Abstract
The annulus‐endplate anchorage system plays a vital role in structurally linking the compliant disc to its adjacent much more rigid vertebrae. Past literature has identified the endplate as a region of weakness, not just in the mature spine but also in the immature spine. The aim of this structural study was to investigate in detail the morphological changes associated with annulus‐endplate integration through different stages of maturity. Ovine lumbar motion segments were collected from two immature age groups: (i) newborn and (ii) spring lamb (roughly 3 months old); these were compared with a third group of previously analysed mature ewe samples (3–5 years). Sections from the posterior region of each motion segment were obtained for microstructural analysis and imaged in their fully hydrated state via differential interference contrast (DIC) optical microscopy. Selected slices were further prepared and imaged via scanning electron microscopy (SEM) to analyse fibril‐level modes of integration. Despite significant changes in endplate morphology, the annular fibre bundles in all three age groups displayed a similar branching mechanism, with the main bundle splitting into several sub‐bundles on entering the cartilaginous endplate. This morphology, previously described in the mature ovine disc, is thought to strengthen significantly annulus‐endplate integration. Its prevalence from an age as young as birth emphasizes the critical role that it plays in the anchorage system. The structure of the branched sub‐bundles and their integration with the surrounding matrix were found to vary with age due to changes in the cartilaginous and vertebral components of the endplate. Microscopically, the sub‐bundles in both immature age groups appeared to fade into the surrounding tissue due to their fibril‐level integration with the cartilaginous endplate tissue, this mechanism being particularly complex in the spring lamb disc. However, in the fully mature disc, the sub‐bundles remained as separate entities throughout the full depth of their anchorage into the cartilaginous endplate. Cell morphology was also found to vary with maturity within the cartilaginous matrix and it is proposed that this relates to endplate development and ossification.
Keywords: annulus‐endplate anchorage, fibril‐level integration, intervertebral disc, maturity, microstructure
Introduction
The healthy intervertebral disc consists of three main regions – the soft and highly hydrated central nucleus, the compliant multi‐layered annulus surrounding it, and the endplates. The latter consist of a partially calcified layer of hyaline cartilage (cartilaginous endplate) into which the annulus and nucleus are anchored. The cartilaginous endplates are, in turn, integrated with the vertebral ends via a layer of cortical bone termed the vertebral endplate. Although it is now known that there is actual structural integration between the nucleus and cartilaginous endplate (Wade et al. 2011, 2012), it is the annulus‐endplate anchorage system that is the dominant source of mechanical strength in the spine, while at the same time providing that critical element of functional flexibility.
Previous studies have identified the endplate or more specifically the junction between the cartilaginous endplate and the ossified tissue of the vertebral body – the osteocartilaginous junction – as an area of predominant weakness in the growing spine (Schmorl & Junghanns, 1971; Keller, 1974; Epstein & Epstein, 1991; Savini et al. 1991; Martínez‐Lage et al. 1998). Several clinical studies have reported on the high incidence of endplate damage in the form of cartilaginous endplate‐bone separation (Takata et al. 1988; Jónsson et al. 1991; Savini et al. 1991; Lee et al. 2000), ruptures within the cartilaginous endplate and underlying growth plate (Aufdermaur, 1974; de Gauzy et al. 2007), and bony avulsions of the vertebral rim (Keller, 1974; Ehni & Schneider, 1988; Takata et al. 1988; Epstein & Epstein, 1991; Beggs & Addison, 1998).
Mechanical studies on human adolescent spines have similarly identified the endplate as a region of high vulnerability under overload conditions. When subjected to axial tension and forced bending, these adolescent spinal columns nearly always rupture within the growth zone of the cartilaginous endplate adjacent to the vertebral body (Aufdermaur, 1974). Likewise, when exposed to excessive compressive loads, a large proportion of adolescent motion segments display cartilaginous endplate fractures and/or the separation of this layer from the vertebral bone (Karlsson et al. 1998). Mechanical experiments on immature animal spines such as adolescent pigs show similar fractures of the endplate and growth zone (Lundin et al. 1998, 2000; Baranto et al. 2004; Brown et al. 2008; Thoreson et al. 2010).
The resistance of the endplate to failure strongly depends on its structural architecture and its integration with the other components of the disc, particularly the annulus, which transmits large tensile forces into the endplate in the outer regions of the disc. The structure of the human disc is known to vary with age, especially during maturation, where the endplate undergoes dramatic changes (Coventry et al. 1945a,b; Peacock, 1952; Coventry, 1969; Schmorl & Junghanns, 1971; Francois & Dhem, 1974; Francois, 1975; Bernick & Cailliet, 1982; Buckwalter, 1995; Urban & Roberts, 1995; Roberts, 2002; Roberts et al. 2006; Paietta et al. 2013). It appears that only a handful of previous studies have commented on the mode of annulus‐endplate anchorage in the maturing disc, and in limited detail (Coventry et al. 1945a,b; Peacock, 1952; Coventry, 1969; Hashizume, 1980; Paietta et al. 2013).
Early light microscopy studies of a 10‐month‐old and a 6‐year‐old human disc by Coventry (1969) and Coventry et al. (1945a,b) have shown, at low magnification, the merging of annular lamellae into the thick cartilage of the endplate. Examination of adolescent and mature samples revealed that with further vertebral development the annular fibres in the outer regions of the disc anchor to the vertebral bone of the developing epiphyseal ring, the latter being eventually fused with the vertebrae (Coventry et al. 1945b). Peacock (1952) similarly observed that microscopically the annular fibres in the outer regions of the disc in a full‐term fetus curve and disappear into the cartilaginous endplate matrix, whereas fibres from the inner annulus turn and merge with the horizontal fibres of the endplate.
Hashizume (1980) provided a more detailed account of the mechanism of structural anchorage. Using both polarized light microscopy and scanning electron microscopy (SEM), he observed the micro‐ and fibril‐level integration of the annulus and endplate tissues in human fetuses. He found no fibrillar interconnections between the annular lamellae and cartilaginous endplate at the 5th embryonic month. However, by the 7th month, cartilaginous endplate fibrils were transversely aligned and merged with fibrils from the annular lamellae. Hashizume described the mode of annulus‐endplate integration at the fibril level in the full‐term fetus as similar to that seen in the adult disc – fibrils from the inner annulus blend with those from the cartilaginous endplate, whereas fibrils from the outer annulus merge with the peripheral cartilaginous cell layer corresponding to the future epiphyseal ring. However, unlike the adult disc this peripheral layer was yet to be ossified in full‐term fetal vertebrae (Hashizume, 1980).
In a more recent study, Paietta et al. (2013) examined the endplate morphology in an infant (56 days old) and an adolescent (17 years) human disc using micro‐level confocal microscopy, and included an analysis of the mineralized component using quantitative back‐scattered SEM and micro‐CT. In both developing spines these investigators observed collagen fibres from the annulus and cartilaginous endplate anchoring directly into a layer of calcified cartilage present between the disc and vertebral bone. The study was, however, unable to identify the mode of integration between the outer annulus and vertebral body due to difficulties in imaging the fibres in this region of the disc (Paietta et al. 2013).
Employing an ovine model and specialised microscopy techniques, Rodrigues et al. (2012, 2015) have shown that in the mature ovine disc, the annular fibre bundles branch into multiple sub‐bundles which penetrate the entire depth of the mostly calcified cartilaginous endplate. These authors proposed that this branching morphology serves to strengthen the anchorage by increasing the interface area over which shear forces are distributed (Rodrigues et al. 2012). More recently, both Liu et al. (2015) and Brown et al. (2016) have demonstrated at the microscopic level a similar mode of anchorage in mature human lumbar discs. Rodrigues et al. (2015) also conducted a fibril‐level structural analysis and provided evidence of short range integration between the branched annular sub‐bundles and the cartilaginous and vertebral endplate fibrils (Rodrigues et al. 2015).
Although the studies of Rodrigues et al. (2012, 2015) have provided significant detail and clarity of this junction in the mature disc, a gap still exists in the current literature concerning how the structure of the annulus‐endplate anchorage system changes with maturity. Therefore, by employing high resolution multi‐scale imaging techniques and immature ovine discs the aim of this study was to investigate in detail the structural changes associated with annulus‐endplate integration through early development and maturity, particularly in the mechanically critical outer‐to‐mid regions of the annulus.
