Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy with poor prognosis. Despite improvements in its diagnosis and therapy, the prognosis for ICC patients remains poor. An improved understanding of ICC pathogenesis and consequential identification of novel therapeutic targets would improve the prognosis of ICC patients. MicroRNAs (miRNAs) are a class of highly conserved, endogenous, small non‐coding RNA molecules of 18–23 nucleotides in length, which regulate gene expression through complementary base‐pairing with target messenger RNAs and subsequent gene silencing. Several studies have shown deregulated expression of miRNAs in ICC cell lines and tissues, in which these miRNAs play important roles in ICC apoptosis, cell proliferation, invasion, migration and metastasis. In this review, we illustrate the potential role of miRNA in the pathogenesis of ICC and explore the possibilities of using miRNAs as prognostic and diagnostic markers, as well as therapeutic targets in ICC.
Keywords: Intrahepatic cholangiocarcinoma, microRNAs, proliferation, apoptosis, metastasis, prognosis
Introduction
Intrahepatic cholangiocarcinoma (ICC) originating from cholangiocytes is the second most common primary tumour of the liver 1, 2, 3, 4. It comprises approximately 5–10% of liver cancers, and both its worldwide incidence and mortality rate have been increasing over the past three decades 5, 6, 7. Surgical resection is still considered to be the only potential curative treatment. However, the prognosis of ICC patients is poor with 5‐year survival of only 25–35% in most studies 8, 9, 10. As there is currently no molecular marker for its early diagnosis, elucidating the molecular pathogenesis of ICC may be crucial for identifying new molecular markers for early diagnosis and ultimately improving the prognosis of ICC patients 11, 12, 13, 14, 15.
MicroRNAs (miRNAs) belong to a new class of small non‐coding, endogenous RNAs comprising 18–23 nucleotides that negatively regulate gene expression through inducing mRNA degradation or repressing translation by annealing with the complementary sites in 3′‐untranslated regions (3′‐UTRs) of target mRNAs 16, 17, 18, 19. Increasing evidence has suggested that miRNAs play crucial roles in many biological processes, such as development, cell proliferation, invasion, migration and differentiation 20, 21, 22, 23, 24. Mutations of miRNA‐encoding genes or aberrant expression of miRNAs have been well described in various tumours, including gastric, breast and lung cancers, as well as glioblastoma and melanoma 25, 26, 27. However, the expression and functional roles of miRNAs in the development of ICC remain elusive. In this review, we focus on recent studies related to miRNAs involved in ICC development and discuss the potential use of miRNAs as prognostic biomarkers and treatment strategies for ICC.
Biogenesis of miRNA
Previous data showed that miRNA synthesis and maturation consists of a stepwise process that is compartmentalized between the nucleus and the cytoplasm 28, 29, 30. The pri‐miRNA (primary miRNA transcript) is transcribed by RNA polymerase II in the nucleus, resulting in a transcript with a stem‐loop structure of about 70 nucleotides, a 5′ 7‐methylguanylate cap and a 3′‐polyadenylated tail 30, 31, 32. A ribonucleoprotein RNase III enzyme, Drosha, and its cofactor DiGeorge syndrome critical region 8 (DGCR8) mediate the release of the stem‐loop intermediate known as the pre‐miRNA (precursor miRNA) 33, 34, 35. The pre‐miRNA is then shuttled from the nucleus to the cytoplasm via exportin‐5 36, 37, 38. The terminal loop is cleaved from the pre‐miRNA by Dicer (another ribonucleoprotein RNase III enzyme) in the cytoplasm 39, 40, 41.
miRNAs in intrahepatic cholangiocarcinoma
Deregulated miRNAs in ICC were listed in Table 1. The first report on miRNA expression profiling in human ICC was performed by Chen et al. using 27 ICC tissues, 10 normal cholangiocyte samples and 8 normal liver tissues 42. miRNA profiling revealed 18 and 20 significantly up‐regulated and down‐regulated miRNAs, respectively, in ICC tissues compared with the normal samples. Furthermore, they compared the miRNA expression between cholangiocytes and normal liver tissues to identify some tissue‐specific miRNAs and revealed 21 miRNAs differentially expressed between these two tissue types.
