Abstract
Background and Purpose
Depression is a neuropsychiatric disorder accompanied by a decrease in the brain‐derived neurotrophic factor (BDNF) signalling cascade in the hippocampus. Fenofibrate is a selective agonist of PPAR‐α. In this study, we investigated the antidepressant‐like effects of fenofibrate in C57BL/6J mice.
Experimental Approach
The antidepressant‐like effects of fenofibrate were first identified in the forced swim test (FST) and tail suspension test (TST), and then assessed in the chronic social defeat stress (CSDS) model. The changes in the hippocampal BDNF signalling pathway and adult hippocampal neurogenesis after CSDS and fenofibrate treatment were further investigated. A PPAR‐α inhibitor, cannabinoid system inhibitors and BDNF signalling inhibitors were also used to determine the antidepressant mechanisms of fenofibrate.
Key Results
Fenofibrate administration exhibited antidepressant‐like effects in the FST and TST without affecting the locomotor activity of mice. Chronic fenofibrate treatment also prevented the depressive‐like symptoms induced by CSDS. Moreover, fenofibrate restored the CSDS‐induced decrease in the hippocampal BDNF signalling cascade and adult hippocampal neurogenesis. The antidepressant‐like effects of fenofibrate could be blocked by a PPAR‐α inhibitor and BDNF signalling inhibitors.
Conclusions and Implications
Taken together, these results suggest that fenofibrate has antidepressant‐like effects mediated through the promotion of the hippocampal BDNF signalling cascade.
Abbreviations
- BDNF
brain‐derived neurotrophic factor
- BrdU
5‐bromo‐2‐deoxyuridine
- CREB
cAMP response element‐binding protein
- CSDS
chronic social defeat stress
- DCX
doublecortin
- DG
dentate gyrus
- FST
forced swim test
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- TrkB
tyrosine kinase B
- TST
tail suspension test
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Catalytic receptors c |
| CB1 receptor | TrkB receptor |
| CB2 receptor | Enzymes d |
| Nuclear hormone receptors b | Akt |
| PPAR‐α | ERK1 |
| ERK2 |
| LIGANDS |
|---|
| AM630 |
| BDNF |
| Fenofibrate |
| GW6471 |
| Rimonabant |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a,b,c,d).
Introduction
Depression is a highly debilitating and life‐threatening mental disorder that occurs in about 17% of the general population, and causes huge costs to society (Blazer et al., 1994). In the past few decades, the monoamine hypothesis has been the most widely investigated aetiology of depression, and almost all available antidepressants depend on enhancing the levels of monoamine neurotransmitters (Berton and Nestler, 2006b). However, current antidepressants are only effective in about one‐third of patients, and sometimes have serious side effects (McGrath et al., 2006). Thus, more reliable antidepressants with fewer side effects need to be developed.
Recently, in a leading hypothesis it was suggested that the brain‐derived neurotrophic factor (BDNF) signalling pathway plays a critical role in the pathophysiology of depression (Shelton, 2007; Krishnan and Nestler, 2008). BDNF induces the phosphorylation and activation of cAMP response element‐binding protein (CREB) by combining the tyrosine kinase B (TrkB) receptor and then promoting the downstream MAPK‐ERK and PI3K‐Akt signalling pathways (Shaywitz and Greenberg, 1999; Lim et al., 2008). Previous studies have already demonstrated that the level of activity of the BDNF signalling pathway is decreased in the hippocampus of stressed mice, while these pathological changes can be reversed by antidepressants, like fluoxetine (Blendy, 2006; Castren and Rantamaki, 2010; Razzoli et al., 2011). Heterogeneous BDNF knockout mice show depressive‐like behaviour in the forced swim test (FST) and tail suspension test (TST), while the administration of BDNF/CREB into the hippocampus produces antidepressant‐like effects in animals (Chen et al., 2001; Shirayama et al., 2002; Hoshaw et al., 2005; Gass and Riva, 2007; Advani et al., 2009).
Fenofibrate is a fibric acid derivative used in the treatment of primary hypercholesterolaemia, mixed dyslipidaemia and hypertriglyceridaemia in adults (Keating and Croom, 2007). Recently, more and more fenofibrate‐induced pharmacological effects on the CNS are being reported, such as its neuroprotective effects against Parkinson's disease, its ability to preserve adult hippocampal neurogenesis and prevent the memory impairments in rats following global cerebral ischaemia (Ramanan et al., 2009; Barbiero et al., 2014; Ouk et al., 2014). Fenofibrate is also a selective agonist of PPAR‐α, one of the three subtypes of the nuclear receptor PPAR family. We previously reported that WY14643, another selective agonist of PPAR‐α, produced antidepressant‐like effects in mice by activating the BDNF signalling pathway (Jiang et al., 2015b). We thus assumed that fenofibrate may also have antidepressant‐like effects. In this study, we investigated the antidepressant‐like effects of fenofibrate using various methods, including the FST, TST and chronic social defeat stress (CSDS) model. Furthermore, the molecular mechanisms of these effects were explored.
Methods
Animals
Adult male C57BL/6J mice (8 weeks old) and male CD1 mice (50 weeks old) were obtained from the Experimental Animal Centre of Medical College, Nantong University. Before being used, mice were housed under standard conditions (12 h light/dark cycle; lights on from 07:00 to 19:00; 23 ± 1°C ambient temperature; 55 ± 10% relative humidity) for 1 week with free access to food and water. Each experimental group consisted of 12 mice. Behavioural experiments were carried out during the light phase. The experiment procedures involving animals and their care were approved by the Institutional Animal Care and Use Committee, Nantong University, and conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and with the European Communities Council Directive of 24 November 1986 (86/609/EEC). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Intracerebroventricular infusions
The chicken anti‐BDNF antibody from Promega has been shown to neutralize and be specific for BDNF (Braun et al., 2004; Chen et al., 2005; Zhu et al., 2010; Shichinohe et al., 2015). In brief, C57BL/6J mice were anaesthetized with pentobarbital sodium (50 mg·kg−1, i.p.) and placed in a stereotaxic frame, with the anaesthetizing effects evaluated by muscle relaxation, slow corneal reflex, and no skin pinch reaction. The cannulas were implanted into the left lateral brain ventricle (−0.2 mm anterior and 1.0 mm lateral relative to the bregma and 2.3 mm below the surface of the skull) (Kleinridders et al., 2009). The cannula was cemented in place, and the incision was sutured. The animals were allowed to recover for 3 days. Osmotic minipumps were designed to deliver 0.3 μL·min−1 of K252a/GW6471/rimonabant/AM630/ACSF with 1% DMSO/chicken anti‐BDNF antibody/chicken IgY/ACSF (final volume, 3 μL per mouse) daily for 3 or 14 days. Each osmotic minipump was attached to a brain infusion cannula.
Forced swim test
The FST test was performed according to previously reported methods (Porsolt et al., 1977; Yan et al., 2010), and separate groups of C57BL/6J mice were used for this test. In brief, 30 min after a single injection, mice were individually placed in a glass cylinder (height 45 cm, diameter 20 cm) filled with 25°C water to a depth of 15 cm. The duration of immobility was recorded during the last 4 of the 6 min test period by an investigator blind to the treatment, and the water was replaced after each trial. The immobility time was measured when the mice are floating in the water without struggling and only making movements necessary to keep their heads above the water.
Tail suspension test
The TST test was performed according to previously reported methods (Steru et al., 1985; Sarkisyan et al., 2010), and separate groups of C57BL/6J mice were used for this test. In brief, 30 min after a single injection, mice were suspended 60 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility was recorded for a 6 min period by an investigator blind to the treatment. Mice were considered immobile only when they hung passively and were completely motionless; any mice that did climb were removed from the experimental analysis.
Open field test
Spontaneous locomotor activity of mice was measured in the open field paradigm (Covington et al., 2009; Muller et al., 2009; Cui et al., 2012), and separate groups of animals were used for this test. C57BL/6J mice were placed individually in an open field apparatus (height 40 cm, width 100 cm, length 100 cm) with the floor divided into 25 equal areas (20 × 20 cm). The apparatus was illuminated with a red bulb (50 W) on the ceiling. For open field observations, each mouse was placed in the central area 30 min after a single injection. The squares each mouse crossed were counted over a 5 min period under dim light conditions by an investigator blind to the treatment. The open field apparatus was thoroughly cleaned after each trial.
Chronic social defeat stress, social interaction and sucrose preference experiments
Adult male C57BL/6J mice were the subjects, and CD1 retired breeders were the aggressors. Social defeat stress was performed as described by us previously (Jiang et al., 2015a,b,c). In brief, each C57BL/6J mouse was exposed to a different CD1 aggressor mouse each day for up to 10 min over a total of 10 days. After the contact, C57BL/6J mice were separated from CD1 aggressors by plastic dividers with holes during the next 24 h. To minimize the harm and avoid any physical wounds, plastic dividers were set when C57BL/6J mice displayed submissive behaviour; this included immobility, crouching, trembling, fleeing and an upright posture (usually 8–10 min was required in this study). Undefeated control mice were handled daily. The day after the last stress (Day 11), all the defeated mice were housed individually and received daily injections of fenofibrate/fluoxetine/vehicle for 14 days. Control mice were also given the vehicle.
On Day 25, the social interaction test was performed. The social interaction test comprises two trials of 5 min for each. In the first trial (‘target absent’), each mouse was placed into an open‐field apparatus and allowed to explore a plastic enclosure placed within the predefined interaction zone. In the second trial (‘target present’), each mouse was returned to the open‐field arena containing a plastic enclosure now holding an unfamiliar CD1 mouse. The amount of time in the interaction zone was obtained using Ethovision XT (Noldus, USA) software (in s). The open‐field apparatus was cleaned after each trial to remove olfactory cues.
On Day 26, the sucrose preference test was performed. The test mice were given the choice of drinking from two bottles in individual cages, one contained 1% sucrose solution and the other water. All the mice were acclimatized for 2 days to two‐bottle choice conditions, and the positions of the two bottles were changed every 6 h to prevent the possible effects of side preference in drinking behaviour. Then the test mice were deprived of water and food for 18 h. On Day 29, each mouse was exposed to pre‐weighed bottles for 6 h with their positions interchanged. The sucrose preference was calculated as a percentage of the sucrose solution consumed relative to the total amount of liquid taken in.
