Abstract
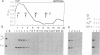
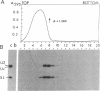
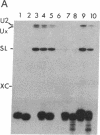
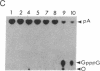
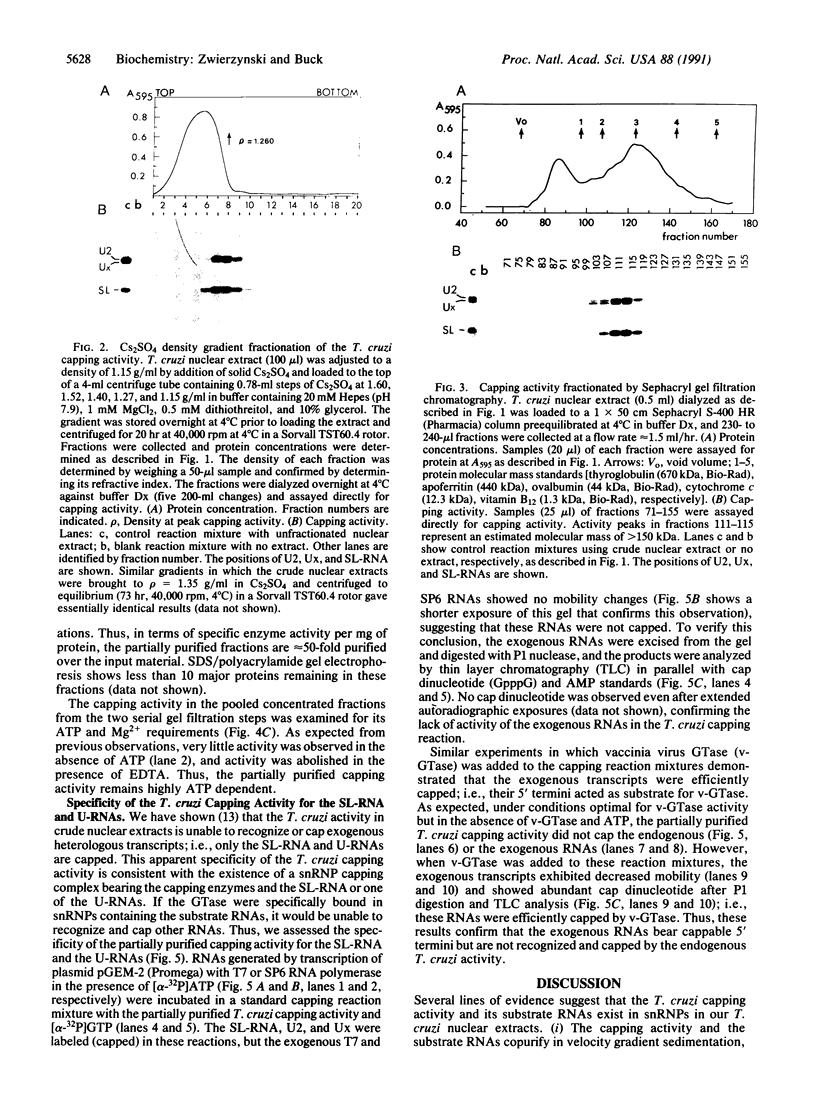
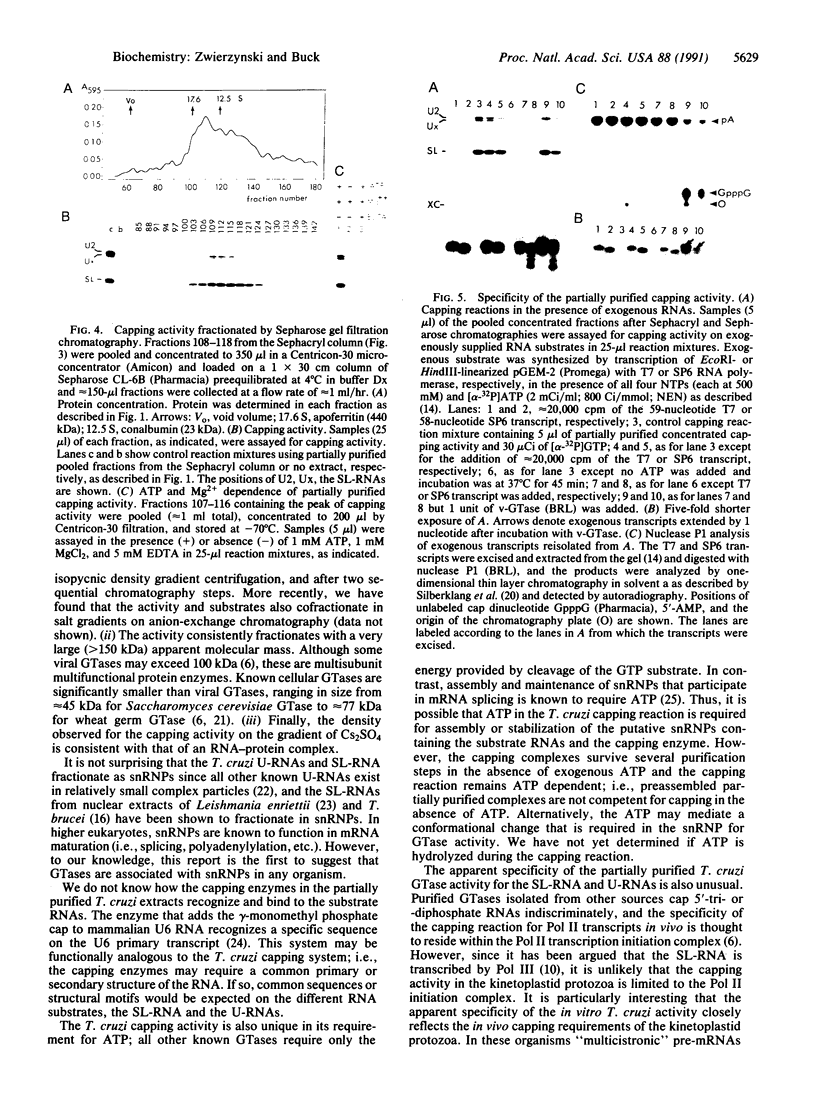
A 39-nucleotide spliced leader (SL) is joined to the 5' ends of trypanosome mRNAs in a bimolecular or trans-splicing process. The SL in Trypanosoma cruzi is transcribed as an approximately 110-nucleotide RNA (SL-RNA or SL primary transcript) bearing the 39-nucleotide SL at the 5' end. The SL-RNA is 5' capped by a guanylyltransferase activity prior to trans-splicing and trypanosome mRNAs thus obtain their mature caps from the SL by trans-splicing. We have previously characterized a guanylyltransferase activity from T. cruzi nuclear extracts and shown that this capping activity has an unusual ATP dependence and an apparent specificity for the SL-RNA and U-RNAs. Herein, we show that the capping activity sediments as a 12-15S particle during velocity sedimentation in glycerol gradients and fractionates as a greater than 150-kDa particle during large-pore gel filtration chromatography. Moreover, the endogenous substrate RNAs--the SL-RNA and U-RNAs--consistently copurify with the capping activity, suggesting that the activity and the substrates form a ribonucleoprotein particle. The capping activity and substrate RNAs are not dissociated in isopycnic Cs2SO4 gradients and band at a density expected for an RNA-protein complex, confirming the existence of ribonucleoprotein particles bearing both the activity and its substrate RNAs. Finally, we partially purified these ribonucleoprotein particles and showed that the capping activity remains ATP dependent and highly specific for the SL-RNA and the U-RNAs. These observations are consistent with the hypothesis that one of the functions of trans-splicing is for mRNA capping.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Bartkiewicz M., Gold H., Altman S. Identification and characterization of an RNA molecule that copurifies with RNase P activity from HeLa cells. Genes Dev. 1989 Apr;3(4):488–499. doi: 10.1101/gad.3.4.488. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Cross G. A., Branch A. D., Robertson H. D. Direct analysis of the mini-exon donor RNA of Trypanosoma brucei: detection of a novel cap structure also present in messenger RNA. Nucleic Acids Res. 1987 Dec 10;15(23):9861–9879. doi: 10.1093/nar/15.23.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Cross G. A., Robertson H. D. Discontinuously synthesized mRNA from Trypanosoma brucei contains the highly methylated 5' cap structure, m7GpppA*A*C(2'-O)mU*A. J Biol Chem. 1988 Oct 15;263(29):15071–15075. [PubMed] [Google Scholar]
- Grondal E. J., Evers R., Kosubek K., Cornelissen A. W. Characterization of the RNA polymerases of Trypanosoma brucei: trypanosomal mRNAs are composed of transcripts derived from both RNA polymerase II and III. EMBO J. 1989 Nov;8(11):3383–3389. doi: 10.1002/j.1460-2075.1989.tb08502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Dorfman D. M., Donelson J. E. The spliced leader sequence of Trypanosoma brucei has a potential role as a cap donor structure. Mol Cell Biol. 1985 Sep;5(9):2487–2490. doi: 10.1128/mcb.5.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Michaeli S., Roberts T. G., Watkins K. P., Agabian N. Isolation of distinct small ribonucleoprotein particles containing the spliced leader and U2 RNAs of Trypanosoma brucei. J Biol Chem. 1990 Jun 25;265(18):10582–10588. [PubMed] [Google Scholar]
- Miller S. I., Wirth D. F. trans splicing in Leishmania enriettii and identification of ribonucleoprotein complexes containing the spliced leader and U2 equivalent RNAs. Mol Cell Biol. 1988 Jun;8(6):2597–2603. doi: 10.1128/mcb.8.6.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kaziro Y. Messenger RNA capping enzymes from eukaryotic cells. Prog Nucleic Acid Res Mol Biol. 1987;34:1–28. doi: 10.1016/s0079-6603(08)60491-2. [DOI] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Polycistronic transcripts in trypanosomes and their accumulation during heat shock: evidence for a precursor role in mRNA synthesis. Mol Cell Biol. 1988 Sep;8(9):3837–3846. doi: 10.1128/mcb.8.