Table 1.
MiRNA expression profiles in intrahepatic cholangiocarcinoma (ICC)
| Num | Method | Sample | Up‐regulated | Down‐regulated | Reference |
|---|---|---|---|---|---|
| 1 | Microarray | Primary ICC |
mir‐21 mir‐142‐3p mir‐25 mir‐15a mir‐193 mir‐17‐5p mir‐374 mir‐106a mir‐224 mir‐130b mir‐19a mir‐331 mir‐324‐5p mir‐20 mir‐17‐3p mir‐223 mir‐15b mir‐103 |
mir‐98 mir‐204 mir‐338 mir‐198 mir‐302d mir‐328 mir‐337 mir‐302b mir‐184 mir‐320 mir‐371 mir‐185mir‐222 mir‐214 mir‐373 mir‐145 miR‐200c let‐7a let‐7b mir‐197 |
42 |
| 2 | Microarray | ICC cell lines (HuCCT1 and MEC) |
miR‐22 miR‐125a miR‐127 miR‐199a miR‐214 miR‐376a miR‐199a* miR‐424 |
43 | |
| 3 | Microarray | ICC tissues |
miR‐660 miR‐425 miR‐93 miR‐494 |
miR‐150 miR‐638 miR‐4459 miR‐4530 miR‐378c |
46 |
| 4 | Microarray | ICC tissues |
miR‐566 miR‐423‐5p miR‐612 miR‐765 miR‐625‐3p miR‐491‐5p miR‐188‐5p miR‐92b‐5p miR‐675‐5p miR‐331‐3p |
miR‐141‐3p miR‐497‐5p miR‐29a‐3p let‐7a‐5p miR‐19b‐3p miR‐103a‐3p miR‐130a‐3p let‐7d‐5p miR‐100‐5p miR‐26b‐5p let‐7e‐5p miR‐24‐3p miR‐101‐3p let‐7f‐5p miR‐99a‐5p miR‐338‐3p miR‐29c‐3p miR‐26a‐5p miR‐451a miR‐143‐3p |
47 |
| 5 | Microarray | ICC tissues |
miR‐141‐3p miR‐141‐5p miR‐200c‐3p miR‐577 miR‐200b‐5p miR‐200a‐5p miR‐135b‐5p miR‐429 miR‐200a‐3p miR‐196a‐1‐5p |
miR‐483‐5p miR‐122‐5p miR‐548aq‐3p miR‐422 miR‐483‐3p miR‐675‐3p miR‐383 miR‐548aq‐3p miR‐885‐5p miR‐1275 |
48 |
Further study on miRNAs expression profiling identified a number of significantly deregulated miRNAs in two ICC cell lines (HuCCT1 and MEC) as compared with a normal intrahepatic biliary epithelial cell line (HIBEpiC) 43. The investigators found 27 miRNAs that were expressed exclusively or predominantly in each cell line, and miR‐22, miR‐125a, miR‐127, miR‐199a, miR‐199a*, miR‐214, miR‐376 and miR‐424 were down‐regulated in the ICC cell lines compared with HIBEpiC using real‐time PCR.
Oishi et al. 44 used the NanoStringn Counter microRNA expression assay platform to examine gene expression profiles of 23 ICC samples with validation in an independent cohort of 68 ICC cases. Unsupervised clustering analysis based on the expression of all 700 human mature miRNAs revealed that ICCs could be divided into two main clusters, namely hepatic stem cell‐like ICC and mature hepatocyte‐like ICC, which could be differentiated by 23 miRNAs.
Plieskatt et al. 45 studied miRNA expression profiling in relation to histologic grades and subtypes of ICC (well‐differentiated, moderately differentiated and papillary ICC) by microarray. They found that each histologic grade and subtype of ICC displayed a distinct miRNA profile, without common deregulated miRNA. Moderately differentiated ICC showed the greatest miRNA deregulation in quantity and magnitude, followed by the papillary subtype, and then the well‐differentiated ICC. Moreover, when ICC tumour tissues were compared with adjacent non‐tumour tissue, similar miRNA dysregulation profiles were observed.
