Abstract
Arthropod-borne viruses (arboviruses) mainly infect people via direct spillover from enzootic cycles. However, dengue, chikungunya, and yellow fever viruses have repeatedly initiated urban transmission cycles involving human amplification and peridomestic mosquito vectors to cause major epidemics. Here, I review these urban emergences and potential strategies for their prevention and control.
Keywords: arbovirus, mosquito, urban, prevention of transmission
Arthropod-borne viruses (arboviruses) are transmitted by mosquitoes and other biting arthropods. They occur nearly worldwide and all were, until recently, zoonotic in nature (circulating enzootically among wild animals but sometimes infecting people). Arboviruses have undoubtedly caused human disease in people for at least millennia, but a few have increased in importance during recent decades because of human population expansion and activities that have increased exposure to infection. Some have simply expanded the geographic range of their enzootic cycles, often due to human trade or travel, to infect more people via direct spillover (transmission from animals via enzootic or bridge vectors to humans, who typically do not develop sufficient viremia to extend the transmission chain and are thus dead-end hosts). An example is West Nile virus (WNV; Flaviviridae: Flavivirus), which circulates in an avian–mosquito cycle and has caused small epidemics for many decades, because of spillover, in the Old World. However, in 1999 WNV was introduced into New York, where it amplified and spread rapidly to cause the largest epidemic of arboviral encephalitis ever documented in the USA, followed by a major resurgence in 2012 [1]. Several other arboviruses may have comparable abilities to invade new geographic regions; the evidence that little or no adaptive evolution was needed for the WNV epidemic to spread across the Americas [2] suggests that such events could occur with other deadly flaviviruses that exploit multiple vectors and hosts [3].
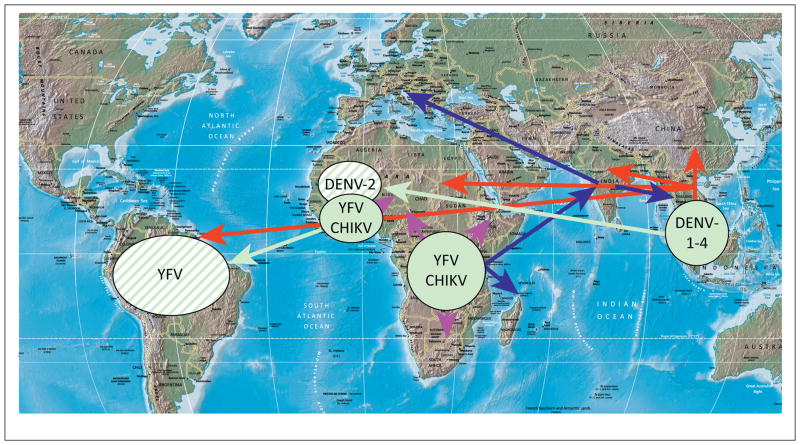
Several of the most important arboviral pathogens are those that not only infect people via enzootic spillover but also use humans as amplification hosts. The most important is the flavivirus dengue virus (DENV), which infects more than 50 million people annually nearly throughout the tropics and subtropics, with secondary infections carrying a high risk of hemorrhagic disease that can be fatal [4]. DENV, which includes four serotypes with limited antigenic cross-reactivity, evolved thousands of years ago in Southeast Asia, where divergence of the four serotypes probably occurred in arboreal nonhuman primate (NHP)–mosquito cycles (Figure 1). Later, DENV-2 was introduced into an African enzootic cycle (probably via spillback or infection of arboreal vectors or NHPs by human strains) and all four serotypes ultimately emerged into independent endemic or epidemic cycles involving humans and the anthropophilic mosquito vectors Aedes aegypti and Aedes albopictus [5]. Extensive urbanization, the nearly global tropical invasion by A. aegypti, and increased commerce and travel led to an explosion of urban dengue fever after World War II [4,6]. Evidence suggests that there was little or no adaptation when enzootic DENV-2 first emerged in the urban cycle, and that human DENV infections with enzootic strains in West Africa and Southeast Asia can be highly pathogenic, like urban infections [5].
Figure 1.
Map showing approximate enzootic origins of dengue virus (DENV), chikungunya virus (CHIKV), and yellow fever virus (YFV) (green circles), introductions (green arrows) that established additional documented sites of enzootic transmission (striped green circles), and general patterns (not comprehensive) of the urbanized spread of DENV (red), CHIKV (blue), or YFV (magenta) leading to extant endemic or epidemic strains.
Another flavivirus with a long history of emergence into a human–A. aegypti cycle is yellow fever virus (YFV), which is highly virulent (case fatality rate of 20–50%) [7]. YFV originated in Africa (Figure 1) in a mosquito–NHP arboreal transmission cycle nearly indistinguishable from that of DENV [8]. Like DENV, YFV caused periodic epidemics in port cities of the Americas, including temperate regions, during the 17th to early 20th centuries, as well as in many parts of Africa. Urban YFV epidemics involving A. aegypti transmission have recently been infrequently reported, especially in Latin America. However, unlike DENV, YFV developed enzootic transmission cycles in the Neotropics, probably via spillback, during the early years of the slave trade from West Africa. Also unlike DENV, YFV has no documented history of spread into Asia, and its urban cycle has never become permanent (Figure 1). An effective YFV vaccine has been available since the 1930s. Unfortunately, incomplete coverage in regions at risk of enzootic spillover and urban epidemics allows hundreds to thousands of human infections, many of which are fatal, to continue annually in Africa and South America, with many more undoubtedly unreported [7].