Methods
Immature ovine spines were collected from two newborn lambs and two spring lambs approximately 3 months old. No ethics approval was required as the newborn lambs were retrieved soon after their natural death, and the spring lambs were obtained from a local meat‐processing slaughterhouse. Individual lumbar motion segments were extracted from the spines, chemically fixed in formalin, and then decalcified in formic acid. For all samples, oblique sections were obtained by cryo‐sectioning the posterior region along one of the inclined annular fibre angles (Fig. 1). Sections were then imaged in their fully hydrated state using differential interference contrast (DIC) optical microscopy and compared with a third group of previously examined samples obtained from the lumbar spines of mature ewes (3–5 years old) using identical procedures (Rodrigues et al. 2012, 2015). It should be noted that DIC optical microscopy can image the collagen component only at the level of the fibre, the fibre consisting of aggregates of fibrils sufficiently large to be resolved optically.
Figure 1.
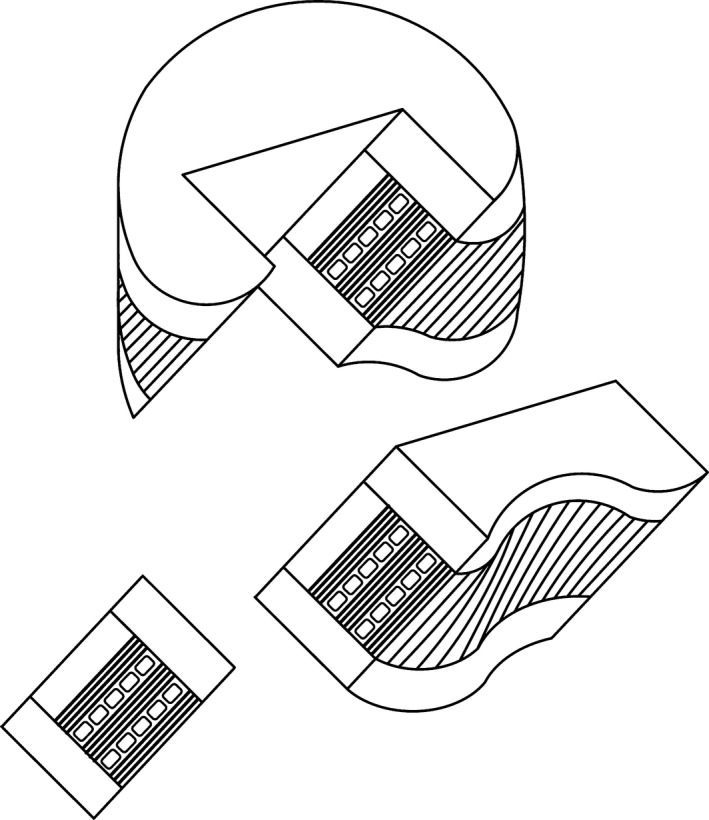
Schematic illustrating the oblique sectioning of a block of tissue from the posterior region of the motion segment. Note that this inclined sectioning plane follows one of the oblique fibre angles in the annulus.
Selected newborn and spring lamb sections were further examined via scanning electron microscopy (SEM) for fibril‐level analysis. The SEM sample preparation process required several additional steps. The sections were first thoroughly rinsed in hexane to remove fat; this was followed by progressive ethanol dehydration, critical point drying, then platinum coating.
A total of five newborn discs and six spring lamb discs were studied via DIC microscopy, with seven slices from the five newborn discs and four slices from three spring lamb discs analysed further at the fibril level using SEM.
Results
Overall comparison
A macro‐level comparison of cross‐sectioned motion segments from each age group (Fig. 2) highlights the significant changes that take place in ovine endplate morphology with increasing maturity. While the newborn endplate comprises a relatively thick peripheral layer of cartilage that extends from the base of the annulus down to the growth plate, in the adolescent spring lamb, much of this cartilage has been replaced by osseous tissue from the developing vertebrae. This leads to both the spring lamb and mature ewe discs containing a thinner layer of cartilage at the outer disc–vertebra junction. Further, the morphology of the growth plate (marked by the asterisk) provides a good indication of the skeletal maturity of the sample, forming a distinct white layer within the vertebral body in immature discs and an osseous‐fused structure in mature discs.
Figure 2.
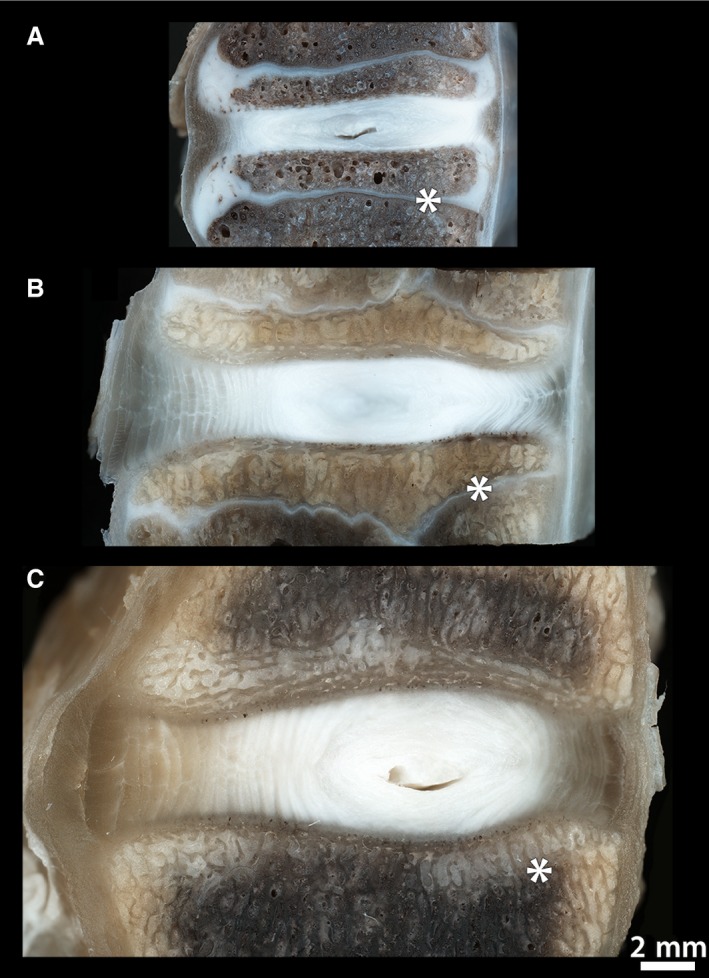
Macroscopic mid‐sagittal views of motion segments from (A) newborn, (B) spring lamb and (C) mature spines, each displaying the anterior annulus on the left and the posterior annulus on the right. Clearly visible in the newborn sample is the thick layer of cartilage (white) that still comprises the peripheral regions of the endplate. By the spring lamb maturity stage most of this cartilage has been replaced by osseous tissue. The growth plate (marked by the asterisk) provides an indication of skeletal maturity, this appearing unfused in the immature spines and fully fused in the mature sample.
The low magnification microstructural images in Fig. 3 show in more detail the differences in the cartilaginous and vertebral (osseous) endplate microstructure with increasing maturity. The newborn endplate (Fig. 3A) is composed predominantly of a thick layer of chondrocyte‐rich cartilage, with masses of porous osseous tissue forming in the centre of the vertebral body beneath the nucleus and inner annulus. This results in the thickness of the cartilaginous endplate visibly decreasing from outer to inner annulus. The cartilaginous layer is also perforated by several holes which represent cross‐sections of the many vascular channels that permeate and nourish the developing disc. At the annulus‐endplate junction, the lamellae appear to merge with the cartilaginous matrix across the thickness of the annulus.
Figure 3.