Wang et al. 46 profiled miRNA expression in three pairs of ICC tissues and peritumoral normal tissues using the microarray platform. They found 10 deregulated miRNAs in the ICC tissues, in which the most significantly down‐regulated miRNAs were miR‐150, miR‐638, miR‐4459, miR‐4530 and miR‐378c, while the overexpressed miRNAs involved miR‐660, miR‐425, miR‐93 and miR‐494.
Karakatsanis et al. 47 demonstrated that miR‐21, miR‐31 and miR‐223 were overexpressed in 21 patients with primary ICC, whereas miR‐122, miR‐145, miR‐200c, miR‐221 and miR‐222 were down‐regulated. However, miR‐21, miR‐31 and miR‐223 did not show correlation with clinicopathological features.
Recently, Zhang et al. 48 conducted miRNA expression profiling in 63 human ICCs and nine normal intrahepatic bile duct samples (NIBD) using a custom microarray containing 1094 probes. Expression analysis showed 158 differentially expressed miRNAs between ICC and NIBD, with 77 up‐regulated and 81 down‐regulated miRNAs. From the 158 differentially expressed miRNAs, a 30‐miRNA signature consisted of 10 up‐regulated and 20 down‐regulated miRNAs in ICC was established for distinguishing ICC from NIBD with 100% accuracy. A separate 3‐miRNA signature was identified for predicting prognosis in ICC. Based on the 3‐miRNA signature, a formula was constructed to compute a risk score for each patient. Patients with high‐risk scores had significantly lower overall survival and disease‐free survival than those with low risk scores. Moreover, they showed that three miRNAs (miR‐675‐5p, miR‐652‐3p and miR‐338‐3p) were significantly associated with overall survival. Of the three miRNAs, miR‐675‐5p was up‐regulated and negatively associated with overall survival, while the other two (miR‐652‐3p and miR‐338‐3p) were down‐regulated and positively associated with overall survival.
Plieskatt et al. 49 comprehensively profiled miRNA expression in ICC tumour tissues using small RNA sequencing and validated the expression profiles using quantitative PCR on matched plasma samples. Distinct miRNA profiles were associated with increasing histological differentiation of ICC. They observed that histologically normal tissues adjacent to ICC tumours displayed miRNA expression profiles more similar to tumours than liver tissues from healthy donors. In plasma samples, an 8‐miRNA signature was associated with ICC, regardless of the degree of histological differentiation of its matched tissue, forming the basis of a circulating miRNA‐based biomarker for ICC.
A number of aberrantly expressed miRNAs have been reported to function as tumour suppressors or oncogenes in ICC (Table 2) via derepressing or suppressing important signalling mediators along specific signalling pathways pertinent to cancer development.
Table 2.
Functional characterization of the deregulated miRNAs in intrahepatic cholangiocarcinoma (ICC)
| Name | Up‐ or down‐regulation | Target gene | Role | Reference |
|---|---|---|---|---|
| miR‐675‐5p | Up | Oncogene | 47 | |
| miR‐652‐3p | Down | Tumour suppressor | 47 | |
| miR‐338‐3p | Down | Tumour suppressor | 47 | |
| miR‐31 | Up | RASA1 | Oncogene | 49 |
| miR‐150 | Up | Oncogene | 46 | |
| miR‐21 | Up | PTPN14, PTEN | Oncogene | 50 |
| miR‐204 | Down | Mcl‐1, Bcl‐2 | Tumour suppressor | 42 |
| miR‐320 | Down | Mcl‐1, Bcl‐2 | Tumour suppressor | 42 |
| miR‐21 | Up | Oncogene | 51 | |
| miR‐31, miR‐223 | Up | Oncogene | 51 | |
| miR‐122, miR‐145, miR‐200c, miR‐221, miR‐222 | Down | Tumour suppressor | 51 | |
| miR‐214 | Down | Twist | Tumour suppressor | 52 |
| miR‐200c | Down | NCAM1 | Tumour suppressor | 44 |
| miR‐124 | Down | SMYD3 | Tumour suppressor | 53 |
| miR‐376c | Down | GRB2 | Tumour suppressor | 54 |
| miR‐204 | Down | slug | Tumour suppressor | 55 |
| miR‐605 | Down | PSMD10 | Tumour suppressor | 56 |
Up‐regulated miRNAs in intrahepatic cholangiocarcinoma
Hu et al. 50 reported that the expression of miR‐31 was significantly up‐regulated in ICC tissues and the human ICC cell line HCCC‐9810, when compared with normal adjacent tissues. Down‐regulation of miR‐31 significantly inhibited cell proliferation and promoted apoptosis in HCCC‐9810 cells. RAS p21 GTPase activating protein 1 (RASA1) was identified as a direct target of miR‐31, and there was an inverse correlation between miR‐31 and RASA1 expression during ICC development.
