Felix Marr F, Peter G, Schultz PG et al. (2007). Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545. [DOI] [PubMed] [Google Scholar]
- Masuda H, Kihara K, Saito K, Matsuoka Y, Yoshida S, Chancellor M (2007). Reactive oxygen species mediate detrusor overactivity via sensitization of afferent pathway in the bladder of anaesthetized rats. BJU Int 101: 775–780. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D (2002). Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58. [DOI] [PubMed] [Google Scholar]
- Miller BA, Zhang W (2011). TRP channels as mediators of oxidative stress. Adv Exp Med Biol 704: 531–544. [DOI] [PubMed] [Google Scholar]
- Moss NG, Harrington WW, Tucker MS (1997). Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol Regul Integr Comp Physiol 272: R695–R703. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Corcoran S, Selmer I, Smith G, Benham D et al. (2006). Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroglu M, Cig B, Ozgul C (2013). Neuroprotection induced by N‐acethylcysteine against cytosolic glutathione depletion‐induced Ca2 + influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience 242: 151–160. [DOI] [PubMed] [Google Scholar]
- Nocchi L, Daly D, Chapple C, Grundy D (2014). Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8‐mediated mechanism: implications for aging. Aging Cell 13: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiya M, Sagawa K, Yazaki J, Takahashi N, Kushida N, Haga N et al. (2012). Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis‐induced chronic bladder ischemia. NeurourolUrodyn 31: 185–189. [DOI] [PubMed] [Google Scholar]
- Ohta T, Imagawa T, Ito S (2007). Novel agonistic action of mustard oil on recombinant and endogenous porcine transient receptor potential V1 (pTRPV1) channels. Biochem Pharmacol 73: 1646–1656. [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, Claiborne A (2004). Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 44: 325–347. [DOI] [PubMed] [Google Scholar]
- Ruan T, Lin YS, Lin KS, Kou YR (2005). Sensory transduction of pulmonary reactive oxygen species by capsaicin‐sensitive vagal lung afferent fibres in rats. J Physiol 565: 563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers P (2006). Hydrogene peroxide: a feed‐forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Matsumura K, Kobayashi S (2008). Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci 27: 1131–1142. [DOI] [PubMed] [Google Scholar]
- Scheepe J, Amelink A, de Jong B, Wolffenbuttel K, Kok D (2011). Changes in bladder wall oxygen saturation in the overactive obstructed bladder. J Urol 186: 1128–1133. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Ustinova EE (1998). Capsaicin receptors mediate free radical‐induced activation of cardiac afferent endings. Cardiovasc Res 38: 348–355. [DOI] [PubMed] [Google Scholar]
- Schroder E, Eaton P (2008). Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol 8: 153–159. [DOI] [PubMed] [Google Scholar]
- Skryma R, Prevarskaya N, Gkika D, Shuba Y (2011). From urgency to frequency: facts and controversies of TRPs in the lower urinary tract. Nat Rev Urol 8: 617–630. [DOI] [PubMed] [Google Scholar]
- Song X, Chen B, Zagorodnyuk V, Lynn P, Blackshaw L, Grundy D et al. (2009). Identification of medium/high threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 137: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. doi:10.1093/nar/gkv1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong RC, Aarsman CJ, van Oirschot JF, van Asbeck BS (1997). Dimethylthiourea protects rats against gram‐negative sepsis and decreases tumor necrosis factor and nuclear factor κB activity. Chest 116: 1365–1368. [DOI] [PubMed] [Google Scholar]
- Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery B, Xavier M et al. (2004). Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol 172: 1175–1178. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S (2006). Hydrogene peroxide: a signalling messenger. Antioxid Redox Signal 8: 243–270. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR et al. (2003). ANKTM1, a TRP‐like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829. [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson H, Hedlund P, Andersson D, Jordt SE, Bevan S et al. (2008). Distribution and function of the hydrogen sulfide–sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–400. [DOI] [PubMed] [Google Scholar]
- Swindle EJ, Hunt JA, Coleman JW (2002). A comparison of reactive oxygen species generation by rat peritoneal macrophages and mast cells using the highly sensitive real‐time chemiluminescent probe pholasin: inhibition of antigen‐induced mast cell degranulation by macrophage‐derived hydrogen peroxide. J Immunol 169: 5866–5873. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD (1994). Activation of cardiac vagal afferents by oxygen‐derived free radicals in rats. Circ Res 74: 895–903. [DOI] [PubMed] [Google Scholar]
- Xu L, Gebhart GF (2008). Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y et al. (2006). Nitric oxide activates TRP channels by cysteine S‐nitrosylation. Nat Chem Biol 2: 596–607. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Chien CT, Lai YJ, Lai MK, Chen CF, Levin RM et al. (2004). Hypoxia preconditioning attenuates bladder overdistension‐induced oxidative injury by up‐regulation of Bcl‐2 in the rat. J Physiol 554: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V, Brookes S, Spencer N (2010). Structure‐functional relationship of sensory endings in the gut and bladder. Auton Neurosci 153: 3–11. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk V, Brookes S, Spencer N, Gregory S (2009). Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J Physiol 587: 3523–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V, Gibbins I, Costa M, Brookes S, Gregory S (2007). Properties of major classes of bladder mechanoreceptors of the guinea pig in vitro. J Physiol 585: 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J (2013). Molecular mechanism of TRP channels. Compr Physiol 3: 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]