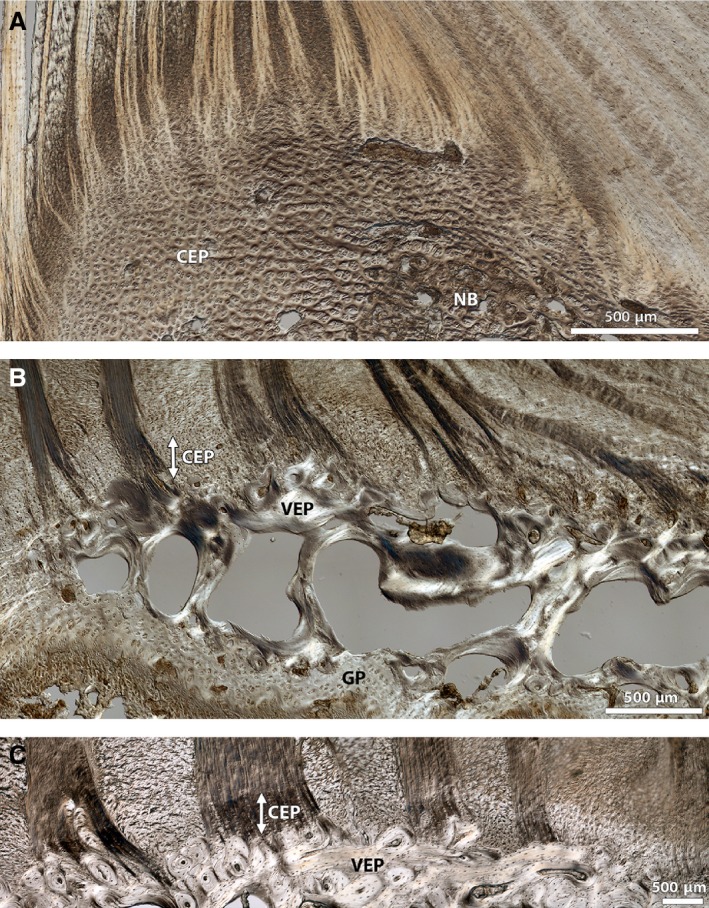
Low magnification DIC images comparing annulus‐endplate microstructure in a typical disc from (A) newborn, (B) spring lamb and (C) mature animal, highlighting the dramatic changes in endplate morphology with increasing maturity. Note that all images show a comparable location in the posterior aspect of the annular‐endplate junction, progressing from outer (left) to inner (right) annulus. The newborn endplate comprises a relatively thick layer of cartilage (CEP) with new bone tissue (NB) forming in the centre of the vertebra. The cartilaginous endplate in the spring lamb is greatly reduced in thickness and a thin continuous layer of dense bone representing the vertebral endplate (VEP) is visible above the trabecular bone and the growth plate (GP). In the mature disc the cartilaginous endplate is mostly calcified (see white arrow) and the vertebral endplate layer is fully developed and thicker. Image (C) is reprinted from Spine Journal, 12(2), Rodrigues SA, Wade KR, Thambyah A, Broom ND, Micromechanics of annulus‐end plate integration in the intervertebral disc, 143–150, Copyright (2012), with permission from Elsevier. DOI: http://dx.doi.org/10.1016/j.spinee.2012.01.003
In contrast, the endplate in the spring lamb disc (Fig. 3B) is much further developed and consists of a considerably reduced thickness of cartilage overlying a thin continuous layer of the dense bone representing the vertebral endplate. This closely resembles the mature endplate (Fig. 3C) where the cartilaginous layer is calcified (almost through its entire depth in the outer‐to‐mid portions of the annulus) and the vertebral endplate is thicker.
Closer inspection of the annular fibre bundles in the outer‐to‐mid regions of the annulus (Fig. 4) reveals a branching morphology where the primary bundle splits into distinct sub‐bundles that gradually integrate with the cartilaginous endplate. This branching mechanism, first reported in earlier studies conducted on mature ovine spine segments (Rodrigues et al. 2012), can now be confirmed as present in all three age groups. However, the morphology of the branched sub‐bundles appears to vary with maturity.
Figure 4.
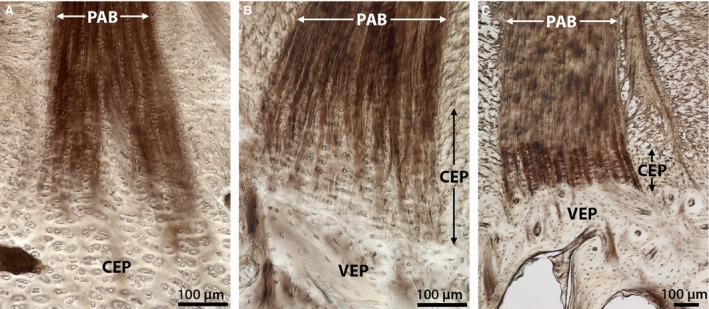
DIC images comparing the mechanism of annular‐endplate anchorage in the outer‐to‐mid annulus within (A) newborn, (B) spring lamb, and (C) mature discs. Despite significant changes in endplate morphology, all three groups illustrate the primary annular bundle (PAB) branching into several sub‐bundles within the cartilaginous endplate (CEP) region. In newborn discs these sub‐bundles seem to disappear into the relatively thick cartilaginous endplate. However, in both spring lamb and mature discs they appear to penetrate almost the entire depth of the cartilaginous endplate down to the vertebral endplate (VEP) surface. Image C is reprinted from The Spine Journal, 12(2), Rodrigues SA, Wade KR, Thambyah A and Broom ND, Micromechanics of annulus‐end plate integration in the intervertebral disc, 143–150, Copyright (2012), with permission from Elsevier. DOI: http://dx.doi.org/10.1016/j.spinee.2012.01.003
Newborn
Figure 5 provides high magnification views of the annular sub‐bundles in the newborn disc at various depths of anchorage and shows more clearly the variation in fibrosity across the anchorage zone. Higher up the anchorage, the sub‐bundles are well‐defined. However, further down, the fibrosity of the sub‐bundles changes from clearly visible fibres (see circled region in Fig. 5B) to a much finer matrix that gradually disappears into the surrounding cartilaginous tissue (Fig. 5C). Additionally, the chondrons within the cartilaginous endplate tissue between the branched sub‐bundles appear to be more or less vertically aligned (Fig. 5B).
Figure 5.
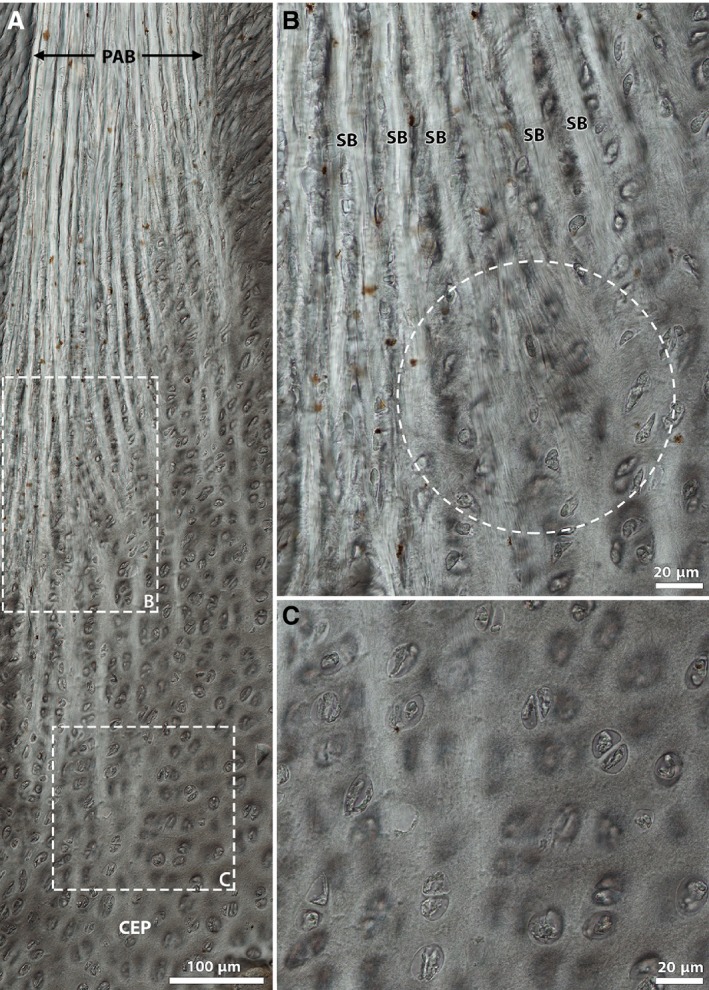
High magnification DIC images of annulus‐cartilaginous endplate integration in the newborn disc. Note that images (B) and (C) are magnified views of boxed regions in image (A), respectively. The circled region in image (B) highlights a visible continuation of fibrosity from the branched sub‐bundles, a feature that is absent further down the anchorage in (C). CEP, cartilaginous endplate; PAB, primary annular bundle; SB, sub‐bundle.
The images in Fig. 6 offer further microstructural insight into the newborn cartilaginous endplate, revealing a region‐to‐region variation in chondron appearance and alignment. Figure 6B captures the progressive enlarging and mass alignment of these cells towards the central region of developing bone under the inner annulus and nucleus. At the growth plate the chondrons appear more spherical than those in the overlying layer immediately above, which are more horizontally aligned (Fig. 6D). This change in cell appearance is accompanied by a variation in the surrounding matrix, as captured by the difference in contrast in Fig. 6D. However, over the remaining bulk of the cartilaginous endplate tissue (for instance, see the chondrons in Fig. 6C), the cells appear undistorted and randomly aligned.
Figure 6.