Wang et al. 46 showed that miR‐150 levels were significantly higher in the ICC plasma samples as compared with the matched control plasma samples. The diagnostic value of plasma miR‐150 was also analysed. For differentiating ICC from age‐ and gender‐matched normal controls, receiver operator curve (ROC) analysis of plasma miR‐150 revealed the area under the curve (AUC) of 0.764 with a sensitivity of 80.6% and a specificity of 58.1%.
Wang et al. 51 showed that miR‐21 plays an important role in the regulation of cell proliferation and tumour growth in ICC, in which miR‐21 could also serve as a diagnostic and prognostic marker as well as a potential therapeutic target. They found that miR‐21 levels were significantly higher in serum of ICC patients. Inhibition of miR‐21 suppressed ICC cell proliferation, induced cell cycle arrest and apoptosis in vitro and impaired tumour growth in vivo. Furthermore, PTPN14 and PTEN were identified as direct and functional targets of miR‐21. High expression levels of miR‐21 were closely related to adverse clinical features, diminished survival and poor prognosis in ICC patients.
Down‐regulated miRNAs in intrahepatic cholangiocarcinoma
The biological functions and/or prognostic significance of two down‐regulated miRNAs, namely miR‐204 and miR‐320, have been studied in details 42. Restored expression of miR‐320 and miR‐204 negatively regulated Mcl‐1 and Bcl‐2 expression, respectively, and facilitated chemotherapeutics‐triggered apoptosis.
Li et al. 52 found that miR‐214 levels were significantly lower in ICC tissues compared with normal tissues. Moreover, miR‐214 expression was remarkably decreased in primary tumours that subsequently metastasized compared with non‐metastatic ICC. Inhibition of miR‐214 promoted metastasis of human ICC cells and increased the transcript levels of the epithelial–mesenchymal transition‐associated gene Twist, and decreased E‐cadherin levels by directly targeting the Twist gene. Oishi et al. 44 also found that the expression level of miR‐200c was lower in ICC and was associated with overall survival and disease‐free survival in ICC cases. Transient transfection of miR‐200c oligos into HuH28 cells induced reversal of epithelial‐mesenchymal transition (EMT) from a mesenchymal‐like to a cobblestone‐like morphology with suppression of genes that mediate EMT. Functionally, miR‐200c overexpression suppressed cell migration and invasion. Moreover, NCAM1 was identified as a direct target of miR‐200c.
Hepatitis C virus core protein (HCVc) plays an important role in the development of ICC. Zeng et al. 53 found that miR‐124 was down‐regulated in HCV‐ICC and the induction of DNMT1 by HCVc mediated the suppression of miR‐124. Ectopic expression of miR‐124 suppressed cell migration and invasion in vitro and reduced the protein levels of SMYD3 and downstream target genes (c‐Myc and matrix metallopeptidase 9). Knockdown of SMYD3 inhibited cell migration and invasion resembling that of miR‐124 overexpression.