Western blotting experiments
Animals were killed the day after the sucrose preference test. To extract the total proteins, tissues were rapidly dissected and homogenized in lyses buffer [50 mM Tris–HCl, pH 7.4; 1 mM EDTA; 100 mM NaCl; 20 mM NaF; 3 mM Na3VO4; 1 mM PMSF with 1% (v.v‐1) Nonidet P‐40; and protease inhibitor cocktail], and then kept on ice for 30 min (Meng et al., 2015; Xu et al., 2015; Yao et al., 2015). The lysates were centrifuged at 12 000 × g for 15 min, and the supernatants were harvested. After denaturation, 30 μg of protein samples were separated by 10% SDS/PAGE gel and then transferred to nitrocellulose membranes (Bio‐Rad, Hercules, CA, USA). After being blocked with 5% non‐fat dried milk powder/Tris‐buffered saline Tween‐20 (TBST) for 1 h, membranes were incubated overnight at 4°C with primary antibodies to BDNF (1:500; Abcam, UK), CREB (1:500; Cell Signalling, MA, USA), phospho‐CREB‐Ser133 (pCREB; 1:500; Cell Signalling, MA, USA), ERK1/2 (1:1000; Cell Signalling, MA, USA), phospho‐ERK1/2 (1:500; Cell Signalling, MA, USA), Akt (1:500; Cell Signalling, MA, USA), phospho‐Akt (pAkt; 1:500; Cell Signalling, MA, USA) or β‐actin (1:5000; Santa Cruz, CA, USA). Then the membranes were washed three times in TBST. The membranes were further incubated for 2 h at room temperature with IRDye 680‐labelled secondary antibodies (1:10 000). Finally immunoblots were visualized by scanning using the Odyssey CLx western blot detection system. The optical density of the bands was determined using Optiquant software (Packard Instruments BV, Groningen, Netherlands).
Immunohistochemical studies
For hippocampal doublecortin (DCX) staining, animals were deeply anaesthetized with pentobarbital sodium and perfused transcardially with 4% paraformaldehyde in 0.01 M phposphate buffer 24 h after the sucrose preference test. The brains were removed and postfixed for 24 h, then dehydrated with 30% sucrose solution. After that, coronal brain sections of hippocampus were cut at 25 μm with a freezing microtome (CM1900, Leica Microsystems, Wetzlar, Germany) and collected serially. The sections were sequentially treated with 0.3% Triton X‐100 in 0.01 M PBS for 30 min and 3% BSA in 0.01 M PBS for another 30 min. Then the sections were incubated with diluted rabbit anti‐DCX antibody (1:100; Cell Signalling, MA, USA) overnight at 4°C. The sections were subsequently washed in 0.01 M PBS and exposed to FITC‐labelled horse anti‐rabbit IgG (1:50; Pierce, Rockford, IL, USA) for 1 h. After that, the sections were washed again and mounted on slides following dehydration, and coverslipped. Sections were visualized with confocal laser scanning system (FV500; Olympus, Tokyo, Japan). Examination of the DCX‐positive (DCX+) cells were confined to the dentate gyrus (DG), in particular the granule cell layer (GCL), including the subgranular zone (SGZ) of hippocampus that defined as a two‐cell body‐wide zone along the border between the GCL and the hilus. Quantifications of the DCX+ cells were respectively conducted from 1‐in‐6 series of hippocampal sections spaced at 150 μm and spanning the rostrocaudal region of the DG bilaterally. Every DCX+ cell within the GCL and SGZ was counted.
For NeuN+/BrdU+ double labelling, animals were injected with BrdU (4 × 75 mg·kg−1 at 2 h intervals day−1) for 2 days after the sucrose preference test. After 28 days, mice were killed and brain sections were then created. DNA denaturation was conducted by incubation in 50% formamide/2 × SSC at 65°C for 2 h, followed by 30 min incubation in 2 N HCl at 37°C, and rinsing in 0.1 M boric acid buffer (pH 8.5) at room temperature. After DNA denaturation, the sections were treated with 0.3% Triton X‐100 in 0.01 M PBS for 30 min and 3% BSA in 0.01 M PBS for another 1 h, and then incubated with mouse monoclonal anti‐BrdU (2 μg·mL−1, Roche) and rabbit monoclonal anti‐NeuN (1:500; Abcam, Cambridge, UK) overnight at 4°C. After being washed, FITC‐conjugated horse anti‐rabbit IgG and rhodamine‐conjugated goat anti‐mouse IgG (1:50; Pierce, Rockford, IL, USA) were applied for 1 h at room temperature. Sections were then washed in 0.01 M PBS and mounted on slides following dehydration, and coverslipped. Examination of the NeuN+/BrdU+ co‐labelling cells was confined to the DG. Quantifications of the NeuN+/BrdU+ cells were respectively conducted from 1‐in‐6 series of hippocampal sections spaced at 150 μm and spanning the rostrocaudal region of the DG bilaterally. Every NeuN+/BrdU+ cell within the GCL and SGZ was counted.
Statistical analysis
All analyses were performed using SPSS 13.0 software (SPSS Inc., USA), and data are presented as mean ± SEM. Differences between mean values were evaluated using one‐way ANOVA or two‐way ANOVA, as appropriate. For all one‐way ANOVAs, post hoc tests were performed using LSD test. For all two‐way ANOVAs, Bonferroni post hoc tests were used to assess isolated comparisons. P < 0.05 was considered statistically significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Materials
Fenofibrate, 5‐bromo‐2‐deoxyuridine (BrdU) and fluoxetine were purchased from Sigma (St. Louis, MO, USA). K252a was purchased from Alomone Laboratories (Jerusalem, Israel). GW6471, rimonabant and AM630 were purchased from Tocris Bioscience (Bristol, UK). Chicken anti‐BDNF neutralizing anti‐body and chicken IgY control Ig were purchased from Promega (Madison, USA). Fenofibrate and fluoxetine were dissolved in 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL. BrdU was dissolved in saline. K252a, GW471, rimonabant and AM630 were dissolved in ACSF with 1% DMSO. Chicken anti‐BDNF neutralizing antibody and chicken IgY control Ig were dissolved in ACSF. The dosages of fenofibrate (50, 100 mg·kg−1), fluoxetine (20 mg·kg−1), BrdU (75 mg·kg−1), K252a (50 ng per mouse), GW6471 (2 μg per mouse), rimonabant (30 μg per mouse), AM630 (10 μg per mouse) and anti‐BDNF neutralizing antibody (60 ng per mouse) were chosen based on previous reports (Niculescu et al., 2008; Hough et al., 2009; Fang et al., 2012; Jiang et al., 2012, 2013, 2015a; Citraro et al., 2013; Barbiero et al., 2014). Fenofibrate, fluoxetine and BrdU were administered i.p. in a volume of 10 mL·kg−1. K252a, GW6471, rimonabant, AM630, chicken anti‐BDNF neutralizing antibody and chicken IgY control Ig were infused i.c.v.
Results
Antidepressant‐like effects of fenofibrate in the FST and TST
The possible antidepressant‐like effects of fenofibrate were first examined in the FST, a most widely used behavioural assay for detecting potential antidepressant‐like activities (Cryan and Holmes, 2005; Cryan and Slattery, 2007). Fenofibrate was injected i.p., with fluoxetine used as the positive control. It was found that a single injection of fenofibrate produced a significant antidepressant‐like effect in the FST (Figure 1A). Data were subjected to one‐way ANOVA with drug treatment as the factor, and a significant main effect of drug treatment was revealed [F(3, 44) = 11.598]. Further analysis indicated that compared with the vehicle group, 100 mg·kg−1 fenofibrate treatment induced a 31.1 ± 3.6% decrease of immobility time in the FST (n = 12, P < 0.05 vs. vehicle; Figure 1A). Similarly, fluoxetine treatment also decreased the immobility time, as expected (n = 12, P < 0.05 vs. vehicle; Figure 1A).
Figure 1.
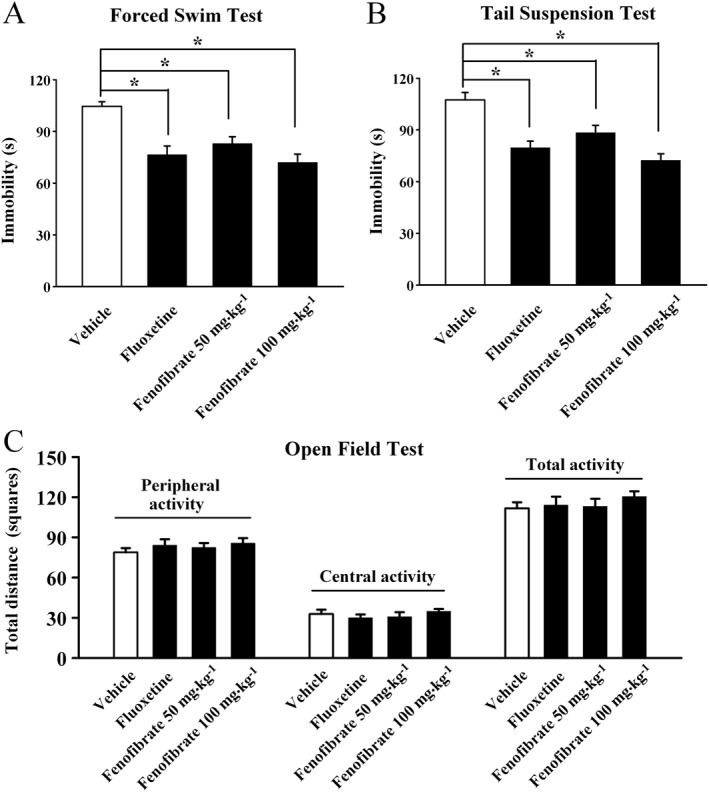
Fenofibrate produces antidepressant‐like effects in the FST and TST tests. C57BL/6J mice were injected i.p. with a single dose of vehicle (Control), fluoxetine (20 mg·kg−1), or fenofibrate (50, 100 mg·kg−1). The behavioural tests were conducted 30 min after the injection. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.). (A) Fenofibrate significantly decreased the immobility time of C57BL/6J mice in the FST test. (B) Fenofibrate significantly decreased the immobility time of C57BL/6J mice in the TST test. (C) Fenofibrate treatment produced no effects on spontaneous locomotor activity of mice in the open‐field test. The data are expressed as mean ± SEM (n = 11–12); * P < 0.05. Comparisons were made by one‐way ANOVA followed by post hoc LSD test.
Then the antidepressant‐like effects of fenofibrate were also assessed in the TST, another rapid and reliable method for screening antidepressants (Cryan and Holmes, 2005; Cryan and Slattery, 2007). Similar to the results of FST, fenofibrate treatment robustly decreased the immobility of mice in the TST, compared with the vehicle group (n = 11–12, P < 0.05 vs. vehicle; Figure 1B). The magnitude of the 100 mg·kg−1 fenofibrate‐induced anti‐immobility effect was comparable with that of 20 mg·kg−1 fluoxetine. A significant main effect of drug treatment [F(3, 42) = 13.544, P < 0.05] was also found.