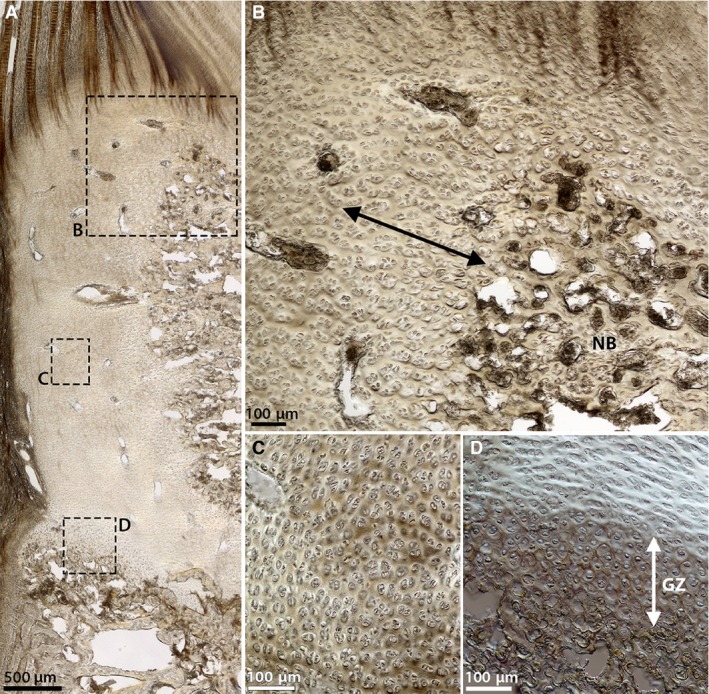
DIC images of the cells within the newborn cartilaginous endplate at (A) low magnification and (B‐D) corresponding high magnification. (B) Region adjacent to the mass of new bone tissue (NB). (C) A peripheral region deeper within the cartilage. (D) The growth plate. Note the changing cell appearance in (B) where the cells grow progressively larger and seem to align near the vertebral bone (see arrow), and in (D) where the cells in the growth zone (GZ) appear more spherical, whereas those immediately above this zone are more horizontally aligned. In (C) and through the rest of the cartilaginous tissue, however, the cells appear undistorted and more randomly arranged.
The precise structural nature of the change in fibrosity over the newborn annulus‐cartilaginous endplate anchorage was verified via fibril‐level structural analysis using scanning electron microscopy (SEM). The series of images in Fig. 7 captures the gradual transition from coarse, strongly aligned fibrils within the branched annular sub‐bundle (Fig. 7B) to a fine multidirectional network of fibrils at the end of the sub‐bundle deeper within the cartilaginous endplate (Fig. 7E). The annular sub‐bundle fibrils appear to branch into even smaller units over this endplate transition. Figure 7C in particular captures a group of sub‐bundle fibrils diverging and blending in with the surrounding fibrils (see white arrows). The images in Fig. 8 provide further examples of fibril‐level interaction between the annular sub‐bundles and the cartilaginous endplate, the boxed regions highlighting an intermingling of the two fibril populations.
Figure 7.
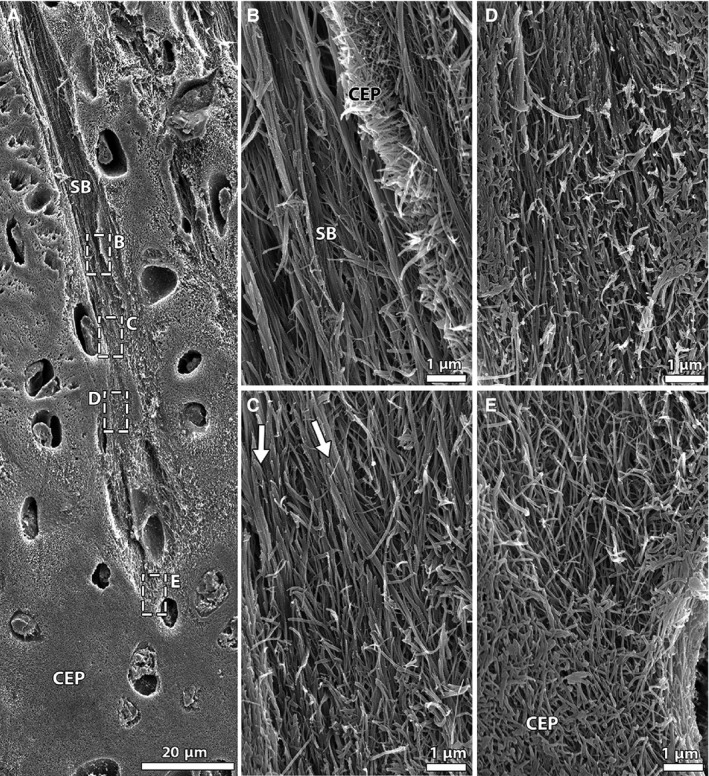
SEM images capturing fibrillar integration between annular sub‐bundles and cartilaginous endplate in the newborn disc. (A) Low magnification; (B‐E) high magnification views of boxed regions in image (A) at various depths of anchorage. Note the change in fibril appearance, from thick aligned fibrils higher up within the sub‐bundle (B) to a fine network of randomly arranged fibrils at its end (E). The arrows in (C) highlight a group of sub‐bundle fibrils splitting into smaller units that merge with the surrounding fibrils. CEP, cartilaginous endplate; SB, sub‐bundle.
Figure 8.
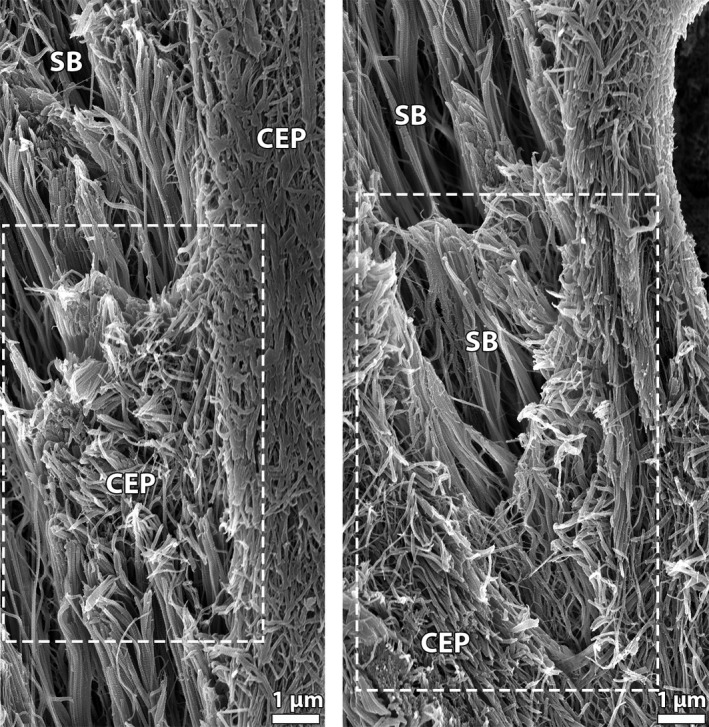
SEM images illustrating interactions between the branched annular sub‐bundles (SB) and the cartilaginous endplate (CEP) in newborn discs and showing an intermingling of the two fibril populations.
Spring lamb
In‐depth examination of the spring lamb discs (Fig. 9) reveals a change in fibre morphology across the anchorage. Higher up their cartilaginous endplate anchorage, the annular fibres display a fine crimp, as seen in Fig. 9A. This crimp progressively diminishes with increasing anchorage depth (see lower portion in Fig. 9A) and the branched annular sub‐bundles lose definition close to the vertebral endplate surface (see boxed area in Fig. 9A). There is also a variation in chondron alignment, with all chondrons higher up the anchorage being predominantly vertically aligned and some chondrons closer to the vertebral endplate surface (particularly those in the region immediately adjacent this boundary) displaying a horizontal alignment (Fig. 9A).
Figure 9.