Iwaki et al. 54 demonstrated that miR‐376c was down‐regulated in ICC cell line (HuCCT1) compared with normal intrahepatic biliary epithelial cells (HIBEpiC). Growth factor receptor‐bound protein 2 (GRB2) was identified as a direct target of miR‐376c. miR‐376c overexpression reduced epidermal growth factor (EGF)‐dependent cell migration in HuCCT1 cells. Interleukin 1β and matrix metallopeptidase 9 were possible participants in EGF‐dependent migration of HuCCT1 cells as determined by microarray and subsequent pathway analysis. Bisulfite sequencing showed higher methylation levels of CpG sites upstream of the miR‐376c‐encoding gene in HuCCT1 relative to HIBEpiC cells. Combined treatment with the DNA‐demethylating agent 5‐aza‐2′‐deoxycytidine and the histone deacetylase inhibitor trichostatin A significantly up‐regulated the expression of miR‐376c in HuCCT1 cells.
Qiu et al. 55 reported that the expression of miR‐204 was frequently down‐regulated in ICC tissues and the low‐level expression of miR‐204 was significantly associated with lymph node metastasis. miR‐204 overexpression suppressed ICC cell migration and invasion, as well as EMT by regulating slug expression.
Li et al. 56 showed that the expression of miR‐605 was expressed at low levels and inversely correlated with the expression of proteasome 26S subunit non‐ATPase 10 (PSMD10) in ICC. Overexpression of miR‐605 inhibited ICC cell proliferation and invasion by regulating PSMD10 expression. Restoration of PSMD10 reversed the phenotypic alteration caused by miR‐605 in ICC cells.
Serum miRNAs in intrahepatic cholangiocarcinoma
Bernuzzi et al.57 performed miRNA expression profiling in 90 serum samples [30 primary sclerosing cholangitis (PSC), 30 cholangiocarcinoma and 30 control cases] to found disease‐associated miRNAs (discovery phase). They found that 33 in cholangiocarcinoma, 21 miRNAs differentially expressed in PSC and 26 in both in comparison to control cases and 24 miRNAs differentially expressed between cholangiocarcinoma and PSC. Furthermore, they demonstrated that miR‐194 and miR‐483‐5p showed deregulated expression in cholangiocarcinoma compared with controls.
Concluding remarks and future perspectives
The dismal prognosis and aggressive progression associated with ICC have led researchers and clinicians to explore new avenues of potential treatment for ICC patients 58, 59, 60. Increasing evidence demonstrated that miRNAs are involved in important biological processes, including cell proliferation, differentiation, migration, invasion and apoptosis 61, 62, 63, 64, 65. As illustrated in Figure 1, altered expression of miRNAs has significant effects on intracellular signalling network and thereby promoting malignant phenotypes in the development and progression of ICC. However, we are still facing many difficulties in miRNA research. In particular, miRNA‐based therapy is not currently available in clinic settings. Nevertheless, with more research efforts to put forth the development of miRNA‐based therapeutics and delivery system, it is hopeful that miRNAs may be used to target specific traits of ICC.
Figure 1.
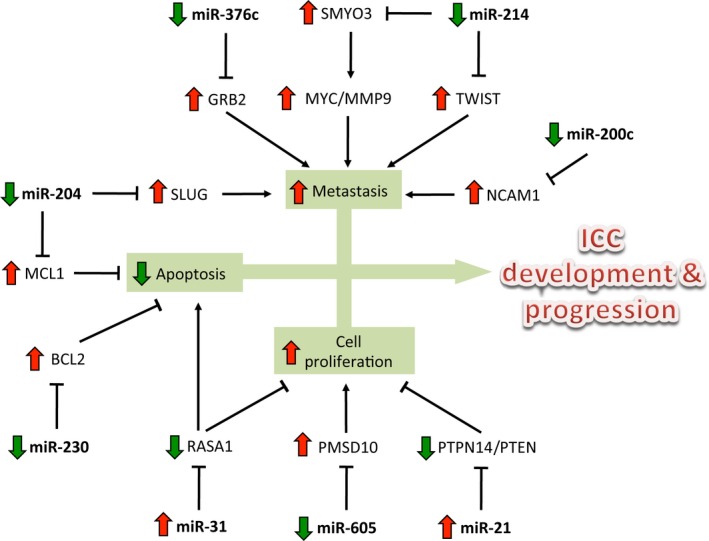
Regulation of tumour cell proliferation, apoptosis and metastasis by miRNAs in the development and progression of intrahepatic cholangiocarcinoma (ICC). miR‐21 and miR‐31 were up‐regulated in the ICC and promote the ICC cell proliferation and invasion and repress the ICC cell apoptosis; miR‐376c, miR‐214, miR‐204, miR‐200c, miR‐230 and miR‐605 were down‐regulated in the ICC and inhibit the ICC cell proliferation and invasion and promote the ICC cell apoptosis.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by grant from the National Natural Science Foundation of China (NSFC) (grant number: 81401847).