The third major arboviral disease with a history of urbanization is chikungunya virus (CHIKV; Togaviridae: Alphavirus), which originated in Africa (Figure 1) and, like DENV and YFV, was probably transported by sailing ships to port cities during the 18th and 19th centuries [3]. However, CHIKV has spread from eastern Africa into Asia at least twice to establish permanent urban transmission. One emergence occurred during or before the early 1950s. The most recent emergence began in coastal Kenya in 2004, before spreading into islands of the Indian Ocean and into Asia to cause severe, often chronic arthralgia in millions of people (Figure 1). The recent epidemics were facilitated by A. albopictus-adaptive mutations that led to more efficient interhuman transmission, even in temperate regions of Europe where this mosquito survives cold winters.
History suggests that enzootic arboviruses that use NHPs as hosts probably represent the greatest risk for urbanization. This group includes the arthralgic alpha-virus Mayaro (MAYV), which circulates enzootically in South America among NHPs, transmitted by arboreal mosquitoes in the genera Haemagogus and Sabethes. Another threat is the flavivirus Zika, which circulates widely in Africa and Asia, probably using NHPs as enzootic hosts, and which recently caused an epidemic of febrile illness, apparently involving human amplification, on Yap Island in the Pacific [9]. Although its enzootic cycle does not appear to involve NHPs, Oropouche virus (OROV; Bunyaviridae: Bunyavirus), an enzootic in South America, has caused major epidemics of febrile illness involving human amplification and urban transmission by the midge Culicoides paraensis [10].
Five strategies for preventing or mitigating arbovirus urbanization are outlined below.
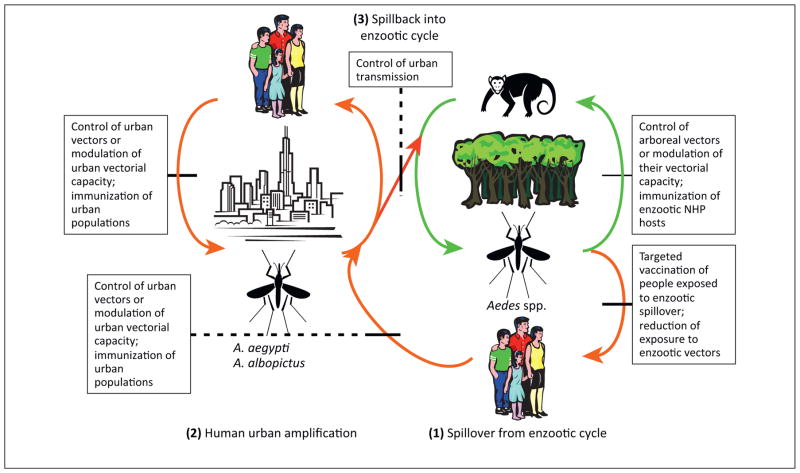
Intervention in enzootic cycles. In theory, the prevention of arbovirus urbanization could be mitigated at several stages of the emergence process (Figure 2). Reduction or elimination of enzootic circulation via vector control or prevention of reservoir and amplification host infection would be highly challenging considering the widespread and often remote locations of sylvatic foci, the environmental concerns of large-scale insecticide use, and the extreme difficulty of vaccinating wild animals. Modulation of mosquito transmission by the introduction of endosymbiotic Wolbachia spp. bacteria, which suppress the replication of some arboviruses, holds promise for reducing urban transmission of DENV, YFV, and CHIKV [11]. However, it is unknown if this approach could be applied to the more diverse enzootic vectors that are poorly understood.
Prevention of enzootic spillover. If enzootic transmission of arboviruses within their sylvatic NHP cycles offers a poor target for interruption, then prevention of introductions into the urban cycle represents the next step potentially amenable to intervention (Figure 2). DENV, YFV, and CHIKV apparently emerge from enzootic cycles by infecting people who live near forested habitats and who then initiate the human–mosquito cycle, and ultimately transport it to urban centers. Thus, reductions in the exposure of these human populations to enzootic vectors, or vaccination of such persons, could reduce the risk of further urbanization. Some measures successfully implemented to reduce infection with malaria parasites and other vector-borne pathogens, such as bed nets, may offer some protection against enzootic arbovirus spillover infections. Nevertheless, anthropogenic factors such as increased human settlement and activity in forests, and deforestation itself, may increase enzootic human exposure over time. Better targeted vaccination of people who live near African enzootic YFV foci could decrease the risk of its reemergence, but licensed vaccines to protect against DENV and CHIKV are not available, and a tetravalent DENV vaccine was disappointing in recent human efficacy trials [12]. CHIKV, owing to its limited antigenic variation and lack of immune enhancement, offers a simpler target for vaccine development, and several promising candidates have been described [13]. Further development of these vaccines, as well as efforts to improve the safety of the YFV 17D vaccine, should be prioritized. Vaccines for other viruses with urbanization potential, such MAYV and OROV, which also cause a large burden of enzootic spillover disease, should also be developed.