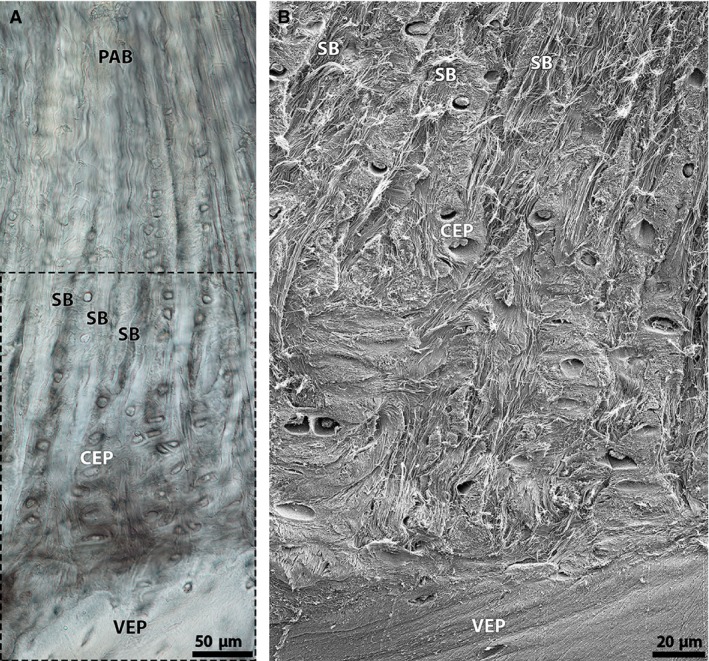
(A) High magnification DIC image of the spring lamb anchorage site, showing a subtle fading of annular sub‐bundle fibres within the cartilaginous endplate further down the anchorage (see boxed region). Note also a general disappearance of fibre crimp with increasing anchorage depth, and the appearance of horizontally aligned chondrons close to the vertebral endplate surface. (B) Corresponding SEM image of the boxed region in (A), showing the branched annular sub‐bundles disappearing into the surrounding cartilaginous endplate matrix close to the vertebral bone interface. Note that some groups of the sub‐bundle fibrils reach the vertebral endplate interface. CEP, cartilaginous endplate; PAB, primary annular bundle; SB, sub‐bundle; VEP, vertebral endplate.
Ultrastructural analysis of the anchorage system close to the vertebral endplate (see boxed region in Fig. 9A) reveals a complex arrangement of fibrils with groups of the branched annular sub‐bundles fibrils disappearing into the surrounding tissue (Fig. 9B). Closer examination shows a complex interweaving and merging of the sub‐bundle fibrils with the cartilaginous endplate network (Fig. 10). The latter appear to be undergoing matrix changes possibly in preparation for ossification – see for example the horizontal re‐alignment of the cartilaginous endplate fibrillar matrix within the boxed region in Fig. 10.
Figure 10.
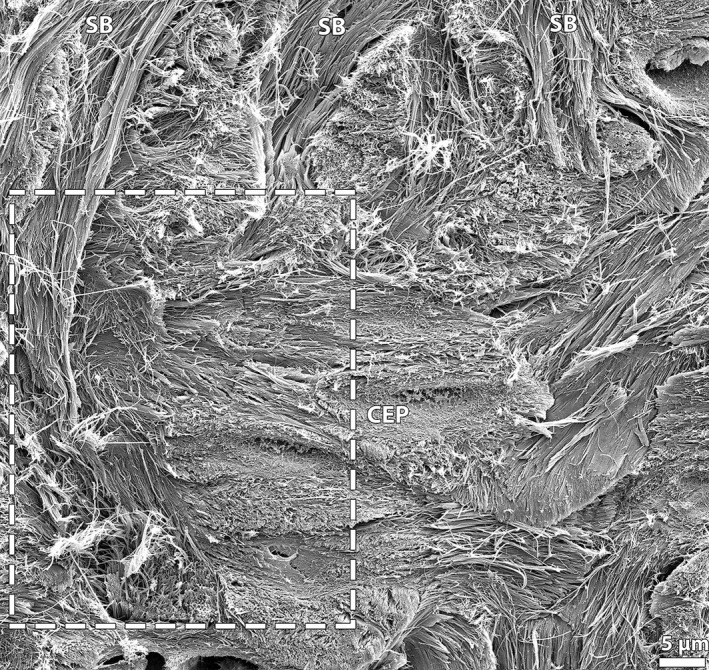
High magnification SEM image capturing structural interactions between the branched annular sub‐bundles (SB) and the cartilaginous endplate (CEP) in the spring lamb disc, and showing a complex interweaving and blending (see boxed region) of the two fibril populations. Also note the apparent horizontal realignment of the cartilaginous endplate fibrillar matrix.
Despite the interweaving of the annulus and cartilaginous endplate tissues in the spring lamb disc, groups of annular sub‐bundle fibrils can be observed at the vertebral bone surface (Figs 9B and 11) – this suggests a full‐depth anchorage of the annular fibres into the cartilaginous endplate. Figure 11 displays, at high magnification, the complex system of fibril‐level integration at this interface. Note that the integration between the cartilaginous endplate and vertebral endplate tissues is particularly subtle, with the somewhat multidirectional cartilaginous endplate fibrils progressively transitioning to the more horizontally aligned fibrils of the vertebral endplate (Figs 11 and 12).
Figure 11.
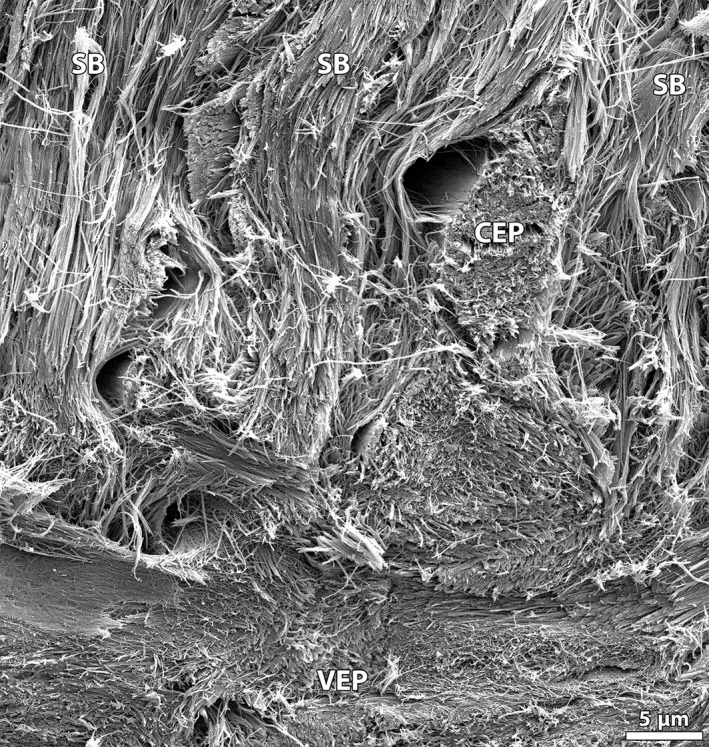
High magnification SEM image of annular sub‐bundles at the vertebral bone junction in the spring lamb disc, showing a complex integration of the various fibril groups. Note in particular the subtle transition of the multidirectional cartilaginous endplate fibrillar network to the more horizontally aligned bone fibrils. CEP, cartilaginous endplate; SB, sub‐bundle;VEP, vertebral endplate.
Figure 12.
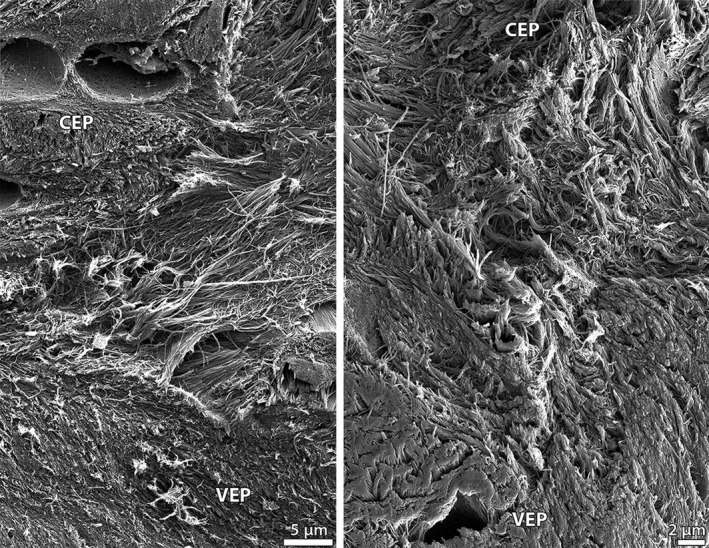
High magnification SEM images capturing the gradual transition of the multidirectional cartilaginous endplate fibrils to the more aligned vertebral endplate fibrils in the spring lamb disc. CEP, cartilaginous endplate; VEP, vertebral endplate.
Mature
Figure 13 provides high magnification views of the mature anchorage system for comparison. As shown in Fig. 13A, the annular fibres display crimp above their endplate anchorage but not below it. A clear boundary, i.e. the tidemark, is visible between the crimped and non‐crimped regions along with a noticeable difference in matrix texture. The branched annular sub‐bundles penetrate the entire depth of cartilaginous endplate (Fig. 13B) down to the vertebral endplate interface and maintain their high definition over this depth. Further, chondrons contained in the intervening regions of cartilaginous endplate tissue between the branched sub‐bundles appear to be only vertically aligned (see Figs 13A,B).
Figure 13.