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R., Griffith J., Maniatis T. Purification and visualization of native spliceosomes. Cell. 1988 Jun 17;53(6):949–961. doi: 10.1016/s0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Rudenko G., Bishop D., Gottesdiener K., Van der Ploeg L. H. Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J. 1989 Dec 20;8(13):4259–4263. doi: 10.1002/j.1460-2075.1989.tb08611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seliger L. S., Zheng K., Shatkin A. J. Complete nucleotide sequence of reovirus L2 gene and deduced amino acid sequence of viral mRNA guanylyltransferase. J Biol Chem. 1987 Dec 5;262(34):16289–16293. [PubMed] [Google Scholar]
- Shea C., Lee M. G., Van der Ploeg L. H. VSG gene 118 is transcribed from a cotransposed pol I-like promoter. Cell. 1987 Aug 14;50(4):603–612. doi: 10.1016/0092-8674(87)90033-x. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Singh R., Gupta S., Reddy R. Capping of mammalian U6 small nuclear RNA in vitro is directed by a conserved stem-loop and AUAUAC sequence: conversion of a noncapped RNA into a capped RNA. Mol Cell Biol. 1990 Mar;10(3):939–946. doi: 10.1128/mcb.10.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. The cap of both miniexon-derived RNA and mRNA of trypanosomes is 7-methylguanosine. Mol Cell Biol. 1988 Jan;8(1):494–496. doi: 10.1128/mcb.8.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Polygene transcripts are precursors to calmodulin mRNAs in trypanosomes. EMBO J. 1988 Feb;7(2):455–463. doi: 10.1002/j.1460-2075.1988.tb02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G. W., Sauterer R. A. Cell biology of the snRNP particles. Crit Rev Biochem Mol Biol. 1990;25(1):1–46. doi: 10.3109/10409239009090604. [DOI] [PubMed] [Google Scholar]
- Zwierzynski T. A., Buck G. A. In vitro capping in Trypanosoma cruzi identifies and shows specificity for the spliced leader RNA and U-RNAs. Nucleic Acids Res. 1990 Jul 25;18(14):4197–4206. doi: 10.1093/nar/18.14.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwierzynski T. A., Widmer G., Buck G. A. In vitro 3' end processing and poly(A) tailing of RNA in Trypanosoma cruzi. Nucleic Acids Res. 1989 Jun 26;17(12):4647–4660. doi: 10.1093/nar/17.12.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]