References
- 1. Altekruse SF, Petrick JL, Rolin AI, et al Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLoS ONE. 2015; 10: e0120574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo S, Liu HD, Liu YF, et al Hepatoma‐derived growth factor: a novel prognostic biomarker in intrahepatic cholangiocarcinoma. Tumour Biol. 2015; 36: 353–64. [DOI] [PubMed] [Google Scholar]
- 3. Tian X, Wang Q, Li Y, et al The expression of S100A4 protein in human intrahepatic cholangiocarcinoma: clinicopathologic significance and prognostic value. Pathol Oncol Res. 2015; 21: 195–201. [DOI] [PubMed] [Google Scholar]
- 4. Krige JE, Kahn D. Determining chances for survival in intrahepatic cholangiocarcinoma. JAMA Surg. 2015; 150: 546. [DOI] [PubMed] [Google Scholar]
- 5. Shimada M. Highlights of topic “Intrahepatic cholangiocarcinoma: recent advancements in pathogenesis, diagnosis and treatment”. J Hepatobiliary Pancreat Sci. 2015; 22: 91–3. [DOI] [PubMed] [Google Scholar]
- 6. Haga H, Patel T. Molecular diagnosis of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2015; 22: 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen JB. Molecular pathogenesis of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2015; 22: 101–13. [DOI] [PubMed] [Google Scholar]
- 8. Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015; 22: 94–100. [DOI] [PubMed] [Google Scholar]
- 9. Hwang S, Lee YJ, Song GW, et al Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single‐center experience of 659 cases. J Gastrointest Surg. 2015; 19: 1291–304. [DOI] [PubMed] [Google Scholar]
- 10. Tabrizian P, Jibara G, Hechtman JF, et al Outcomes following resection of intrahepatic cholangiocarcinoma. HPB (Oxford). 2015; 17: 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh CN, Hsieh FJ, Chiang KC, et al Clinical effect of a positive surgical margin after hepatectomy on survival of patients with intrahepatic cholangiocarcinoma. Drug Des Devel Ther. 2015; 9: 163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spolverato G, Yakoob MY, Kim Y, et al Impact of complications on long‐term survival after resection of intrahepatic cholangiocarcinoma. Cancer. 2015; 121: 2730–9. [DOI] [PubMed] [Google Scholar]
- 13. Miura JT, Johnston FM, Tsai S, et al Chemotherapy for surgically resected intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015; 22: 3716–23. [DOI] [PubMed] [Google Scholar]
- 14. Kim Y, Spolverato G, Amini N, et al Surgical management of intrahepatic cholangiocarcinoma: defining an optimal prognostic lymph node stratification schema. Ann Surg Oncol. 2015; 22: 2772–8. [DOI] [PubMed] [Google Scholar]
- 15. Adachi T, Eguchi S, Beppu T, et al Prognostic impact of preoperative lymph node enlargement in intrahepatic cholangiocarcinoma: a multi‐institutional study by the Kyushu Study Group of Liver Surgery. Ann Surg Oncol. 2015; 22: 2269–78. [DOI] [PubMed] [Google Scholar]
- 16. Li Z, Yu X, Shen J, et al MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015; 48: 278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Yu X, Shen J, et al MicroRNA expression and its clinical implications in Ewing's sarcoma. Cell Prolif. 2015; 48: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015; 48: 271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu LL, Zhao X, Xu HL, et al Identification of microRNA‐regulated autophagic pathways in plant lectin‐induced cancer cell death. Cell Prolif. 2012; 45: 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review). Int J Mol Med. 2014; 34: 923–33. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Yu X, Wang Y, et al By downregulating TIAM1 expression, microRNA‐329 suppresses gastric cancer invasion and growth. Oncotarget. 2014; 6: 17559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu X, Li Z, Shen J, et al MicroRNA‐10b promotes nucleus pulposus cell proliferation through RhoC‐Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS ONE. 2013; 8: e83080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Li M, Yu M, Liu C, et al miR‐34c works downstream of p53 leading to dairy goat male germline stem‐cell (mGSCs) apoptosis. Cell Prolif. 2013; 46: 223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo X, Dong Z, Chen Y, et al Enrichment of ovarian cancer stem‐like cells is associated with epithelial to mesenchymal transition through an miRNA‐activated AKT pathway. Cell Prolif. 2013; 46: 436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Lei H, Luo M, et al DNA methylation downregulated mir‐10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015; 18: 43–54. [DOI] [PubMed] [Google Scholar]