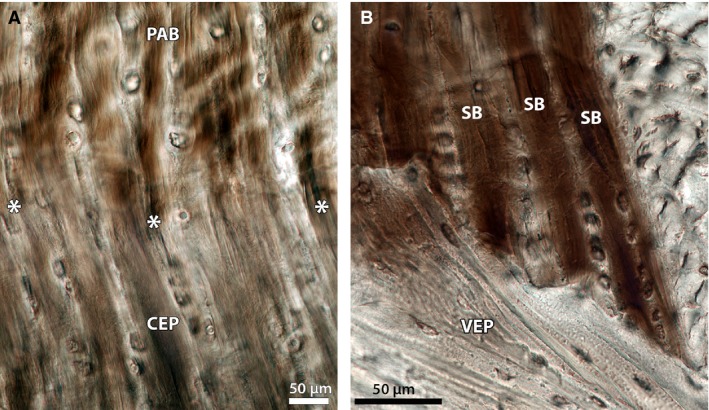
High magnification views of the mature annulus‐endplate anchorage system, showing: (A) a sudden absence of crimp in the annular fibres on their insertion into the cartilaginous endplate, and (B) the clear continuation of well‐defined sub‐bundles extending to the vertebral endplate surface further down the anchorage depth. Note that in both images, the chondrons within the cartilaginous endplate are only vertically aligned. CEP, cartilaginous endplate; PAB, primary annular bundle; SB, sub‐bundle; VEP, vertebral endplate. Image A is reprinted from Spine Journal, 12(2), Rodrigues SA, Wade KR, Thambyah A and Broom ND, Micromechanics of annulus‐end plate integration in the intervertebral disc, 143–150, Copyright (2012), with permission from Elsevier. DOI: http://dx.doi.org/10.1016/j.spinee.2012.01.003
Discussion
The detailed structural analysis conducted in this study adds new clarity to our understanding of the mechanisms governing annular‐endplate anchorage as a function of maturity. Our results confirm that the ovine endplate and its mode of anchoring the annulus undergo significant structural changes with maturity. In the newborn disc, the endplate comprises a relatively thick layer of cartilage that, in the outer to mid portions of the annulus, extends down to the growth plate (Figs 2A and 3A). The spring lamb endplate, however, is noticeably different to that of the newborn despite being only a few months more mature. Unlike the latter, the centre of ossification in the spring lamb motion segments is further developed and includes a thin layer of dense subchondral bone to support the much‐reduced cartilaginous endplate (Figs 2B and 3B). This rapid growth is presumably a result of the spine being exposed to higher and more complex modes of mechanical loading after birth, and leads developmentally to the microstructure of the spring lamb endplate closely resembling that of the mature endplate (cf. Figs 3B,C).
Despite these large differences in endplate microstructure, the annular fibre bundles in all three sample groups display a tendency to split into multiple sub‐bundles on entering the cartilaginous endplate (Fig 4). Although not fully formed in the immature disc, the sub‐bundles in the newborn and spring lamb disc (Fig. 4A and B, respectively) foreshadow the well‐defined anchorage system that eventually develops in the mature disc (Fig. 4C). As discussed by Rodrigues et al. (2012), this branching morphology increases the anchorage resistance to tensile failure by increasing the interface area between the annulus and endplate tissues over which shear forces are distributed (Rodrigues et al. 2012). The observation that it develops from an age as young as birth reinforces the critical role that this mechanism plays in aiding integration and strengthening the annulus‐endplate junction.
Further, the morphology of the branched annular sub‐bundles, and their integration with the surrounding tissue, changes with maturity. Microscopically, the annular sub‐bundles in the mature tissue are generally strongly imaged as distinct entities throughout the full thickness of the cartilaginous endplate (Figs 4C and 13B). By contrast, in the newborn and spring lamb discs the sub‐bundles display a subtle fading within the cartilaginous endplate tissue close to the vertebral endplate (Figs 5 and 9, respectively). This loss of sub‐bundle definition reflects a fibril‐level integration with the cartilaginous endplate in the immature disc. Our SEM analysis of the newborn discs shows that the group of highly aligned fibrils within the sub‐bundles divide and progressively transform into finer fibrils of a similar alignment before eventually blending into a fine multi‐directional network characteristic of the cartilaginous endplate (Fig. 7). This progressive transition, along with the intermingling of the two fibril populations observed in Fig. 8, presumably leads to a more mechanically robust mode of integration.
Likewise, SEM analysis of the spring lamb anchorage site indicates that the branched annular sub‐bundles intertwine and, in some cases, merge with the fibrils of the surrounding cartilaginous endplate tissue (Figs 9B and 10). The presence of aligned sub‐bundle fibrils at the spring lamb vertebral endplate surface (Figs 9B and 11) suggests that despite this complex integration the annular fibres still anchor through the entire depth of the cartilaginous endplate in the spring lamb discs, as in the mature (Fig. 13B).
Additionally, it is highly probable that the changing chondron morphology observed in the newborn and spring lamb samples is linked to the mechanism of calcification and ossification in the maturing disc. It is now well‐established that the phenotype of chondrocytes changes during cartilage calcification and endochondral ossification (Coventry et al. 1945b; Bonucci, 1967; Bernick & Cailliet, 1982; Buckwalter et al. 1986; Hunziker et al. 1987; Anderson, 1989; Martin et al. 1998). Cells within the zone of calcification (closest to the newly formed bone) become ‘hypertrophic’ and generally appear enlarged (Buckwalter et al. 1986; Hunziker et al. 1987; Anderson, 1989; Martin et al. 1998). They assume a more spherical shape compared with their general spindle shape (Bernick & Cailliet, 1982; Buckwalter et al. 1986) and acquire a columnar alignment (Buckwalter et al. 1986; Hunziker et al. 1987; Anderson, 1989; Martin et al. 1998).
Such chondrocyte morphologies were displayed in the present analysis of newborn discs at the growth plate (Fig. 6D) and in regions bordering the developing mass of bone in the centre of the vertebra (Fig. 6B). This provides a glimpse of how the cells in these regions prepare the cartilaginous tissue for calcification and ossification. The fact that the cells in the bulk of the cartilage layer, such as those occurring across the annulus‐cartilage tissue transition (Fig. 5) and those located in the peripheral regions further down the cartilage (Fig. 6C), do not appear enlarged suggests that a large depth of the cartilaginous endplate and the annulus‐endplate anchorage zone is probably not yet calcified immediately following birth.
Further, the horizontal realignment of chondrons within the cartilaginous endplate tissue observed in the immature disc could be linked to the process of ossification. All three age groups contained vertically aligned chondrons within the cartilaginous endplate tissue between the branched sub‐bundles (Figs 5, 9 and 13). However, in the newborn disc the chondrons located immediately above the growth zone were more horizontally aligned (Fig. 6D). Similarly, the spring lamb endplate contained horizontally aligned chondrons close to the vertebral bone interface (Fig. 9A). Such chondrons were, however, absent in the mature endplate (Fig. 13).
Chondrocyte alignment in articular cartilage is known to be strongly related to the fibrillar alignment of the surrounding extracellular matrix (Kääb et al. 2003). A horizontal alignment of chondrons and therefore the fibrillar matrix may be indicative of a mechanical environment that involves shear. Previous studies on the mechanobiology of bone have suggested that shear stresses are important in promoting bone formation (Carter et al. 1987, 2004; Stevens et al. 1999; Beaupré et al. 2000; Carter & Wong, 2003). The horizontal alignment of the chondrons close to the osteochondral junction, at both the newborn growth plate and the spring lamb cartilaginous endplate‐bone surface, provides an indication of how the matrix may be ossified. It seems plausible to suggest that the region containing the horizontally aligned chondrons in the spring lamb endplate (Fig. 9A) serves as a precursor to new bone development within the corresponding region shown in the mature endplate image (Fig. 13B). This suggests that mechanobiological factors are strongly involved in the advance of the junction between the vertebral and cartilaginous endplates (i.e. cement line) during endplate development and provides some direction for future investigations.
Another factor that requires further study is the degree of endplate calcification in the immature disc. Calcification significantly influences the strength of the annulus‐endplate anchorage system and its mode of failure (Rodrigues et al. 2015). It is also closely linked to vertebral bone formation via the process of endochondral ossification which occurs at the growth zones (Coventry et al. 1945b; Bick & Copel, 1950; Peacock, 1952; Bernick & Cailliet, 1982; Paietta et al. 2013). From the data obtained in the present study, it is difficult to determine conclusively the extent of calcification in both newborn and spring lamb groups due to the decalcification treatment required to achieve the quality of imaging of the soft–hard junction. However, the general diminishing of crimp with increasing depth in the spring lamb anchorage system does suggest present or anticipated calcification. As previously discussed by Rodrigues et al. (2012), a lack of crimp is consistent with the annular fibres being restricted by a hard mineralized phase. It is also possible that this change in crimp morphology is related to some other biochemical factor such as a sudden change in proteoglycan concentration or ratio of Type I/II collagen. Chemical analysis would be required to investigate this issue further.