To exclude the possible effects of fenofibrate on spontaneous locomotor activity that may contribute to immobility in the FST and TST (Bourin et al., 2001), naive mice treated with fenofibrate were exposed to the open‐field test. There were no significant differences found in the number of squares an animal crossed in the centre area or the periphery area between all the groups (n = 12, Figure 1C), and ANOVA revealed no effects for drug treatment [F(3, 44) = 0.518, P = 0.673]. These data indicate that fenofibrate may have antidepressant‐like activities.
Chronic fenofibrate treatment restores the CSDS‐induced depressive‐like symptoms
We further investigated the antidepressant‐like effects of fenofibrate in the CSDS model (Berton et al., 2006a). The social interaction test and sucrose preference test were performed. As shown in Figure 2B, while all the test mice spent a similar amount of time in the interaction zone when the CD1 mouse was absent, CSDS‐defeated mice spent about 64.1 ± 4.5% less time in the interaction zone than vehicle‐treated mice when the CD1 mouse was present (n = 12, P < 0.05 vs. vehicle), in accordance with previous reports (Tsankova et al., 2006). Interestingly, 14 days of treatment with fenofibrate fully reversed the CSDS‐induced decrease in social interaction, especially at 100 mg·kg−1 (n = 12, P < 0.05 vs. CSDS + vehicle), similar to fluoxetine. Two‐way ANOVA analysis revealed a significant interaction [F(3, 88) = 12.336] with significant effects for CSDS [F(1, 88) = 28.426] and drug treatment [F(3, 88) = 5.46]. Fenofibrate produced no significant effects on the social interaction of naive mice (n = 12).
Figure 2.
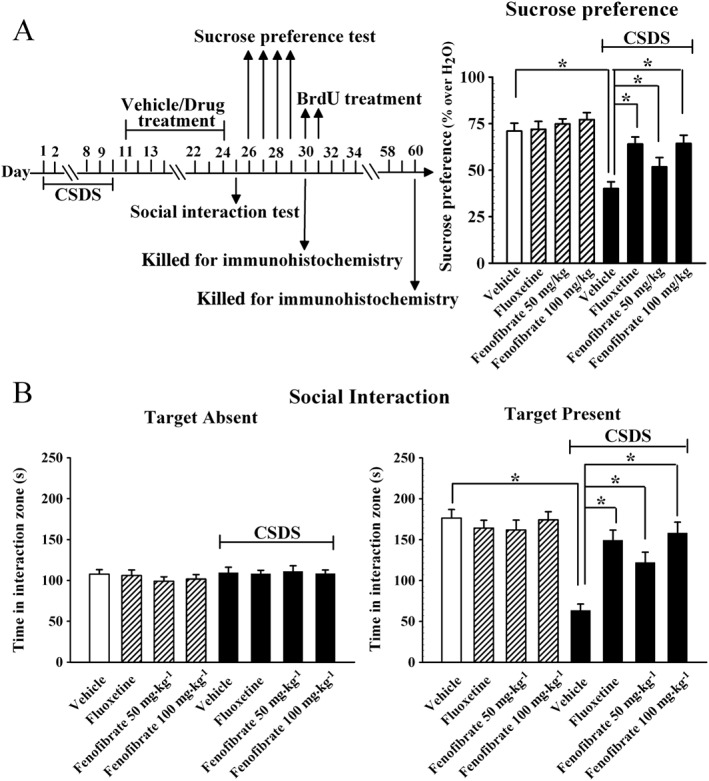
Fenofibrate has antidepressant‐like effects in the CSDS model. CSDS‐stressed mice received daily injections of vehicle, fluoxetine (20 mg·kg−1), or fenofibrate (50, 100 mg·kg−1) for 14 days, behavioural tests were then conducted. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.). (A) The antidepressant‐like effects of fenofibrate in the sucrose preference test. CSDS + fenofibrate mice displayed significantly higher sucrose preference than CSDS + vehicle mice. (B) The antidepressant‐like effects of fenofibrate in the social interaction test. CSDS + fenofibrate mice spent significantly more time engaged in social interaction than CSDS + vehicle mice. Data are expressed as means ± SEM (n = 12); * P < 0.05. Comparisons were made by two‐way ANOVA followed by post hoc Bonferroni's test.
Figure 2A illustrates the sucrose preference data. Two‐way ANOVA reported a significant interaction [F(3, 88) = 8.862] with significant effects for CSDS [F(1, 88) = 37.412] and drug treatment [F(3, 88) = 4.27]. Chronic stress produced a 43.5 ± 4.1% decrease in the sucrose preference of C57BL/6J mice compared with the vehicle group (n = 12, P < 0.05 vs. vehicle), and this change was fully restored by chronic fenofibrate treatment (n = 12, P < 0.05 vs. CSDS + vehicle). Statistical analysis further revealed that the sucrose consumption was increased by 29 ± 3.4% and 60.4 ± 5.8% with administration of 50 and 100 mg·kg−1 fenofibrate respectively. Fenofibrate also had no significant effects on the sucrose preference of naive mice (n = 12). Together, these data suggest that fenofibrate has antidepressant‐like effects.
Chronic fenofibrate treatment reverses the CSDS‐induced decrease in adult hippocampal neurogenesis
It is known that chronic stress induces not only depressive‐like behaviour but also decreased neuronal proliferation and differentiation in the DG of hippocampus (Lagace et al., 2010). Furthermore, adult hippocampal neurogenesis is also required for the effects of common antidepressants, like fluoxetine (Santarelli et al., 2003). We thus examined whether fenofibrate treatment can prevent the CSDS‐induced effects on adult hippocampal neurogenesis. In this study, neuronal proliferation was studied by DCX immunohistochemistry in the DG region, as previously described by us (Jiang et al., 2012). As shown in Figure 3A, CSDS induced a 52.9 ± 4.4% reduction in the number of DCX+ cells in the DG (n = 5, P < 0.05 vs. vehicle), while 100 mg·kg−1 fenofibrate treatment completely restored this change (n = 5, P < 0.05 vs. CSDS + vehicle), similar to fluoxetine. ANOVA indicated a significant difference between groups [F(4, 20) = 18.876].
Figure 3.
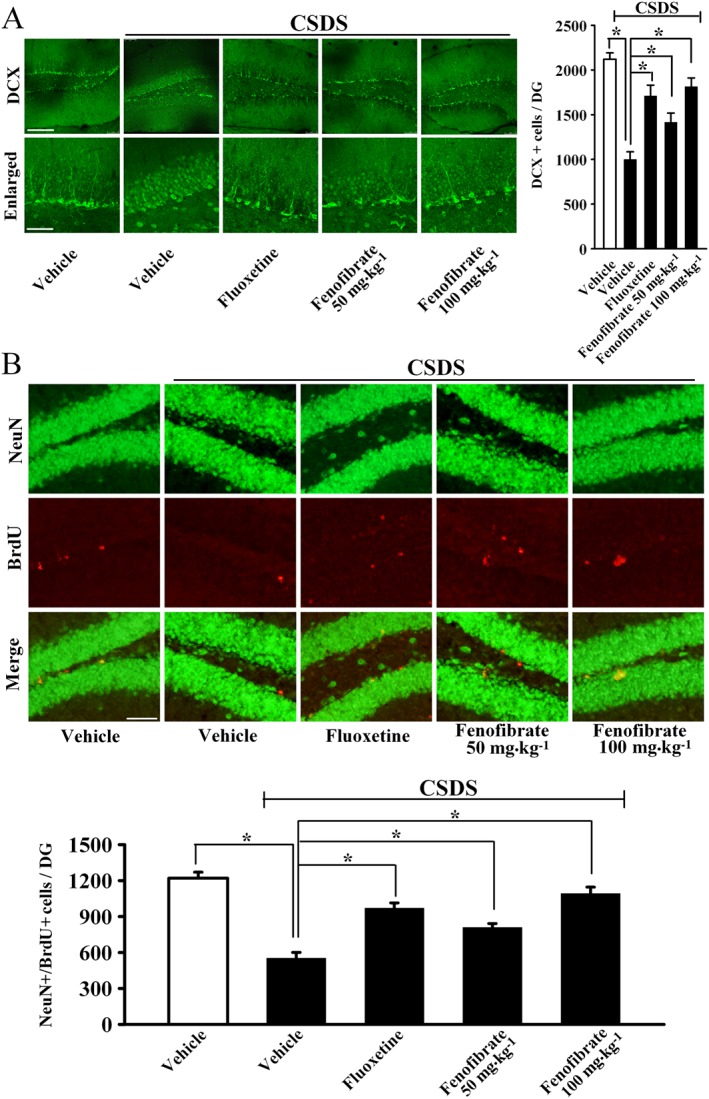
Fenofibrate administration restores the decreased adult hippocampal neurogenesis caused by CSDS stress. (A) Representative confocal microscopic images showing the localization of doublecortin (DCX; green) in the dentate gyrus (DG). The scale bar is 150 μm for representative images and 50 μm for enlarged images respectively. Density statistics showed that chronic fenofibrate treatment significantly increased the number of DCX‐stained cells in the DG of stressed mice. (B) Representative microscopic images showed the co‐staining (yellow) of neuronal nuclei (NeuN) (green) and BrdU (red) in the DG. The majority of BrdU+ cells are doubly labelled with the neuronal marker NeuN and located within the granule cell layer. The scale bar is 75 μm. Density statistics showed that fenofibrate treatment fully reversed the CSDS‐induced decrease in the number of NeuN+/BrdU+ cells in the DG. Data are expressed as means ± SEM (n = 5); * P < 0.05. Comparisons were made by one‐way ANOVA followed by post hoc LSD test.
Newly generated cells in the DG differentiate into mature neurons within 28 days after their birth (Kempermann et al., 2003). To determine whether the fenofibrate‐induced newborn cells differentiated into mature neurons, BrdU was administrated to label the proliferating cells, and neuronal nuclei (NeuN) was employed as a marker for the mature neurons. As shown in Figure 3B, CSDS resulted in a 54.8 ± 5.2% reduction in the number of NeuN+/BrdU+ co‐labelled cells in the DG (n = 5, P < 0.05 vs. vehicle), while this change was fully reversed by 100 mg·kg−1 fenofibrate (n = 5, P < 0.05 vs. CSDS + vehicle). ANOVA revealed a significant difference between groups [F(4, 20) = 21.546]. These data indicate that fenofibrate has protective effects on adult hippocampal neurogenesis.