Prevention of urban introductions. If spillover enzootic infections cannot be prevented or reduced, preemptive (for YFV and CHIKV) reductions in the urban transmission potential represent the next target for intervention via control of A. aegypti and A. albopictus, modulation of their vectorial capacity, and vaccination of urban populations, as discussed below (Figure 2).
-
Reduction or elimination of urban transmission. If urban introductions cannot be prevented, reductions in urban mosquito–human transmission remain the only option to reduce disease burden. Control of the major YFV and DENV vector, A. aegypti, has proved challenging because of its behavioral traits, including its exploitation of human refuse and water storage containers as larval habitats, and the tendency of adult females to remain inside houses, where insecticide applications are intrusive and expensive [6]. A. albopictus, the major CHIKV vector that has invaded the Americas, Africa, and Southern Europe from its native Asia since 1985, also lives in association with people, albeit to a lesser degree than A. aegypti. However, epidemiological studies suggest that DENV infection is spatially distributed based primarily on human movement, suggesting that better targeted vector control, not necessarily in the places with the highest vector density, could impact incidence [14]. Moreover, major differences in DEN incidence and seroprevalence in the adjacent cities of Matamoros, Mexico, and Brownsville, USA, suggest that socioeconomic differences play a major role in DENV transmission [15].
The use of Wolbachia spp. to suppress transmission of all three viruses is one of the most promising strategies on the horizon to reduce mosquito transmission. Ultimately, however, vaccines offer the best hope of controlling the urban cycles and of preventing reemergence. In theory, vaccination could eradicate all of the urban cycles because humans are the only amplification hosts. Furthermore, the prevention or suppression of CHIKV and YFV epidemics via vaccination would greatly reduce the risk of importation into the Americas and Asia, respectively, and potential devastating epidemics. However, even if urban transmission were eradicated, vaccination of urban populations, as well as those exposed to spillover infections near sylvatic foci, would be required to prevent reemergence in the absence of methods to control enzootic circulation.
Prevention of spillback into enzootic cycles. For DENV and CHIKV in the Americas and YFV in Asia, spillback into enzootic cycles (infection of arboreal vectors or NHPs by urban virus strains, leading to stable enzootic transmission) could lead to additional sources of urban reemergence, as well as spillover infections, both of which have major public health consequences. It is likely that the only way to reduce this risk is to control urban transmission (and prevent YFV from being introduced into Asia and CHIKV into the Americas). A far better understanding of the vector competence of neotropical (for CHIKV and DENV) and Asian (for YFV and CHIKV) arboreal primatophilic mosquitoes for these urbanized viruses is needed to estimate the probability of these spillback events.
Figure 2.
Cartoon depicting the emergence of urban transmission cycles for dengue virus (DENV), yellow fever virus (YFV), and chikungunya virus (CHIKV) from enzootic cycles. Lines through arrows indicate potential points for intervention in enzootic circulation, spillover infections of humans, introductions into the urban cycle, and spillback from urban cycles to initiate arboreal enzootic cycles. The line thickness reflects the likelihood of success of these interventions (a thicker line indicates a greater likelihood of success).
Acknowledgments
I thank Nikos Vasilakis for critical reading of the manuscript. My research on arboviruses is supported by NIH grants R01-AI071192, R01-AI069145, and R01AI093491 and by the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (NIH grant U54 AIO57156).
References
- 1.Arnold C. West Nile virus bites back. Lancet Neurol. 2012;11:1023–1024. doi: 10.1016/S1474-4422(12)70278-8. [DOI] [PubMed] [Google Scholar]
- 2.Coffey LL, et al. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol. 2013;8:155–176. doi: 10.2217/fmb.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2009;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons CP, et al. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 5.Vasilakis N, et al. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jentes ES, et al. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- 8.Bryant JE, et al. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3:e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy MR, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 10.Tesh RB. The emerging epidemiology of Venezuelan hemorrhagic fever and Oropouche fever in tropical South America. Ann N Y Acad Sci. 1994;740:129–137. doi: 10.1111/j.1749-6632.1994.tb19863.x. [DOI] [PubMed] [Google Scholar]
- 11.Maciel-de-Freitas R, et al. Why do we need alternative tools to control mosquito-borne diseases in Latin America? Mem Inst Oswaldo Cruz. 2012;107:828–829. doi: 10.1590/s0074-02762012000600021. [DOI] [PubMed] [Google Scholar]
- 12.Sabchareon A, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled Phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 13.Weaver SC, et al. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines. 2012;11:1087–1101. doi: 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honorio NA, et al. Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2009;3:e545. doi: 10.1371/journal.pntd.0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos MM, et al. Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg. 2008;78:364–369. [PubMed] [Google Scholar]