Comparison with human discs
A comparison of the present findings with those previously reported for the immature human disc reveals certain similarities in fundamental structure. Like sheep, the newborn human endplate also comprises a relatively thick layer of cartilage (Coventry et al. 1945a,b; Coventry, 1969; Bernick & Cailliet, 1982; Buckwalter, 1995; Urban & Roberts, 1995; Roberts, 2002; Roberts et al. 2006; Paietta et al. 2013) containing several vascular channels (Coventry et al. 1945a,b; Bernick & Cailliet, 1982; Edelson & Nathan, 1988; Buckwalter, 1995; Urban & Roberts, 1995; Roberts, 2002; Roberts et al. 2006) and spindle‐shaped cells (Bernick & Cailliet, 1982). In terms of anchorage, fibrils from the human outer annulus penetrate and disappear into the layer of cartilage which is yet to be ossified and form the future epiphyseal ring (Peacock, 1952; Hashizume, 1980; Buckwalter, 1995), whereas fibrils from the inner annulus intermingle with those from the cartilaginous endplate (Hashizume, 1980).
During childhood and adolescence, the thickness of the human cartilaginous endplate decreases (Coventry et al. 1945b; Coventry, 1969; Urban & Roberts, 1995) and the underlying bone becomes denser and more uniform (Coventry et al. 1945b; Coventry, 1969) with further bone deposition (Coventry et al. 1945b) and the formation of a subchondral bone layer (Bick & Copel, 1950). The blood vessels in the cartilaginous endplate also diminish in size and number (Coventry et al. 1945b; Buckwalter, 1995; Urban & Roberts, 1995; Roberts et al. 2006). These trends are similar to those observed in the present ovine study.
There are some differences, however: previous literature indicates that there are two independent systems of endplate development in the human vertebrae, one located in the peripheral region and the other in the central portion. The base of the cartilaginous endplate forms the central growth zone of the vertebral body (Coventry et al. 1945a,b; Hashizume, 1980; Bernick & Cailliet, 1982; Urban & Roberts, 1995; Roberts, 2002; Roberts et al. 2006; Alini et al. 2008) but a separate ossification centre arises in the peripheral region (i.e. beneath the outer annulus) to form the future epiphyseal ring (Coventry et al. 1945a; Bick & Copel, 1950, 1951; Peacock, 1952). This latter region does not participate actively in the longitudinal growth of the vertebral body; instead, it is ossified separately and fuses with the body once growth is complete (Coventry et al. 1945a; Bick & Copel, 1950, 1951). This leads to different modes of endplate integration in the outer and inner annulus, not just in the immature human disc but also in the final mature structure.
In contrast, sheep endplates have a more uniform growth pattern across the annulus. Unlike in humans, the secondary ossification centre is not limited to the peripheral areas of the vertebral body but occurs at its caudal and cranial ends and spans the whole width of the body (Alini et al. 2008). This means that unlike immature human vertebrae, a separate growth plate persists within the vertebral bodies of sheep throughout immaturity (Alini et al. 2008). These morphological differences need to be considered when findings from the ovine disc model are applied to the human disc.
How the detailed structure of the disc‐endplate junction changes with maturity and thus how it might influence where failure occurs across this junction is potentially relevant to the issue of disc herniation and its associated pain symptoms. A study by Willburger et al. (2004) found that patient age influenced the histological composition of the extruded material – there was a more frequent occurrence of cartilage endplate in the extrusion with increasing age which was associated with increased symptoms of back pain and nerve root compression. It is thought that cartilage endplate fragments may be more resistant to resorption and thus increase the risk of persisting pain symptoms (Lama et al. 2013). Conversely, where there is pure nucleus migration to the periphery of the disc without any attached endplate cartilage there may be a relatively rapid resolution of both the inflammatory process and pain symptoms (Autio et al. 2006; Lama et al. 2013; Shan et al. 2014). Other studies have argued that any potential positive correlation between cartilage endplate herniations and pain and clinical symptoms will be confounded by the inflammatory effect of the nucleus material in the extrusion (Joe et al. 2015).
A related effect is thought to arise from the loss of any endplate cartilage associated with herniation. This will increase the permeability of the endplate, allowing easier migration of biological agents that can stimulate Modic changes in the endplate and vertebral body (Rodriguez et al. 2011; Shan et al. 2014). It has been suggested that non‐virulent anaerobic bacteria can infect the disc at the time of herniation. The infection can then trigger inflammation in the adjacent vertebra via the disrupted endplate leading to persisting low back pain (Albert et al. 2008, 2013).
The above clinically directed comments serve to emphasize the importance of gaining a more rigorous understanding of the structure of the disc‐endplate junction, and where and how failure occurs within it.
Conclusions
This study provides new insights into the development of the annulus‐endplate anchorage system. The clarity of the structural detail is largely a consequence of using carefully chosen sectioning planes that capture much more effectively the modes of integration between the annulus and endplate tissues. As described by previous researchers, endplate morphology changes dramatically from newborn lamb to spring lamb and mature ewe. In all three age groups, however, a similar branching morphology was observed at the anchorage site, with the annular bundles splitting into multiple sub‐bundles on their insertion into the cartilaginous endplate. Although the morphology of the sub‐bundles and their integration with the surrounding cartilaginous endplate does vary with maturity, the prevalence of this mechanism of integration from an age as young as birth reinforces the critical role that it plays in strengthening the anchorage site. Further, analysis of the changing arrangement of chondrons within the cartilaginous matrix provides additional clues concerning the mechanism of endplate development and ossification.
Author contributions
S.A.R. carried out all experiments and wrote the primary draft. A.T. contributed to both the planning of the research and analysis of the experimental data. N.D.B. contributed to the planning of the research, analysis of the experimental data, and drafting of the manuscript and its final submitted form.
Acknowledgements
The first author acknowledges funding provided by a University of Auckland Doctoral Scholarship.
References
- Albert HB, Kjaer P, Jensen TS, et al. (2008) Modic changes, possible causes and relation to low back pain. Med Hypotheses 70, 361–368. [DOI] [PubMed] [Google Scholar]
- Albert HB, Lambert P, Rollason J, et al. (2013) Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 22, 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alini M, Eisenstein SM, Ito K, et al. (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17, 2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HC (1989) Mechanism of mineral formation in bone. Lab Invest 60, 320–330. [PubMed] [Google Scholar]
- Aufdermaur M (1974) Spinal injuries in juveniles: necropsy findings in twelve cases. J Bone Joint Surg Br 56B, 513–519. [PubMed] [Google Scholar]
- Autio RA, Karppinen J, Niinimaki J, et al. (2006) Determinants of spontaneous resorption of intervertebral disc herniations. Spine 31, 1247–1252. [DOI] [PubMed] [Google Scholar]
- Baranto A, Ekström L, Hellström M, et al. (2004) Fracture patterns of the adolescent porcine spine: an experimental loading study in bending‐compression. Spine 30, 75–82. [DOI] [PubMed] [Google Scholar]
- Beaupré GS, Stevens SS, Carter DR (2000) Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev 37, 145–151. [PubMed] [Google Scholar]
- Beggs I, Addison J (1998) Posterior vertebral rim fractures. Br J Radiol 71, 567–572. [DOI] [PubMed] [Google Scholar]
- Bernick S, Cailliet R (1982) Vertebral end‐plate changes with aging of human vertebrae. Spine 7, 97–102. [DOI] [PubMed] [Google Scholar]
- Bick EM, Copel JW (1950) Longitudinal growth of the human vertebra: a contribution to human osteogeny. J Bone Joint Surg Am 32‐A, 803–814. [PubMed] [Google Scholar]