The CSDS‐induced decrease in the hippocampal BDNF signalling pathway is reversed by fenofibrate
To investigate the possible mechanisms underlying the antidepressant‐like and neurogenic effects of fenofibrate, we examined the expression of BDNF, which is critical for adult hippocampal neurogenesis and is also closely involved in the pathogenesis of depression (Gourley et al., 2008; Lee and Son, 2009; Castren and Rantamaki, 2010). Therefore, western blotting experiments were performed to measure the hippocampal BDNF expression following CSDS and fenofibrate treatment, and β‐actin was selected as the internal control. Figure 4A shows that the BDNF level was significantly decreased in the hippocampus of mice exposed to CSDS compared with unstressed mice (n = 5, P < 0.05 vs. vehicle), while chronic fenofibrate treatment enhanced the BDNF expression by 44.7 ± 6.2% and 95.8 ± 7.4% at dosages of 50 and 100 mg·kg−1 respectively (n = 5, P < 0.05 vs. CSDS + vehicle). ANOVA indicated a significant difference between groups [F(4, 20) = 11.702]. Next, we examined the downstream signalling pathway molecules of BDNF: the activated (and phosphorylated) forms of ERK1/2 (pERK1/2), Akt (pAkt) and CREB (pCREB) (Lim et al., 2008). As shown in Figure 4A, chronic fenofibrate administration significantly reversed the CSDS‐induced decrease in the hippocampal pERK1/2 [ANOVA: F(4, 20) = 6.232], pAkt [ANOVA: F(4, 20) = 9.413] and pCREB [ANOVA: F(4, 20) = 19.475] expression (n = 5, P < 0.05 vs. CSDS). In contrast, the total ERK1/2, Akt and CREB levels were unchanged among all the groups.
Figure 4.
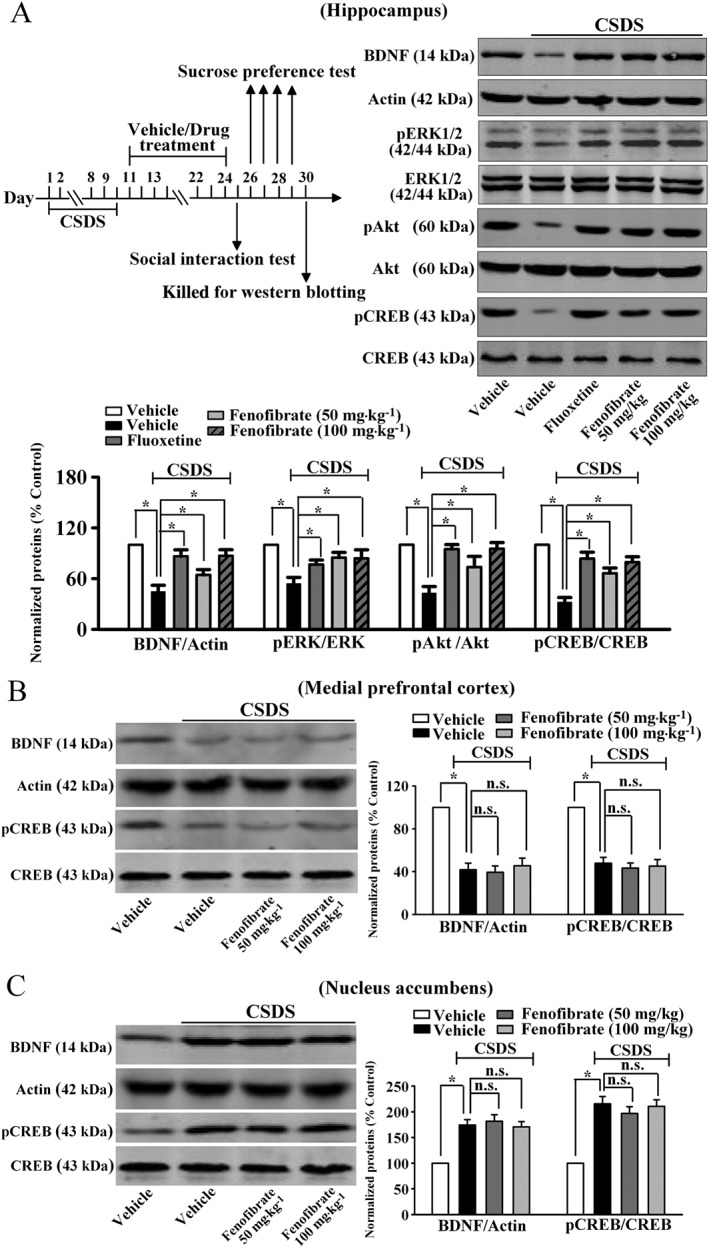
Fenofibrate treatment reverses the CSDS‐induced decrease in the hippocampal BDNF signalling pathway. (A) Our western blotting data showed that fenofibrate treatment restored the CSDS‐induced decrease in BDNF, pERK1/2, pAkt and pCREB protein levels in the hippocampus. CSDS + fenofibrate mice displayed significantly higher expression of BDNF, pERK1/2, pAkt and pCREB in the hippocampus than in that of CSDS + vehicle mice. (B) Fenofibrate treatment could not reverse the CSDS‐induced decrease in BDNF and pCREB expression in the mPFC. (C) Fenofibrate treatment produced no effects on the CSDS‐induced enhancement of BDNF and pCREB levels in the NAc. Data are expressed as means ± SEM (n = 5); * P < 0.05; n.s., no significance. Comparisons were made by one‐way ANOVA followed by post hoc LSD test.
Other brain regions, especially the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc), are also implicated in depression (Di Chiara et al., 1999; Li et al., 2010). Previous reports showed that chronic stress reduced the expression of BDNF and pCREB in the mPFC (Gourley et al., 2008; Castren and Rantamaki, 2010), while the expression of BDNF and pCREB were enhanced in the NAc (Eisch et al., 2003; Krishnan et al., 2007). However, it was found that fenofibrate treatment could not restore the stress‐induced changes in the BDNF signalling pathway in the two regions (Figure 4B and C). Collectively, these results indicate that the hippocampal BDNF signalling cascade may be involved in the antidepressant‐like effects of fenofibrate.
The antidepressant‐like effects of fenofibrate require central PPAR‐α
To explore whether PPAR‐α is required for the antidepressant‐like effects of fenofibrate, the selective PPAR‐α inhibitor GW6471 was used. Mice were first daily infused with GW6471 for 3 days, then injected with fenofibrate (100 mg·kg−1), and followed by the FST. Figure 5A shows that GW6471 pretreatment fully blocked the effects of fenofibrate in the FST (n = 12), and ANOVA showed a significant interaction [F(1, 44) = 10.669] with significant effects for GW6471 [F(1, 44) = 5.439] and fenofibrate [F(1, 44) = 12.842]. Similarly, GW6471 pretreatment also prevented the effects of fenofibrate in the TST (n = 11–12, Figure 5B), and ANOVA revealed a significant interaction [F(1, 43) = 9.305] with significant effects for GW6471 [F(1, 43) = 6.817] and fenofibrate [F(1, 43) = 16.036]. GW6471 alone produced no effects in the FST or TST (n = 12), and the open field test results showed that mice in all the groups had equal locomotor activity (n = 12, Figure 5C). Moreover, CSDS‐stressed mice were co‐treated with fenofibrate (100 mg·kg−1) and GW6471 for 14 days, and followed by behavioural tests. Figure 5E and D showed that GW6471 significantly blocked the effects of fenofibrate in the social interaction test [ANOVA: fenofibrate, F(1, 44) = 25.228; GW6471, F(1, 44) = 20.014; interaction, F(1, 44) = 32.426; n = 12] and sucrose preference test [ANOVA: fenofibrate, F(1, 44) = 15.537; GW6471, F(1, 44) = 8.375; interaction, F(1, 44) = 11.906; n = 12] respectively.
Figure 5.
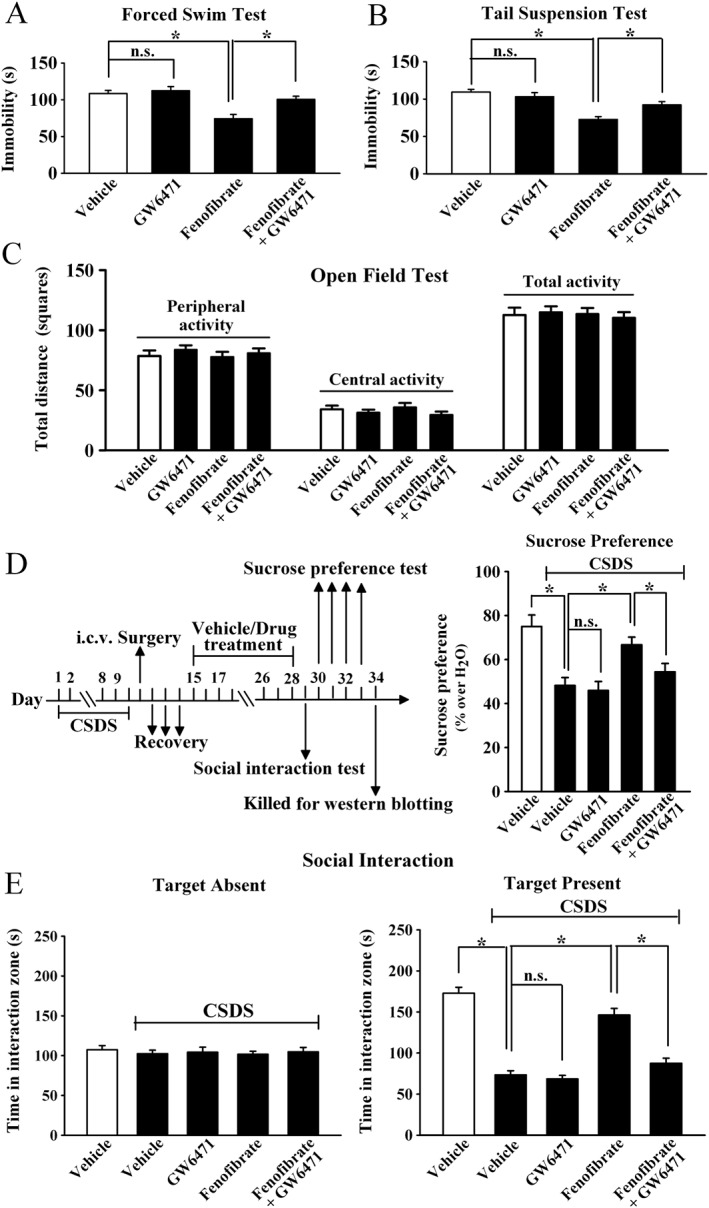
Blockade of PPAR‐α by GW6471 prevents the antidepressant effects of fenofibrate. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.) + ACSF with 1% DMSO (i.c.v.). (A) GW6471 pretreatment before fenofibrate administration prevented the fenofibrate‐induced decrease in immobility in the FST. (B) GW6471 pretreatment also prevented the fenofibrate‐induced decrease in immobility in the TST test. (C) GW6471 did not influence the locomotor activity of mice in the open field test. (D) CSDS mice were co‐injected with fenofibrate and GW6471 for 14 days. CSDS + fenofibrate + GW6471 mice displayed significantly lower sucrose preference than CSDS + fenofibrate mice. (E) CSDS + fenofibrate + GW6471 mice also displayed significantly lower social interaction than CSDS + fenofibrate mice. Results are expressed as means ± SEM (n = 11–12); * P < 0.05; n.s., no signifcance. Comparisons were made by two‐way ANOVA followed by post hoc Bonferroni's test.