- 26. Li Z, Yu X, Shen J, et al MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015; 6: 4562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohdaira H, Sekiguchi M, Miyata K, et al MicroRNA‐494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif. 2012; 45: 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu WK, Lee CW, Cho CH, et al MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010; 29: 5761–71. [DOI] [PubMed] [Google Scholar]
- 29. Tivnan A, McDonald KL. Current progress for the use of miRNAs in glioblastoma treatment. Mol Neurobiol. 2013; 48: 757–68. [DOI] [PubMed] [Google Scholar]
- 30. Lopez‐Ramirez MA, Nicoli S. Role of miRNAs and epigenetics in neural stem cell fate determination. Epigenetics. 2014; 9: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR‐103/107 regulates human metabolic pathways. Mol Genet Metab. 2007; 91: 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Githinji J, McLaughlin B, et al Role of miRNAs in neuronal differentiation from human embryonic stem cell‐derived neural stem cells. Stem Cell Rev. 2012; 8: 1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Z, Wu J, Yang C, et al DiGeorge syndrome critical region 8 (DGCR8) protein‐mediated microRNA biogenesis is essential for vascular smooth muscle cell development in mice. J Biol Chem. 2012; 287: 19018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan P, Chen Z, Tian P, et al miRNA biogenesis enzyme Drosha is required for vascular smooth muscle cell survival. PLoS ONE. 2013; 8: e60888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sohn EJ, Park J, Kang SI, et al Accumulation of pre‐let‐7 g and downregulation of mature let‐7 g with the depletion of EWS. Biochem Biophys Res Commun. 2012; 426: 89–93. [DOI] [PubMed] [Google Scholar]
- 36. Okubo M, Tahara T, Shibata T, et al Association between common genetic variants in pre‐microRNAs and the clinicopathological characteristics and survival of gastric cancer patients. Exp Ther Med. 2010; 1: 1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li SC, Liao YL, Ho MR, et al miRNA arm selection and isomiR distribution in gastric cancer. BMC Genom. 2012; 13(Suppl 1): S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kunej T, Godnic I, Ferdin J, et al Epigenetic regulation of microRNAs in cancer: an integrated review of literature. Mutat Res. 2011; 717: 77–84. [DOI] [PubMed] [Google Scholar]
- 39. Vaksman O, Hetland TE, Trope CG, et al Argonaute, Dicer, and Drosha are up‐regulated along tumor progression in serous ovarian carcinoma. Hum Pathol. 2012; 43: 2062–9. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Z, O'Rourke JR, McManus MT, et al The microRNA‐processing enzyme Dicer is dispensable for somite segmentation but essential for limb bud positioning. Dev Biol. 2011; 351: 254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saurat N, Andersson T, Vasistha NA, et al Dicer is required for neural stem cell multipotency and lineage progression during cerebral cortex development. Neural Dev. 2013; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen L, Yan HX, Yang W, et al The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009; 50: 358–69. [DOI] [PubMed] [Google Scholar]
- 43. Kawahigashi Y, Mishima T, Mizuguchi Y, et al MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell‐specific microRNAs. J Nippon Med Sch. 2009; 76: 188–97. [DOI] [PubMed] [Google Scholar]
- 44. Oishi N, Kumar MR, Roessler S, et al Transcriptomic profiling reveals hepatic stem‐like gene signatures and interplay of miR‐200c and epithelial‐mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012; 56: 1792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Plieskatt JL, Rinaldi G, Feng Y, et al Distinct miRNA signatures associate with subtypes of cholangiocarcinoma from infection with the tumourigenic liver fluke Opisthorchis viverrini . J Hepatol. 2014; 61: 850–8. [DOI] [PubMed] [Google Scholar]