- Bick EM, Copel JW (1951) The ring apophysis of the human vertebra: contribution to human osteogeny, II. J Bone Joint Surg Am 33‐A, 783–787. [PubMed] [Google Scholar]
- Bonucci E (1967) Fine structure of early cartilage calcification. J Ultrastruct Res 20, 33–50. [DOI] [PubMed] [Google Scholar]
- Brown SHM, Gregory DE, McGill SM (2008) Vertebral end‐plate fractures as a result of high rate pressure loading in the nucleus of the young adult porcine spine. J Biomech 41, 122–127. [DOI] [PubMed] [Google Scholar]
- Brown S, Rodrigues S, Sharp C, et al. (2016) Staying connected: structural integration at the intervertebral disc‐vertebra interface of human lumbar spines. Eur Spine J, 1–11. Epub ahead of print. doi: 10.1007/s00586‐016‐4560‐y [DOI] [PubMed] [Google Scholar]
- Buckwalter JA (1995) Spine update: aging and degeneration of the human intervertebral disc. Spine 20, 1307–1314. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Mower D, Ungar R, et al. (1986) Morphometric analysis of chondrocyte hypertrophy. J Bone Joint Surg Am 68‐A, 243–255. [PubMed] [Google Scholar]
- Carter DR, Wong M (2003) Modelling cartilage mechanobiology. Philos Trans R Soc Lond B Biol Sci 358, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DR, Orr TE, Fyhrie DP, et al. (1987) Influences of mechanical stress on prenatal and postnatal skeletal development. Clin Orthop Relat Res 219, 237–250. [PubMed] [Google Scholar]
- Carter DR, Beaupré GS, Wong M, et al. (2004) The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res 427, S69–S77. [DOI] [PubMed] [Google Scholar]
- Coventry MB (1969) Anatomy of the intervertebral disk. Clin Orthop Relat Res 67, 9–15. [PubMed] [Google Scholar]
- Coventry MB, Ghormley RK, Kernohan JW (1945a) The intervertebral disc: its microscopic anatomy and pathology. Part I. Anatomy, development, and physiology. J Bone Joint Surg Am 27, 105–112. [Google Scholar]
- Coventry MB, Ghormley RK, Kernohan JW (1945b) The intervertebral disc: its microscopic anatomy and pathology. Part II. Changes in the intervertebral disc concomitant with age. J Bone Joint Surg Am 27, 233–247. [Google Scholar]
- Edelson JG, Nathan H (1988) Stages in the natural history of the vertebral end‐plates. Spine 13, 21–26. [DOI] [PubMed] [Google Scholar]
- Ehni G, Schneider SJ (1988) Posterior lumbar vertebral rim fracture and associated disc protrusion in adolescence. J Neurosurg 68, 912–916. [DOI] [PubMed] [Google Scholar]
- Epstein NE, Epstein JA (1991) Limbus lumbar vertebral fractures in 27 adolescents and adults. Spine 16, 962–966. [DOI] [PubMed] [Google Scholar]
- Francois RJ (1975) Ligament insertions into the human lumbar vertebral body. Acta Anat 91, 467–480. [PubMed] [Google Scholar]
- Francois RJ, Dhem A (1974) Microradiographic study of the normal human vertebral body. Acta Anat 89, 251–265. [DOI] [PubMed] [Google Scholar]
- de Gauzy JS, Jouve JL, Violas P, et al. (2007) Classification of chance fracture in children using magnetic resonance imaging. Spine 32, E89–E92. [DOI] [PubMed] [Google Scholar]
- Hashizume H (1980) Three‐dimensional architecture and development of lumber intervertebral discs. Acta Med Okayama 34, 301–314. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK, Cruz‐Orive LM (1987) Quantitation of chondrocyte performance in growth‐plate cartilage during longitudinal bone growth. J Bone Joint Surg Am 69‐A, 162–173. [PubMed] [Google Scholar]
- Joe E, Lee JW, Park KW, et al. (2015) Herniation of cartilaginous endplates in the lumbar spine: MRI findings. Am J Roentgenol 204, 1075–1081. [DOI] [PubMed] [Google Scholar]
- Jónsson H, Bring G, Rauschning W, et al. (1991) Hidden cervical spine injuries in traffic accident victims with skull fractures. J Spinal Disord 4, 251–263. [DOI] [PubMed] [Google Scholar]
- Kääb MJ, Richards RG, Ito K, et al. (2003) Deformation of chondrocytes in articular cartilage under compressive load: a morphological study. Cells Tissues Organs 175, 133–139. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Lundin O, Ekström L, et al. (1998) Injuries in adolescent spine exposed to compressive loads: an experimental cadaveric study. J Spinal Disord 11, 501–507. [PubMed] [Google Scholar]
- Keller RH (1974) Traumatic displacement of the cartilagenous vertebral rim: a sign of intervertebral disc prolapse. Radiology 110, 21–24. [DOI] [PubMed] [Google Scholar]
- Lama P, Le Maitre CL, Dolan P, et al. (2013) Do intervertebral discs degenerate before they herniate, or after? Bone Joint J 95‐B, 1127–1133. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ernestus RI, Schröder R, et al. (2000) Histological study of lumbar intervertebral disc herniation in adolescents. Acta Neurochir 142, 1107–1110. [DOI] [PubMed] [Google Scholar]
- Liu J, Mei Z, Shan Z, et al. (2015) Anchorage of annulus fibrosus within the vertebral endplate with reference to disc herniation. Microsc Res Tech 78, 754–760. [DOI] [PubMed] [Google Scholar]
- Lundin O, Ekström L, Hellström M, et al. (1998) Injuries in the adolescent porcine spine exposed to mechanical compression. Spine 23, 2574–2579. [DOI] [PubMed] [Google Scholar]
- Lundin O, Ekström L, Hellström M, et al. (2000) Exposure of the porcine spine to mechanical compression: differences in injury pattern between adolescents and adults. Eur Spine J 9, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RB, Burr DB, Sharkey NA (1998) Skeletal Tissue Mechanics. New York: Springer. [Google Scholar]
- Martínez‐Lage JF, Poza M, Arcas P (1998) Avulsed lumbar vertebral rim plate in an adolescent: trauma or malformation? Childs Nerv Syst 14, 131–134. [DOI] [PubMed] [Google Scholar]
- Paietta RC, Burger EL, Ferguson VL (2013) Mineralization and collagen orientation throughout aging at the vertebral endplate in the human lumbar spine. J Struct Biol 184, 310–320. [DOI] [PubMed] [Google Scholar]
- Peacock A (1952) Observation on the postnatal structure of the intervertebral disc in man. J Anat 86, 162–179. [PMC free article] [PubMed] [Google Scholar]
- Roberts S (2002) Disc morphology in health and disease. Biochem Soc Trans 30, 864–869. [DOI] [PubMed] [Google Scholar]
- Roberts S, Evans H, Trivedi J, et al. (2006) Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 88‐A(Suppl 2), 10–14. [DOI] [PubMed] [Google Scholar]
- Rodrigues SA, Wade KR, Thambyah A, et al. (2012) Micromechanics of annulus‐end plate integration in the intervertebral disc. Spine J 12, 143–150. [DOI] [PubMed] [Google Scholar]
- Rodrigues SA, Thambyah A, Broom ND (2015) A multiscale structural investigation of the annulus‐endplate anchorage system and its mechanisms of failure. Spine J 15, 405–416. [DOI] [PubMed] [Google Scholar]
- Rodriguez AG, Slichter CK, Acosta FL, et al. (2011) Human disc nucleus properties and vertebral endplate permeability. Spine 36, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savini R, Di Silvestre M, Gargiulo G, et al. (1991) Posterior lumbar apophyseal fractures. Spine 16, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Schmorl G, Junghanns H (1971) The Human Spine in Health and Disease, 2nd edn New York: Grune & Stratton. [Google Scholar]
- Shan Z, Fan S, Xie Q, et al. (2014) Spontaneous resorption of lumbar disc herniation is less likely when Modic changes are present. Spine 39, 736–744. [DOI] [PubMed] [Google Scholar]
- Stevens SS, Beaupre GS, Carter DR (1999) Computer model of endochondral growth and ossification in long bones: biological and mechanobiological influences. J Orthop Res 17, 646–653. [DOI] [PubMed] [Google Scholar]
- Takata K, Inoue S, Takahashi K, et al. (1988) Fracture of the posterior margin of a lumbar vertebral body. J Bone Joint Surg Am 70‐A, 589–594. [PubMed] [Google Scholar]
- Thoreson O, Baranto A, Ekström L, et al. (2010) The immediate effect of repeated loading on the compressive strength of young porcine lumbar spine. Knee Surg Sports Traumatol Arthrosc 18, 694–701. [DOI] [PubMed] [Google Scholar]
- Urban JPG, Roberts S (1995) Development and degeneration of the intervertebral discs. Mol Med Today 1, 329–335. [DOI] [PubMed] [Google Scholar]
- Wade KR, Robertson PA, Broom ND (2011) A fresh look at the nucleus‐endplate region: new evidence for significant structural integration. Eur Spine J 20, 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade KR, Robertson PA, Broom ND (2012) On how nucleus‐endplate integration is achieved at the fibrillar level in the ovine lumbar disc. J Anat 221, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willburger RE, Ehiosun UK, Kuhnen C, et al. (2004) Clinical symptoms in lumbar disc herniations and their correlation to the histological composition of the extruded disc material. Spine 29, 1655–1661. [DOI] [PubMed] [Google Scholar]