Furthermore, the usage of GW6471 also blocked the effects of fenofibrate on the hippocampal BDNF signalling pathway, as fenofibrate + GW6471 + CSDS mice had significantly less BDNF [ANOVA: fenofibrate, F(1, 16) = 13.065; GW6471, F(1, 16) = 6.223; interaction, F(1, 16) = 10.847] and pCREB [ANOVA: fenofibrate, F(1, 16) = 12.616; GW6471, F(1, 16) = 7.144; interaction, F(1, 16) = 14.107] expression in the hippocampus than fenofibrate + CSDS mice (n = 5, Figure 6). Together, the antidepressant‐like effects of fenofibrate require PPAR‐α in the brain.
Figure 6.
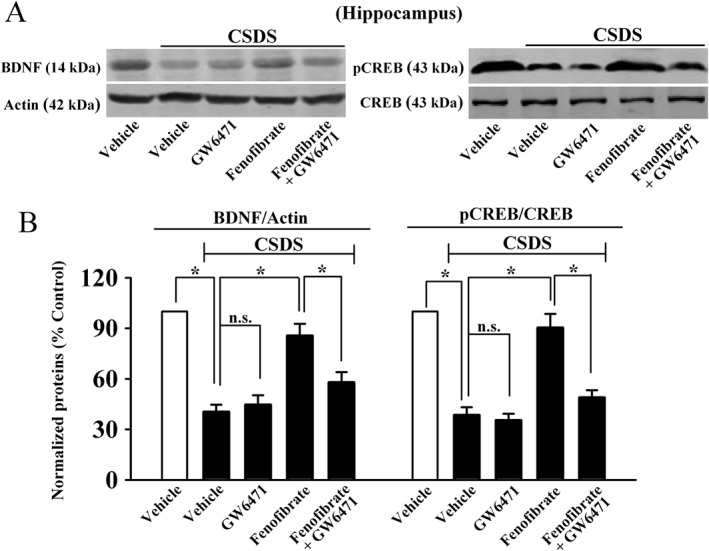
GW6471 also prevents the effects of fenofibrate on the hippocampal BDNF signalling pathway. (A) Representative images of our western blotting data. (B) Statistical analysis revealed that GW6471 abolished the fenofibrate‐induced promotion of hippocampal BDNF and pCREB expression, as CSDS + fenofibrate + GW6471 mice displayed significantly lower BDNF and pCREB levels in the hippocampus than CSDS + fenofibrate mice. Data are expressed as means ± SEM (n = 5); * P < 0.05; n.s., no signifcance. Comparisons were made by two‐way ANOVA followed by post hoc Bonferroni's test.
The antidepressant‐like effects of fenofibrate do not require the cannabinoid system
Recent studies have shown that in addition to PPAR‐α, fenofibrate is also an agonist of the cannabinoid receptors (Nickolls et al., 2015). Since the cannabinoid system is also implicated in depression (Hill et al., 2009), the selective CB1 receptor antagonist rimonabant and CB2 receptor antagonist AM630 were used. Mice were first daily infused with rimonabant or AM630 for 3 days, then injected with fenofibrate (100 mg·kg−1), and followed by the FST. Figure S1A shows that neither rimonabant [ANOVA: fenofibrate, F(1, 44) = 46.682, P < 0.05; rimonabant, F(1, 44) = 0.256, P = 0.616] nor AM630 [ANOVA: fenofibrate, F(1, 44) = 52.957, P < 0.05; AM630, F(1, 44) = 0.334, P = 0.572] prevented the effects of fenofibrate in the FST (n = 12). Similarly. neither rimonabant [ANOVA: fenofibrate, F(1, 43) = 44.882, P < 0.05; rimonabant, F(1, 43) = 0.549, P = 0.426] nor AM630 [ANOVA: fenofibrate, F(1, 41) = 39.585, P < 0.05; AM630, F(1, 41) = 0.879, P = 0.341] blocked the effects of fenofibrate in the TST (n = 10–12, Figure S1B). The open field test results show that mice in all the groups had equal locomotor activity (n = 12, Figure S1C). Furthermore, CSDS‐stressed mice were co‐treated with fenofibrate (100 mg·kg−1) and rimonabant/AM630 for 14 days, and we found that both rimonabant [ANOVA for Figure S1E: fenofibrate, F(1, 43) = 60.355, P < 0.05; rimonabant, F(1, 43) = 0.776, P = 0.378; ANOVA for Figure S1D: fenofibrate, F(1, 43) = 36.854, P < 0.05; rimonabant, F(1, 43) = 1.112, P = 0.167] and AM630 [ANOVA for Figure S1E: fenofibrate, F(1, 43) = 53.702, P < 0.05; AM630, F(1, 43) = 0.904, P = 0.312; ANOVA for Figure S1D: fenofibrate, F(1, 43) = 29.449, P < 0.05; AM630, F(1, 43) = 1.084, P = 0.181] did not influence the antidepressant‐like effects of fenofibrate in the social interaction test (n = 12, Figure S1E) and sucrose preference test (n = 12, Figure S1D). These results suggest that the antidepressant‐like effects of fenofibrate do not require the cannabinoid system.
Fenofibrate produces antidepressant‐like effects through the hippocampal BDNF signalling pathway
To determine whether the antidepressant effects of fenofibrate require the BDNF system, the potent pharmacological inhibitor of the BDNF receptor TrkB, K252a (Yan et al., 2010), was applied. C57BL/6J mice were first daily infused with K252a for 3 days, then treated with fenofibrate (100 mg·kg−1), and followed by the FST or TST. While K252a alone produced no effects in the FST or TST (n = 12), K252a pretreatment significantly blocked the fenofibrate‐induced antidepressant‐like effects in the FST [ANOVA: fenofibrate, F(1, 44) = 17.223; K252a, F(1, 44) = 8.418; interaction, F(1, 44) = 14.036; n = 12, Figure S2A] and TST [ANOVA: fenofibrate, F(1, 44) = 19.204; K252a, F(1, 44) = 6.993; interaction, F(1, 44) = 10.655; n = 12, Figure S2B]. The open field test results showed that mice in all the groups had equal locomotor activity (n = 12, Figure S2C). Moreover, CSDS‐stressed mice were co‐treated with fenofibrate (100 mg·kg−1) and K252a for 14 days, and behavioural tests were then performed. Co‐treatment K252a with fenofibrate significantly prevented the antidepressant‐like effects of fenofibrate in the social interaction test [ANOVA: fenofibrate, F(1, 44) = 24.117; K252a, F(1, 44) = 19.757; interaction, F(1, 44) = 26.489; n = 12, Figure S2E] and sucrose preference test [ANOVA: fenofibrate, F(1, 44) = 15.077; K252a, F(1, 44) = 8.336; interaction, F(1, 44) = 7.671; n = 12, Figure S2D].
In a parallel series, an anti‐BDNF antibody was used to specifically block the BDNF signalling pathway (Zhu et al., 2010). Mice were first daily infused with anti‐BDNF antibody for 3 days, then treated with fenofibrate (100 mg·kg−1), and followed by the FST or TST. It was found that infusion of the anti‐BDNF antibody alone enhanced the immobility of test mice in the FST and TST (n = 11–12, P < 0.05 vs. vehicle; Figure 7A and B), consistent with previous reports showing that a deficiency in BDNF induces depressive‐like behaviour in mice (Monteggia et al., 2007). More importantly, infusion of the anti‐BDNF antibody fully abolished the antidepressant‐like effects of fenofibrate in the FST [ANOVA: fenofibrate, F(1, 44) = 14.34; anti‐BDNF, F(1, 44) = 36.477; interaction, F(1, 44) = 17.903; n = 12, Figure 7A] and TST [ANOVA: fenofibrate, F(1, 43) = 6.397; anti‐BDNF, F(1, 43) = 33.968; interaction, F(1, 43) = 14.875; n = 11–12, Figure 7B]. The open field test results also indicate that mice in all the groups had equal locomotor activity (n = 11–12, Figure 7C). Moreover, CSDS‐stressed mice were co‐treated with fenofibrate (100 mg·kg−1) and anti‐BDNF antibody for 14 days, and behavioural tests were then performed. As expected, infusion of the anti‐BDNF antibody also blocked the antidepressant‐like effects of fenofibrate in the social interaction test [ANOVA: fenofibrate, F(1, 42) = 33.85; anti‐BDNF, F(1, 42) = 15.407; interaction, F(1, 42) = 29.769; n = 11–12, Figure 7E] and sucrose preference test [ANOVA: fenofibrate, F(1, 42) = 12.811; anti‐BDNF, F(1, 42) = 4.901; interaction, F(1, 42) = 6.78; n = 11–12, Figure 7D].
Figure 7.