- 46. Wang S, Yin J, Li T, et al Upregulated circulating miR‐150 is associated with the risk of intrahepatic cholangiocarcinoma. Oncol Rep. 2015; 33: 819–25. [DOI] [PubMed] [Google Scholar]
- 47. Karakatsanis A, Papaconstantinou I, Gazouli M, et al Expression of microRNAs, miR‐21, miR‐31, miR‐122, miR‐145, miR‐146a, miR‐200c, miR‐221, miR‐222, and miR‐223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013; 52: 297–303. [DOI] [PubMed] [Google Scholar]
- 48. Zhang MY, Li SH, Huang GL, et al Identification of a novel microRNA signature associated with intrahepatic cholangiocarcinoma (ICC) patient prognosis. BMC Cancer. 2015; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plieskatt J, Rinaldi G, Feng Y, et al A microRNA profile associated with Opisthorchis viverrini‐induced cholangiocarcinoma in tissue and plasma. BMC Cancer. 2015; 15: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu C, Huang F, Deng G, et al miR‐31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Therap Med. 2013; 6: 1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang LJ, He CC, Sui X, et al MiR‐21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget. 2015; 6: 5932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li B, Han Q, Zhu Y, et al Down‐regulation of miR‐214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012; 279: 2393–8. [DOI] [PubMed] [Google Scholar]
- 53. Zeng B, Li Z, Chen R, et al Epigenetic regulation of miR‐124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012; 586: 3271–8. [DOI] [PubMed] [Google Scholar]
- 54. Iwaki J, Kikuchi K, Mizuguchi Y, et al MiR‐376c down‐regulation accelerates EGF‐dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS ONE. 2013; 8: e69496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qiu YH, Wei YP, Shen NJ, et al miR‐204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell Physiol Biochem. 2013; 32: 1331–41. [DOI] [PubMed] [Google Scholar]
- 56. Li J, Tian F, Li D, et al MiR‐605 represses PSMD10/Gankyrin and inhibits intrahepatic cholangiocarcinoma cell progression. FEBS Lett. 2014; 588: 3491–500. [DOI] [PubMed] [Google Scholar]
- 57. Bernuzzi F, Marabita F, Lleo A, et al Serum microRNAs as novel biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Clin Exp Immunol. 2016; 185: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrone CR, Ting DT, Shahid M, et al The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA‐enhanced albumin RNA in situ hybridization technology. Ann Surg Oncol. 2016; 23: 290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dong ZR, Zhang C, Cai JB, et al Role of 5‐hydroxymethylcytosine level in diagnosis and prognosis prediction of intrahepatic cholangiocarcinoma. Tumour Biol. 2015; 36: 2763–71. [DOI] [PubMed] [Google Scholar]
- 60. Sirica AE, Almenara JA, Li C. Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Exp Mol Pathol. 2014; 97: 515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peng HH, Zhang YD, Gong LS, et al Increased expression of microRNA‐335 predicts a favorable prognosis in primary gallbladder carcinoma. Onco Targets Ther. 2013; 6: 1625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu XY, Zhang Z, Liu J, et al MicroRNA‐141 is downregulated in human renal cell carcinoma and regulates cell survival by targeting CDC25B. Onco Targets Ther. 2013; 6: 349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee HK, Finniss S, Cazacu S, et al Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self‐renewal. Oncotarget. 2013; 4: 346–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bier A, Giladi N, Kronfeld N, et al MicroRNA‐137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP‐1. Oncotarget. 2013; 4: 665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tang M, Lin L, Cai H, et al MicroRNA‐145 downregulation associates with advanced tumor progression and poor prognosis in patients suffering osteosarcoma. Onco Targets Ther. 2013; 6: 833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