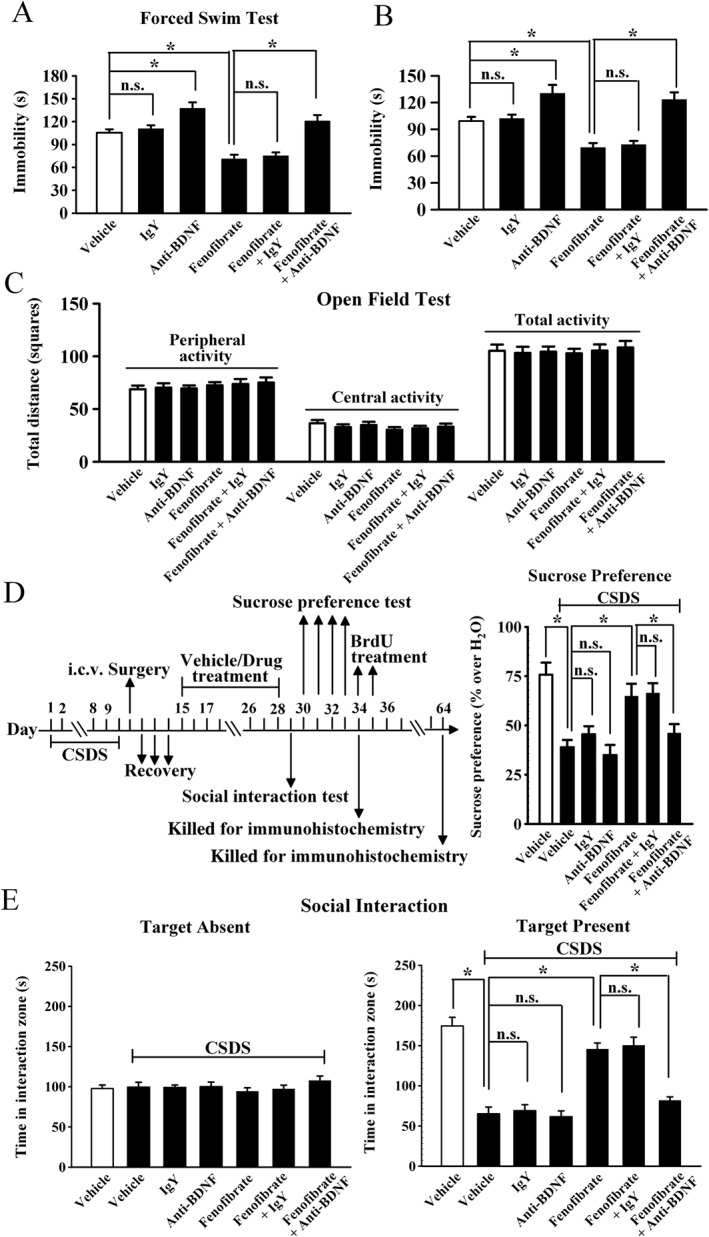
Blockade of the BDNF signalling cascade by anti‐BDNF infusion abolishes the antidepressant effects of fenofibrate. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.) + ACSF (i.c.v.). (A) Pre‐infusion of anti‐BDNF antibody significantly blocked the fenofibrate‐induced decrease in immobility in the FST. (B) Pre‐infusion of anti‐BDNF antibody also prevented the fenofibrate‐induced decrease in immobility in the TST. (C) Anti‐BDNF infusion did not influence the locomotor activity of mice in the open field test. (D) CSDS mice were co‐treated with fenofibrate and anti‐BDNF antibody for 14 days. CSDS + fenofibrate + anti‐BDNF mice displayed signifcantly lower sucrose preference than CSDS + fenofibrate mice. (E) Co‐treatment of fenofibrate with anti‐BDNF antibody also blocked the antidepressant effects of fenofibrate in the social interaction test. CSDS + fenofibrate + anti‐BDNF mice displayed signifcantly lower social interaction than CSDS + fenofibrate mice. Results are expressed as means ± SEM (n = 11–12); * P < 0.05; n.s., no signifcance. Comparisons were made by two‐way ANOVA followed by post hoc Bonferroni's test.
Furthermore, we examined whether the anti‐BDNF infusion blocked the effects of fenofibrate on the BDNF signalling cascade and adult hippocampal neurogenesis. The immunohistochemical data are summarized in Figure 8, and similar to the behavioural data, anti‐BDNF infusion significantly prevented the protective effects of fenofibrate on neuronal proliferation [ANOVA: fenofibrate, F(1, 16) = 31.104; anti‐BDNF, F(1, 16) = 37.101; interaction, F(1, 16) = 31.83; n = 5, Figure 8A] and differentiation [ANOVA: fenofibrate, F(1, 16) = 25.923; anti‐BDNF, F(1, 16) = 28.418; interaction, F(1, 16) = 23.705; n = 5, Figure 8B] in the DG. The western blotting data are summarized in Figure 9, and it was found that CSDS + fenofibrate + anti‐BDNF mice displayed significantly less BDNF [ANOVA: fenofibrate, F(1, 16) = 13.294; anti‐BDNF, F(1, 16) = 5.296; interaction, F(1, 16) = 12.754; n = 5], pERK1/2 [ANOVA: fenofibrate, F(1, 16) = 5.367; anti‐BDNF, F(1, 16) = 5.897; interaction, F(1, 16) = 6.366; n = 5], pAkt [ANOVA: fenofibrate, F(1, 16) = 15.435; anti‐BDNF, F(1, 16) = 5.244; interaction, F(1, 16) = 9.352; n = 5] and pCREB [ANOVA: fenofibrate, F(1, 16) = 10.973; anti‐BDNF, F(1, 16) = 6.181; interaction, F(1, 16) = 14.619; n = 5] expression in the hippocampus than CSDS + fenofibrate mice. Collectively, these results indicate that the BDNF signalling cascade is necessary for the antidepressant effects of fenofibrate.
Figure 8.
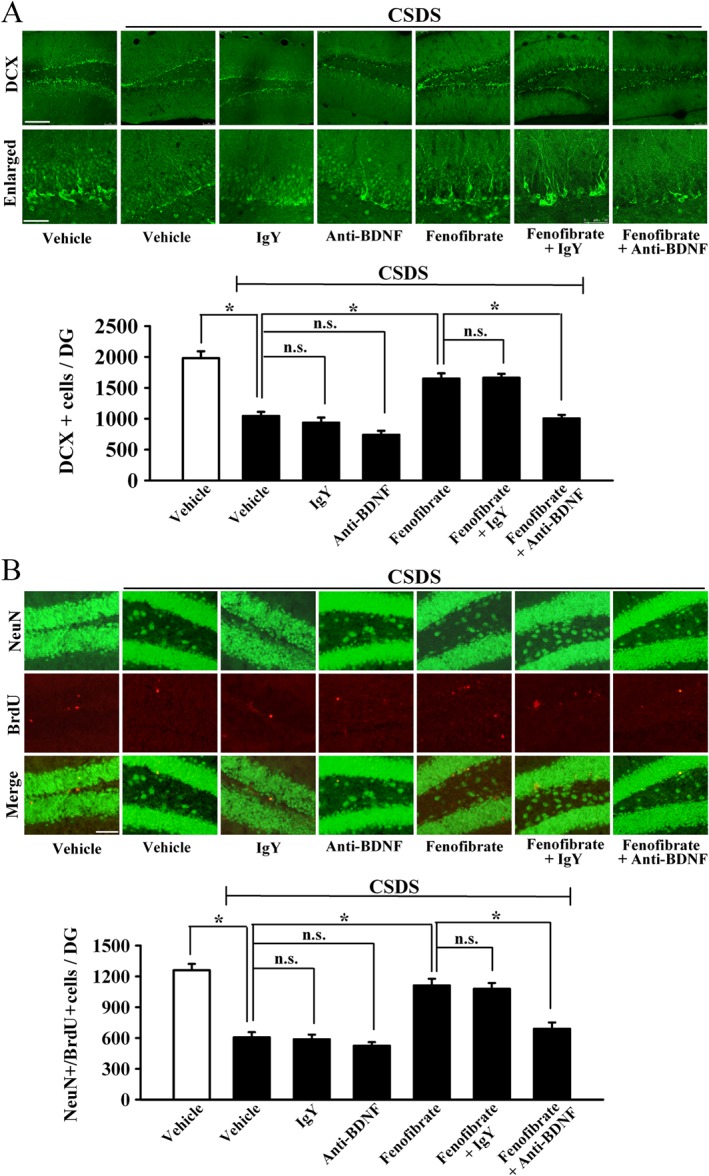
Anti‐BDNF infusion prevents the neurogenic effects of fenofibrate. (A) Representative confocal microscopic images show the staining of doublecortin (DCX; green) in the dentate granule cell layer. The scale bar is 150 μm for representative images and 50 μm for enlarged images respectively. Density statistics show that the increased number of DCX‐positive cells induced by fenofibrate was blocked by an infusion of anti‐BDNF antibody. (B) Representative microscopic images show the co‐staining (yellow) of neuronal nuclei (NeuN) (green) and BrdU (red) in the DG. The scale bar is 75 μm. Density statistics show that co‐treatment with anti‐BDNF antibody abolished the fenofibrate‐induced effects on the amount of NeuN+/BrdU+ cells in the DG. Data are expressed as means ± SEM (n = 5); * P < 0.05; n.s., no signifcance. Comparisons were made by two‐way ANOVA followed by post hoc Bonferroni's test.
Figure 9.
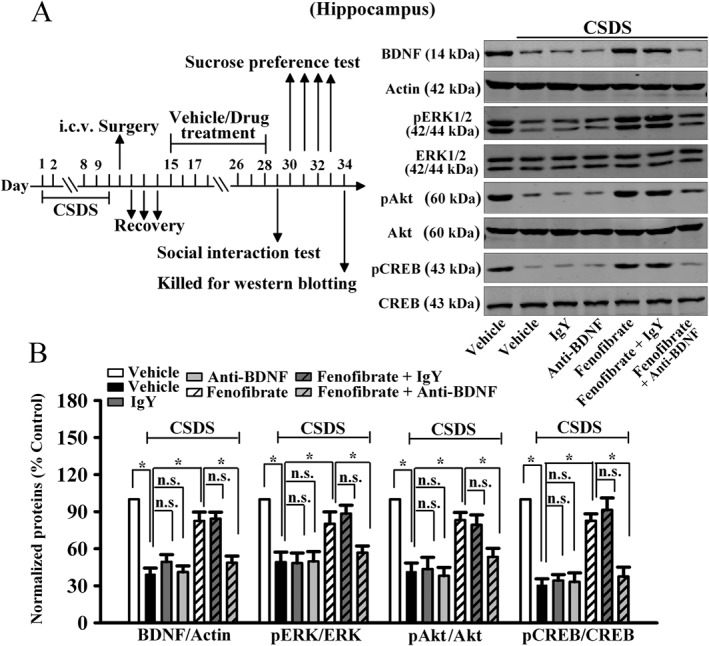
Anti‐BDNF infusion also blocks the effects of fenofibrate on the hippocampal BDNF signalling pathway. (A) Representative images of our western blotting data. (B) Quantitative analysis indicated that anti‐BDNF infusion prevented the fenofibrate‐induced enhancement of hippocampal BDNF, pERK1/2, pAkt and pCREB expression. CSDS + fenofibrate + anti‐BDNF mice displayed significantly lower BDNF, pERK1/2, pAkt and pCREB levels in the hippocampus than CSDS + fenofibrate mice. Data are expressed as means ± SEM (n = 5); * P < 0.05; n.s., no signifcance. Comparisons were made by two‐way ANOVA followed by post hoc Bonferroni's test.
Discussion
The major findings of this study are as follows. Firstly, fenofibrate has antidepressant‐like effects in the FST, TST and CSDS model. Secondly, the antidepressant‐like effects of fenofibrate require PPAR‐α and the BDNF signalling cascade in the brain.
Although fenofibrate is clinically used as a hypolipidaemic drug, more and more reports have been demonstrating the effects of fenofibrate on the CNS. To the best of our knowledge, our study is the most comprehensive study showing that fenofibrate has beneficial effects against depression, a most burdensome neuropsychiatric disease worldwide. This finding is very interesting and exciting as it has identified a new potential antidepressant. Our data revealed that fenofibrate has properties common to classical antidepressants in the FST and TST, two tests with high predictive validity for detecting antidepressant activities (Cryan and Holmes, 2005; Cryan and Slattery, 2007). Moreover, the reduction of immobility in the FST and TST induced by a single injection of fenofibrate was not paralleled by an increase in locomotor activity of mice, suggesting that fenofibrate may have potential as a treatment for depression. The CSDS model was further used to investigate the effects of fenofibrate. It was found that a consecutive injection of fenofibrate for 14 days significantly ameliorated not only the CSDS‐induced social avoidance and anhedonia but also the CSDS‐induced decrease in the hippocampal BDNF signalling cascade and adult hippocampal neurogenesis. The western blotting results for mPFC and NAc samples are also interesting, indicating that the effects of fenofibrate on the central BDNF system are region‐selective, and this needs more study. Collectively, these findings indicate that fenofibrate could be developed as a novel antidepressant.
Fenofibrate is identified as a selective agonist of PPAR‐α, which is a transcription factor that regulates the genes involved in fatty acid metabolism. In this study, the most important reason for assuming that fenofibrate may possess antidepressant‐like effects comes from our previous report showing that WY14643, another agonist of PPAR‐α, has antidepressant‐like effects in mice (Jiang et al., 2015b). Roy et al. (2013) reported that PPAR‐α is also widely expressed in the nuclei of hippocampal neurons and controls the expression of various plasticity‐related proteins via the direct transcriptional regulation of CREB. Moreover, it was found that simvastatin up‐regulated the hippocampal BDNF expression via the PPAR‐α‐mediated transcriptional activation of CREB (Roy et al., 2015), and simvastatin exerted antidepressant‐like effects in rats exposed to chronic mild stress (Lin et al., 2014). Thus, it is possible that fenofibrate produces antidepressant‐like effects via the PPAR‐α‐mediated transcriptional activation of CREB to enhance the hippocampal BDNF expression. In this study, the PPAR‐α inhibitor GW6471 was found to block not only the effects of fenofibrate in the behavioural tests but also the effects of fenofibrate on hippocampal BDNF expression, as expected. These results are very interesting and indicate that central PPAR‐α could be a novel antidepressant target, which needs further study. In addition, there is other evidence implying that fenofibrate has antidepressant‐like effects. Firstly, Barbiero et al. (2014) demonstrated that fenofibrate significantly reduces the immobility time and increases the swimming time of MPTP‐treated rats in the FST, which is direct evidence showing the potential of fenofibrate as a antidepressant medication. Secondly, Ramanan et al. (2009) reported that fenofibrate prevented the whole‐brain irradiation‐induced decrease in adult hippocampal neurogenesis by promoting the survival of newborn cells in the DG. It is known that adult hippocampal neurogenesis is closely involved in the pathophysiology of depression (Shelton, 2007; Krishnan and Nestler, 2008), and our immunohistochemical results are consistent with those of Ramanan et al. (2009). Lastly, Ji et al. (2015) found that the activation of hippocampal PPAR‐δ (also called PPAR‐β) prevented stress‐induced depressive‐like behaviour and enhanced the adult hippocampal neurogenesis. Since PPAR‐α and PPAR‐β share similar biological structures, the activation of the two nuclear receptors may produce similar effects including antidepressant‐like effects.
Recently, Nickolls et al. (2015) found that fenofibrate also acts as an agonist of the CB2 receptor and a partial agonist of the CB1 receptor. It has been demonstrated that chronic stress leads to widespread reductions in anandamide concentrations throughout the brain, together with decreased CB1 receptor signal transduction in subcortical structures, such as the hippocampus, while the direct pharmacological activation of the CB1 receptor produces antidepressant‐like responses in the FST (Hill et al., 2005, 2008, 2009; Jiang et al., 2005; Bambico et al., 2007). Thus, it is possible that like PPAR‐α, the cannabinoid system also plays a role in the antidepressant‐like effects of fenofibrate. However, we found that the cannabinoid receptor antagonists, rimonabant and AM630, did not influence the effects of fenofibrate. One explanation of these results may be that the partial activating effects of fenofibrate on the CB1 receptor are not sufficient to induce an antidepressant‐like effect.
Moreover, the finding that fenofibrate could reverse the CSDS‐induced effects on the hippocampal BDNF signalling cascade is exciting. This study is the first to provide experimental evidence showing that fenofibrate has effects on the BDNF system. It is known that BDNF has a lot of physiological effects in the brain, playing critical roles in learning and memory, neurogenesis, neuronal survival and so on (Ghosh et al., 1994; Radecki et al., 2005; Rossi et al., 2006; Lipsky and Marini, 2007; Cunha et al., 2010). BDNF is implicated in the pathophysiology of not only depression but also other neurological disorders, such as Alzheimer's disease (Budni et al., 2015). Thus, fenofibrate may have more pharmacological effects involving BDNF.
In addition, pharmacokinetic studies have revealed that fenofibrate is rapidly metabolized to fenofibric acid in vivo, which is responsible for the majority of its clinical effects (Strain et al., 2010). Fenofibric acid can also activate the PPAR‐α. Therefore, the antidepressant‐like effects of fenofibrate observed in our study may actually be due to fenofibric acid, and this needs to be investigated further.
Collectively, the results of this study reveal that fenofibrate induces antidepressant‐like effects in mice via the PPAR‐α‐mediated promotion of the hippocampal BDNF signalling pathway, providing a new insight into understanding the pharmacological effects of fenofibrate and shedding light on the development of new antidepressants with higher efficacy and fewer side effects.
Author contributions
B.J. and W.Z. conceived and designed this study. B.J., Y.‐J.W., H.W. and L.S. performed the experiments. B.J. and W.Z. wrote the manuscript. B.J., Q.Z. and F.W. reviewed and edited this manuscript. All the authors read and approved this manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 The antidepressant‐like actions of fenofibrate occur independent of the cannabinoid system. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.) + ACSF with 1% DMSO (i.c.v.). (A) Blocking cannabinoid system with rimonabant/AM630 had no influence on the antidepressant‐like effects of fenofibrate in the FST. (B) Rimonabant/AM630 pretreatment could not eliminate the antidepressant‐like effects of fenofibrate in the TST. (C) Rimonabant and AM630 did not influence the locomotor activity of mice in the open field test. (D) Co‐treatment fenofibrate with rimonabant/AM630 did not block the effects of fenofibrate in the sucrose preference test, since CSDS + fenofibrate + rimonabant/AM630 mice had as much sucrose consumption as CSDS + fenofibrate mice. (E) Also, in the social interaction test, CSDS + fenofibrate + rimonabant/AM630 mice did not differ significantly from CSDS + fenofibrate mice. Results are expressed as means ± S.E.M. (n = 10–12); ** P < 0.01; n.s., no signifcance. Comparison was made by two‐way ANOVA followed by post hoc Bonferroni's test.
Figure S2 Blockade of BDNF signalling cascade by K252a prevents the antidepressant actions of fenofibrate. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.) + ACSF with 1% DMSO (i.c.v.). (A) K252a pretreatment before fenofibrate administration prevented the fenofibrate‐induced decrease of immobility in the FST test. (B) K252a pretreatment also prevented the fenofibrate‐induced decrease of immobility in the TST test. (C) K252a did not influence the locomotor activity of mice in the open field test. (D) CSDS mice were co‐injected with fenofibrate and K252a for 14 days. CSDS + fenofibrate + K252a mice displayed significantly lower sucrose preference than CSDS + fenofibrate mice. (E) Co‐treatment fenofibrate with K252a also blocked the effects of fenofibrate in the social interaction test. CSDS + fenofibrate + K252a mice displayed significantly lower social interaction than CSDS + fenofibrate mice. Results are expressed as means ± S.E.M. (n = 12); * P < 0.05; ** P < 0.01; n.s., no signifcance. Comparison was made by two‐way ANOVA followed by post hoc Bonferroni's test.
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China to B J (no. 81401116), a grant from the Provincial Natural Science Foundation of Jiangsu Province (China) to B J (no. 14KJB310013), and a grant from the Provincial Natural Science Foundation of Jiangsu Province (China) to W Z (no. BK20151276).
Jiang, B. , Wang, Y. ‐J. , Wang, H. , Song, L. , Huang, C. , Zhu, Q. , Wu, F. , and Zhang, W. (2017) Antidepressant‐like effects of fenofibrate in mice via the hippocampal brain‐derived neurotrophic factor signalling pathway. British Journal of Pharmacology, 174: 177–194. doi: 10.1111/bph.13668.
Contributor Information
Bo Jiang, Email: jiangbo78099@ntu.edu.cn.
Wei Zhang, Email: huanghezhi36020@126.com.
References
- Advani T, Koek W, Hensler JG (2009). Gender differences in the enhanced vulnerability of BDNF+/− mice to mild stress. Int J Neuropsychopharmacol 12: 583–588. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G (2007). Cannabinoids elicit antidepressant‐like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci 27: 11700–11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiero JK, Santiago R, Tonin FS, Boschen S, da Silva LM, Werner MF et al. (2014). PPAR‐α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry 53: 35–44. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ et al. (2006a). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–868. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ (2006b). New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7: 137–151. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Kessler RC, McGonagle KA, Swartz MS (1994). The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry 151: 979–986. [DOI] [PubMed] [Google Scholar]
- Blendy JA (2006). The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59: 1144–1150. [DOI] [PubMed] [Google Scholar]
- Bourin M, Fiocco AJ, Clenet F (2001). How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16: 9–21. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Neuhaus‐Steinmetz U, Quarcoo D, Glaab T, McGregor GP et al. (2004). Brain‐derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol 141: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budni J, Bellettini‐Santos T, Mina F, Garcez ML, Zugno AI (2015). The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis 6: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E, Rantamaki T (2010). The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol 70: 289–297. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS (2001). Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry 49: 753–762. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A et al. (2005). Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood FlowMetab 25: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro R, Russo E, Scicchitano F, van Rijn CM, Cosco D, Avagliano C et al. (2013). Antiepileptic action of N‐palmitoylethanolamine through CB1 and PPAR‐α receptor activation in a genetic model of absence epilepsy. Neuropharmacology 69: 115–126. [DOI] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O et al. (2009). Antidepressant actions of histone deacetylase inhibitors. J Neurosci 29: 11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4: 775–790. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA (2007). Animal models of mood disorders: recent developments. Curr Opin Psychiatry 20: 1–7. [DOI] [PubMed] [Google Scholar]
- Cui J, Jiang L, Xiang H (2012). Ginsenoside Rb3 exerts antidepressant‐like effects in several animal models. J Psychopharmacol 26: 697–713. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL (2010). A simple role for BDNF in learning and memory? Front Mol Neurosci 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G (1999). Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry 46: 1624–1633. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M et al. (2003). Brain‐derived neurotrophic factor in the ventral midbrain‐nucleus accumbens pathway: a role in depression. Biol Psychiatry 54: 994–1005. [DOI] [PubMed] [Google Scholar]
- Fang Q, Han ZL, Li N, Wang ZL, He N, Wang R (2012). Effects of neuropeptide FF system on CB(1) and CB(2) receptors mediated antinociception in mice. Neuropharmacology 62: 855–864. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA (2007). CREB, neurogenesis and depression. Bioessays 29: 957–961. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME (1994). Requirement for BDNF in activity‐dependent survival of cortical neurons. Science 263: 1618–1623. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS et al. (2008). Regionally specific regulation of ERK MAP kinase in a model of antidepressant‐sensitive chronic depression. Biol Psychiatry 63: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ et al. (2008). Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem 106: 2322–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G (2009). The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci 30: 484–493. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ et al. (2005). Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 30: 508–515. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I (2005). Central administration of IGF‐I and BDNF leads to long‐lasting antidepressant‐like effects. Brain Res 1037: 204–208. [DOI] [PubMed] [Google Scholar]
- Hough LB, Svokos K, Nalwalk JW (2009). Non‐opioid antinociception produced by brain stem injections of improgan: significance of local, but not cross‐regional, cannabinoid mechanisms. Brain Res 1247: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji MJ, Yu XB, Mei ZL, An YQ, Tang SS, Hu M et al. (2015). Hippocampal PPARδ overexpression or activation represses stress‐induced depressive behaviors and enhances neurogenesis. Int J Neuropsychopharmacol 19. doi: 10.1093/ijnp/pyv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang C, Chen XF, Tong LJ, Zhang W (2015a). Tetramethylpyrazine produces antidepressant‐like effects in mice through promotion of BDNF signaling pathway. Int J Neuropsychopharmacol 18. doi: 10.1093/ijnp/pyv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang C, Zhu Q, Tong LJ, Zhang W (2015b). WY14643 produces anti‐depressant‐like effects in mice via the BDNF signaling pathway. Psychopharmacology (Berl) 232: 1629–1642. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang F, Yang S, Fang P, Deng ZF, Xiao JL et al. (2015c). SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF–TrkB pathway. Int J Neuropsychopharmacol 18. doi: 10.1093/ijnp/pyu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Wang W, Wang F, Hu ZL, Xiao JL, Yang S et al. (2013). The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol Psychiatry 74: 145–155. [DOI] [PubMed] [Google Scholar]
- Jiang B, Xiong Z, Yang J, Wang W, Wang Y, Hu ZL et al. (2012). Antidepressant‐like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol 166: 1872–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G et al. (2005). Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic‐ and antidepressant‐like effects. J Clin Invest 115: 3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM, Croom KF (2007). Fenofibrate: a review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs 67: 121–153. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003). Early determination and long‐term persistence of adult‐generated new neurons in the hippocampus of mice. Development 130: 391–399. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T et al. (2009). MyD88 signaling in the CNS is required for development of fatty acid‐induced leptin resistance and diet‐induced obesity. Cell Metab 10: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2008). The molecular neurobiology of depression. Nature 455: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O et al. (2010). Adult hippocampal neurogenesis is functionally important for stress‐induced social avoidance. Proc Natl Acad Sci U S A 107: 4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Son H (2009). Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 42: 239–244. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. (2010). mTOR‐dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Park SI, Oh JH, Kim SM, Jeong CH, Jun JA et al. (2008). Brain‐derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood‐derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt‐dependent signaling pathways. J Neurosci Res 86: 2168–2178. [DOI] [PubMed] [Google Scholar]
- Lin PY, Chang AY, Lin TK (2014). Simvastatin treatment exerts antidepressant‐like effect in rats exposed to chronic mild stress. Pharmacol Biochem Behav 124: 174–179. [DOI] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM (2007). Brain‐derived neurotrophic factor in neuronal survival and behavior‐related plasticity. Ann N Y Acad Sci 1122: 130–143. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA et al. (2006). Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 163: 1531–1541; quiz 1666. [DOI] [PubMed] [Google Scholar]
- Meng G, Yang S, Chen Y, Yao W, Zhu H, Zhang W (2015). Attenuating effects of dihydromyricetin on angiotensin II‐induced rat cardiomyocyte hypertrophy related to antioxidative activity in a NO‐dependent manner. Pharm Biol 53: 904–912. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S et al. (2007). Brain‐derived neurotrophic factor conditional knockouts show gender differences in depression‐related behaviors. Biol Psychiatry 61: 187–197. [DOI] [PubMed] [Google Scholar]
- Muller CJ, Groticke I, Bankstahl M, Loscher W (2009). Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine‐induced status epilepticus in C57BL/6 mice. Exp Neurol 219: 284–297. [DOI] [PubMed] [Google Scholar]
- Niculescu M, Perrine SA, Miller JS, Ehrlich ME, Unterwald EM (2008). Trk: a neuromodulator of age‐specific behavioral and neurochemical responses to cocaine in mice. J Neurosci 28: 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouk T, Gautier S, Petrault M, Montaigne D, Marechal X, Masse I et al. (2014). Effects of the PPAR‐α agonist fenofibrate on acute and short‐term consequences of brain ischemia. J Cereb Blood Flow Metab 34: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229: 327–336. [PubMed] [Google Scholar]
- Priestley RS, Nickolls SA, Alexander SP, Kendall DA (2015). A potential role for cannabinoid receptors in the therapeutic action of fenofibrate. FASEB J 29: 1446–1455. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ (2005). BDNF protects against stress‐induced impairments in spatial learning and memory and LTP. Hippocampus 15: 246–253. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME (2009). The PPARα agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole‐brain irradiation. Int J Radiat Oncol Biol Phys 75: 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Domenici E, Carboni L, Rantamaki T, Lindholm J, Castren E et al. (2011). A role for BDNF/TrkB signaling in behavioral and physiological consequences of social defeat stress. Genes Brain Behav 10: 424–433. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F et al. (2006). Brain‐derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 24: 1850–1856. [DOI] [PubMed] [Google Scholar]
- Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ et al. (2013). Regulation of cyclic AMP response element binding and hippocampal plasticity‐related genes by peroxisome proliferator‐activated receptor α. Cell Rep 4: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK et al. (2015). HMG‐CoA reductase inhibitors bind to PPARα to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab 22: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Sarkisyan G, Roberts AJ, Hedlund PB (2010). The 5‐HT(7) receptor as a mediator and modulator of antidepressant‐like behavior. Behav Brain Res 209: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME (1999). CREB: a stimulus‐induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68: 821–861. [DOI] [PubMed] [Google Scholar]
- Shelton RC (2007). The molecular neurobiology of depression. Psychiatr Clin North Am 30: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichinohe H, Ishihara T, Takahashi K, Tanaka Y, Miyamoto M, Yamauchi T et al. (2015). Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabil Neural Repair 29: 80–89. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002). Brain‐derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P (1985). The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85: 367–370. [DOI] [PubMed] [Google Scholar]
- Strain JD, Farver DK, Clem JR (2010). A review on the rationale and clinical use of concomitant rosuvastatin and fenofibrate/fenofibric acid therapy. Clin Pharmacol 2: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9: 519–525. [DOI] [PubMed] [Google Scholar]
- Xu X, He M, Liu T, Zeng Y, Zhang W (2015). Effect of salusin‐β on peroxisome proliferator‐activated receptor gamma gene expression in vascular smooth muscle cells and its possible mechanism. Cell Physiol Biochem 36: 2466–2479. [DOI] [PubMed] [Google Scholar]
- Yan HC, Qu HD, Sun LR, Li SJ, Cao X, Fang YY et al. (2010). Fuzi polysaccharide‐1 produces antidepressant‐like effects in mice. Int J Neuropsychopharmacol 13: 623–633. [DOI] [PubMed] [Google Scholar]
- Yao W, Gu C, Shao H, Meng G, Wang H, Jing X et al. (2015). Tetrahydroxystilbene glucoside improves TNF‐α‐induced endothelial dysfunction: involvement of TGFβ/Smad pathway and inhibition of vimentin expression. Am J Chin Med 43: 183–198. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L et al. (2010). Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant‐like effects in adult rats. J Neurosci 30: 12653–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The antidepressant‐like actions of fenofibrate occur independent of the cannabinoid system. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.) + ACSF with 1% DMSO (i.c.v.). (A) Blocking cannabinoid system with rimonabant/AM630 had no influence on the antidepressant‐like effects of fenofibrate in the FST. (B) Rimonabant/AM630 pretreatment could not eliminate the antidepressant‐like effects of fenofibrate in the TST. (C) Rimonabant and AM630 did not influence the locomotor activity of mice in the open field test. (D) Co‐treatment fenofibrate with rimonabant/AM630 did not block the effects of fenofibrate in the sucrose preference test, since CSDS + fenofibrate + rimonabant/AM630 mice had as much sucrose consumption as CSDS + fenofibrate mice. (E) Also, in the social interaction test, CSDS + fenofibrate + rimonabant/AM630 mice did not differ significantly from CSDS + fenofibrate mice. Results are expressed as means ± S.E.M. (n = 10–12); ** P < 0.01; n.s., no signifcance. Comparison was made by two‐way ANOVA followed by post hoc Bonferroni's test.
Figure S2 Blockade of BDNF signalling cascade by K252a prevents the antidepressant actions of fenofibrate. The vehicle refers to 5% dextrose (pH 7.0) with 2.5% DMSO and 10% Cremaphor EL (i.p.) + ACSF with 1% DMSO (i.c.v.). (A) K252a pretreatment before fenofibrate administration prevented the fenofibrate‐induced decrease of immobility in the FST test. (B) K252a pretreatment also prevented the fenofibrate‐induced decrease of immobility in the TST test. (C) K252a did not influence the locomotor activity of mice in the open field test. (D) CSDS mice were co‐injected with fenofibrate and K252a for 14 days. CSDS + fenofibrate + K252a mice displayed significantly lower sucrose preference than CSDS + fenofibrate mice. (E) Co‐treatment fenofibrate with K252a also blocked the effects of fenofibrate in the social interaction test. CSDS + fenofibrate + K252a mice displayed significantly lower social interaction than CSDS + fenofibrate mice. Results are expressed as means ± S.E.M. (n = 12); * P < 0.05; ** P < 0.01; n.s., no signifcance. Comparison was made by two‐way ANOVA followed by post hoc Bonferroni's test.
Supporting info item
Supporting info item
Supporting info item


